1. Introduction
Here, we report a 56-year-old male patient diagnosed with adult-onset, severe, sensory–motor polyneuropathy caused by a unique heterozygous
DNAJB2 c.823+6C>T mutation. The
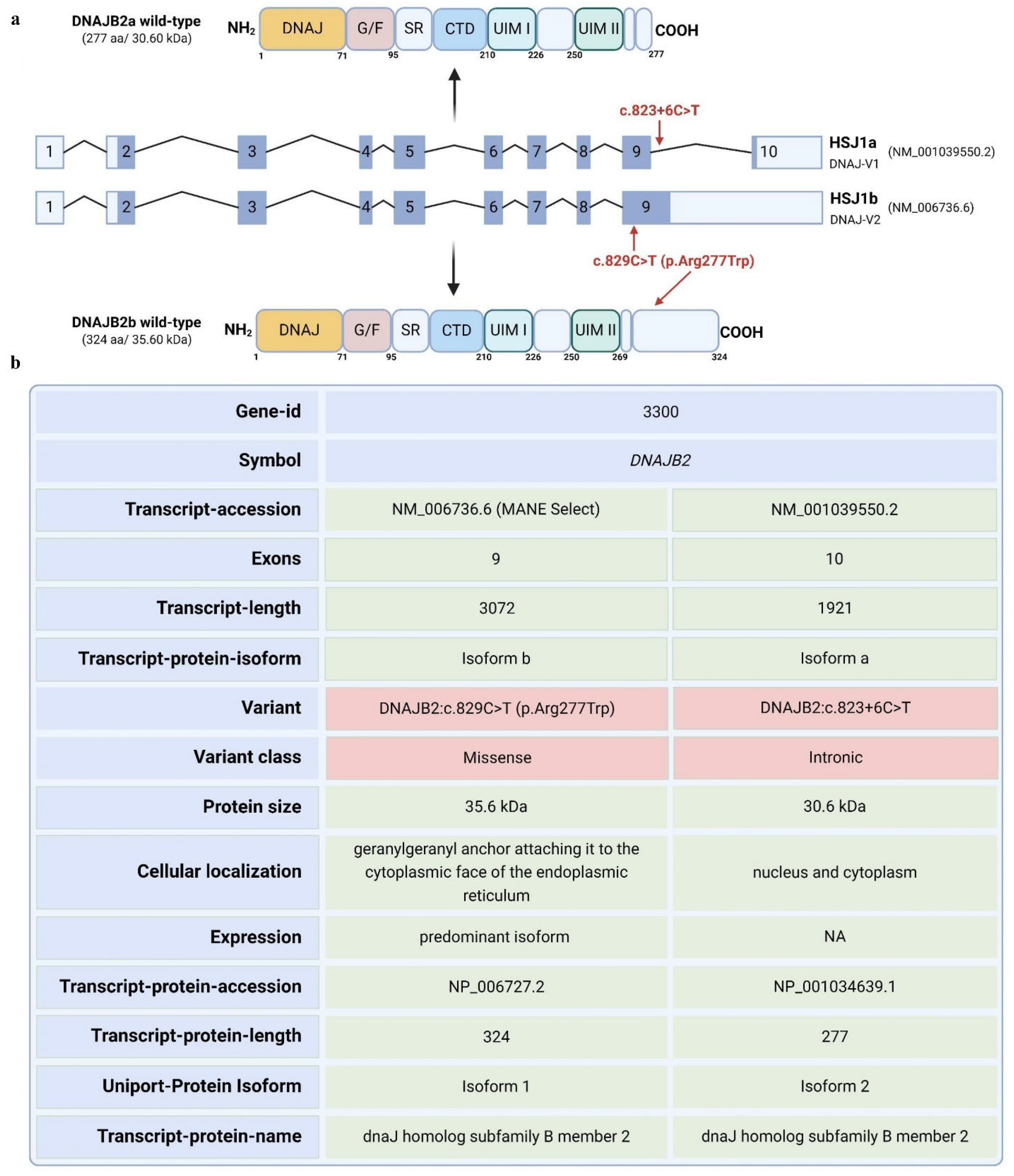
DNAJB2 gene has 10 exons and is expressed in two isoforms. Transcript a contains exons 2–10, while transcript b contains exons 2–9 [
1]. The mutation is predicted to affect the two transcripts differently. For transcript a, the mutation is in a splice site, and algorithms predict that it will strengthen the splice site, possibly causing aberrant splicing. For transcript b, the mutation falls in the coding sequence and will lead to an amino acid change from arginine to tryptophan at position 277 of the amino acid sequence. Arginine is highly conserved among species at that site, indicating a potential significance of this mutation (
Figure S1).
Mutations in DNAJB2 have been linked to neuromuscular diseases such as distal hereditary motor neuropathy (dHMN) and Charcot-Marie-Tooth disease type 2 (CMT2) [
1,
2,
3,
4,
5]. These mutations often result in a loss of DNAJB2 expression, leading to peripheral axonal neuropathy with symptoms typically appearing in the second decade of life [
1,
2,
3,
4,
5]. Some patients also exhibit central nervous system symptoms, including early-onset Parkinsonism [
4,
5,
6]. Moreover, in an in vitro model, it has been shown that these mutations disrupt DNAJB2 expression in fibroblasts, with a presumed similar effect in neurons [
1,
2]. The exact client proteins and cellular processes affected remain to be determined. Overall, DNAJB2 plays a crucial role in protein quality control, particularly in neurons, by directing misfolded or aggregated proteins to degradation pathways, thereby maintaining cellular homeostasis.
DNAJB2 (HSP40), initially described as HSJ1, belongs to class II (DNAJB subfamily) of J proteins [
7]. It contains an N-terminal J-domain followed by a G/F region. J domain is crucial for binding Hsp70 and activating its ATPase activity [
8]. A distinctive feature of HSJ1 is its C-terminal region, which includes two ubiquitin interaction motifs (UIMs) that facilitate binding to polyubiquitylated proteins and the proteasome [
9,
10]. The glycine/phenylalanine-rich region and ubiquitin-interacting motifs (UIMs) also contribute to specific substrate recognition and targeting for degradation whenever necessary [
8,
10]. HSJ1 undergoes alternative splicing, resulting in two isoforms with different C-termini and subcellular localizations: DNAJB2a (HSJ1a, 277 aa, 31 kDa), which localizes to the cytosol and nucleus, and DNAJB2b (HSJ1b, 324 aa, 36 kDa), which is associated with the cytoplasmic face of the ER via a C-terminal geranylgeranyl anchor (
Figure 1) [
8].
DNAJB2 is predominantly expressed in neurons, particularly in the neocortex, with lower levels detected in other tissues and cells [
1,
2,
7,
8]. The primary isoform in neuronal tissues is HSJ1b [
1,
2,
8,
11]. DNAJB2 has been reported to be expressed at low levels in cardiac and skeletal muscles as well as fibroblasts [
12,
13]. Its presence at neuromuscular junctions in mature muscle fibers and its upregulation after eccentric exercise suggest physiological relevance in muscle [
12,
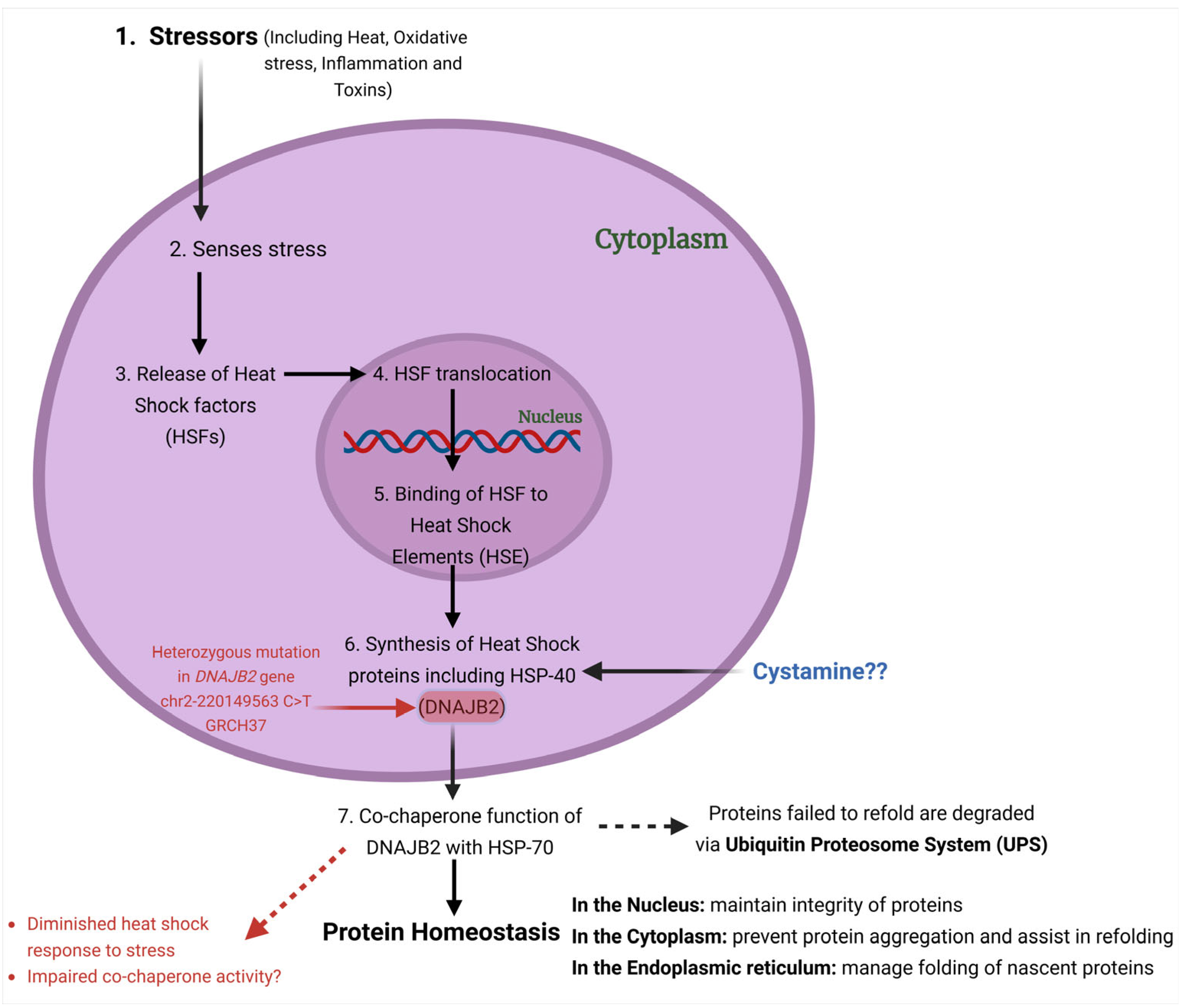
13]. Functionally, DNAJB2 acts as a co-chaperone for HSPA (HSP70), enhancing HSPA’s ATPase activity and modulating its client binding [
14] (
Figure 2). Unlike typical co-chaperones that promote protein refolding, DNAJB2 primarily directs proteins towards degradation by the ubiquitin–proteasome system (UPS) [
10,
15].
In the present study, we aimed to study the effect of the newly identified mutation in our patient on the stress response. We used acute heat shock (at 42 °C) as a source of stress to fibroblasts and assessed the levels of DNAJB2. Additionally, we evaluated the effect of Cystamine on DNAJB2 expression under both baseline and stress conditions in control and mutant DNAJB2 fibroblasts.
Cystamine, an oxidized form of Cysteamine has been reported to increase the levels of isoform HSJ1b in Huntington disease (HD) models [
11,
16]. Cystamine was found to benefit HD mice by increasing the levels of BDNF in both the brain and serum [
11]. The effect was attributed to the inhibition of transglutaminase activity, a process associated with neurodegeneration in HD [
17]. However, later studies revealed that the protective effect of Cystamine is independent of transglutaminase inhibition [
17]. Other hypotheses include its role in inhibiting caspases, which are involved in late-stage HD pathogenesis [
18]. Lastly, Cystamine may modulate cysteine metabolism, increasing antioxidant glutathione (GSH) and hypotaurine levels, further contributing to neuroprotection [
18,
19]. These various mechanisms highlight the multifaceted role of Cystamine in promoting neuronal survival and function in HD models.
3. Discussion
In this work, we explored the effect of a heterozygous DNAJB2 c.823+6C>T mutation on the RNA and protein expression levels. We evaluated the ability of mutant cells to appropriately upregulate expression levels of both the isoforms of DNAJB2 after heat shock and upon Cystamine stimulation. Our results are impacted by working with a heterozygous and not a homozygous cell line, and it is possible that the effects of the mutation in a homozygous cell line would have been more pronounced and easier to delineate. Given that the patient has developed disease despite the presence of one normal allele, it was justifiable to use un-manipulated fibroblasts instead of an engineered homozygous cell line. Although, this is not the best cell line, not a neuronal cell, the ease of obtaining, maintaining, and stimulating fibroblasts with heat shock determined our choice.
Mutations in the
DNAJB2 gene have been linked to various neurodegenerative disorders [
22]. Different specific mutations are associated with distinct neurological conditions [
22]. For example, mutations that affect splicing at introns 4 and 5 of the gene are reported to cause distal hereditary motor neuropathy, indicating that accurate splicing is crucial for normal protein function. Another mutation in the J-domain of DNAJB2 is associated with Charcot Marie Tooth disease type 2 (CMT-2), which causes progressive muscle weakness. Additionally, splice site deletion and a deletion affecting the J-domain have been found in patients with severe motor neuron diseases like spinal muscular atrophy and juvenile Parkinsonism [
1,
2,
3,
5,
23]. These findings highlight the vital role of DNAJB2 in maintaining neuronal integrity and its implication in these debilitating conditions.
The c.823+6C>T variant identified in the DNAJB2 gene in this neuropathy patient is located within the splice site of intron 9. This type of mutation typically results in abnormal splicing, leading to either the production of aberrant proteins or a reduction in functional protein levels. While in silico predictions suggest that this mutation could potentially affect RNA splicing, its direct role in the pathogenesis of DNAJB2-related conditions is still uncertain, as no prior studies have documented the pathogenicity of this specific variant. Since this variant has contributed to the polyneuropathy observed in the patient and this protein generally has a crucial role in protein quality control and cellular stress response, we examined the exact impact of this variant on the expression levels of DNAJB2 under normal and stressful conditions.
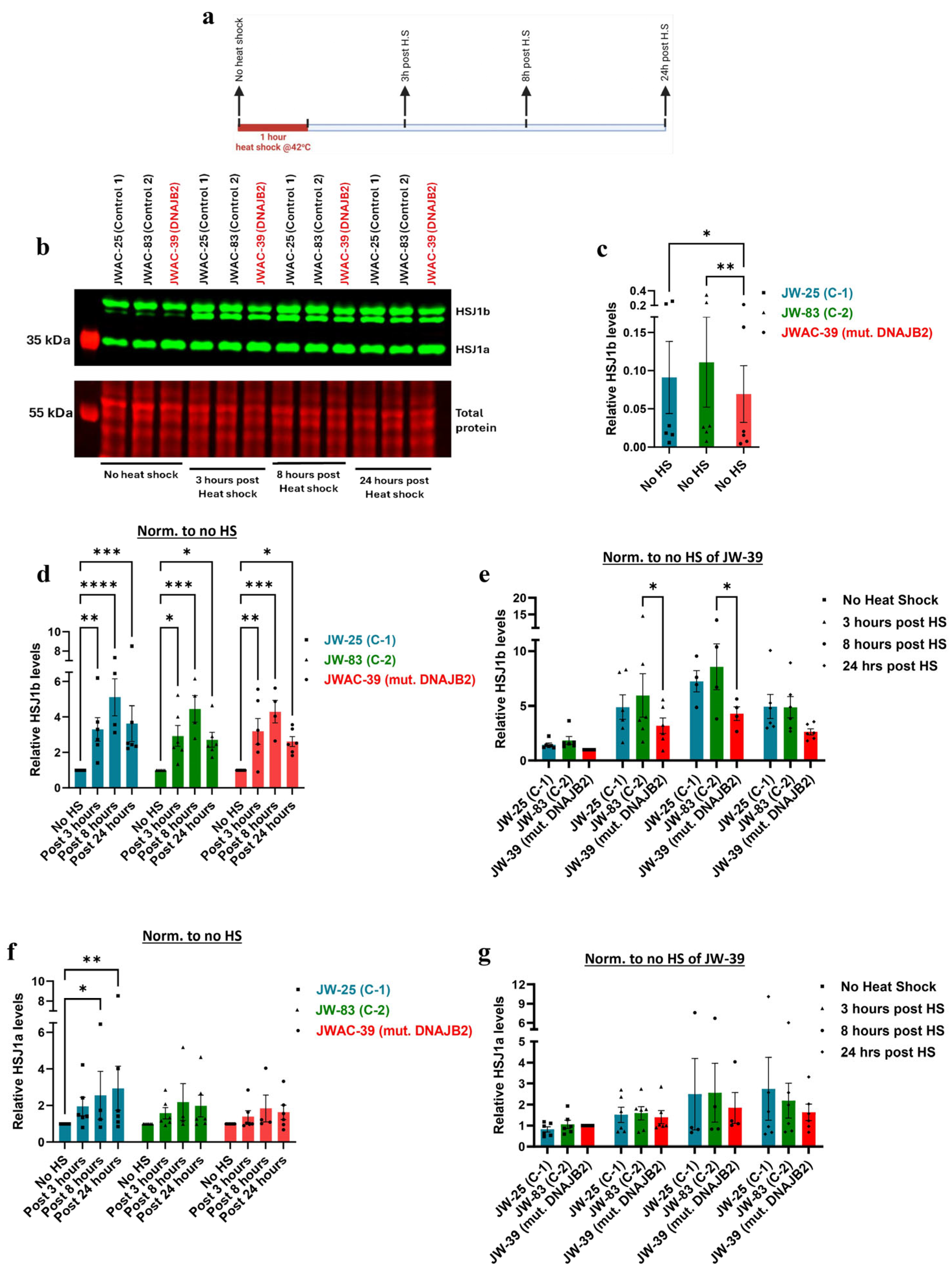
Our findings revealed that at baseline,
HSJ1a gene expression was not significantly different between the DNAJB2-mutated fibroblasts and the controls (
Figure 3b). This suggests that the mutation did not affect HSJ1a expression under non-stressed conditions. The HSJ1a transcripts did show an increase in expression at 3 h post-heat shock, with no observable differences between control and mutant cells (
Figure 3d). This suggests that the mutation does not appear to have a substantial effect on HSJ1a expression or its regulation in response to heat stress. However,
HSJ1b gene expression was significantly lower at baseline in mut. DNAJB2 fibroblasts compared to controls, suggesting that the mutation specifically affects the expression of this isoform (
Figure 3e). After heat shock treatment, HSJ1b expression remained low in the mut. DNAJB2 fibroblasts compared to the control fibroblasts (
Figure 3g). In our patient, although we do not have direct evidence of the effect of the mutation on neuronal cells, the evidence from fibroblasts suggests that a possible reduction in the baseline level of HSJ1b as well as under stress conditions might lead to misfolded protein accumulation due to insufficient folding or failure to direct these proteins to the proteasome.
Supporting these findings, immunoblot analysis revealed that HSJ1b protein expression was significantly lower at baseline in the mutated fibroblasts, and the increase in response to heat shock was substantially weaker compared to the controls (
Figure 4b,c,e). Post-translational geranylgeranylation is essential for the C-terminal end of HSJ1b to mediate its attachment to the cytoplasmic side of the endoplasmic reticulum membrane. Therefore, the mutation that falls in the C-terminal end of the protein, such as in our case, may have prevented proper attachment, thereby disrupting its normal localization and expression [
24]. While the magnitude of the response in DNAJB2-mutated cells was similar to that of the two control lines following heat shock, the lower baseline level of HSJ1b expression in these cells did not reach the abundance levels, as seen in the control cells (
Figure 4b,d,e).
Although outside of the scope of this work, implications from our findings may relate to neuronal processes which will be investigated in future studies. DNAJB2 plays a critical role in protein quality control by directing misfolded or aggregated proteins to the ubiquitin–proteasome system for degradation, thereby preventing toxic accumulation within neurons [
25]. he expression of DNAJB2 in peripheral nerve axons alongside neurofilaments indicates that the mutation in this protein could disrupt axonal transport [
12]. Similarly, the abundant expression of DNAJB2 at the neuromuscular junction (NMJ), where it interacts with other chaperones involved in facilitating the clustering of acetylcholine receptors, suggests possible defects in NMJ maintenance [
26]. Overall, our findings highlight the significant implication of HSJ1b dysfunction both at RNA and protein level. Mutation resulting in impaired DNAJB2 function might have led to the accumulation of misfolded proteins, disrupted axonal transport, and compromised NMJ integrity, collectively contributing to the pathological process.
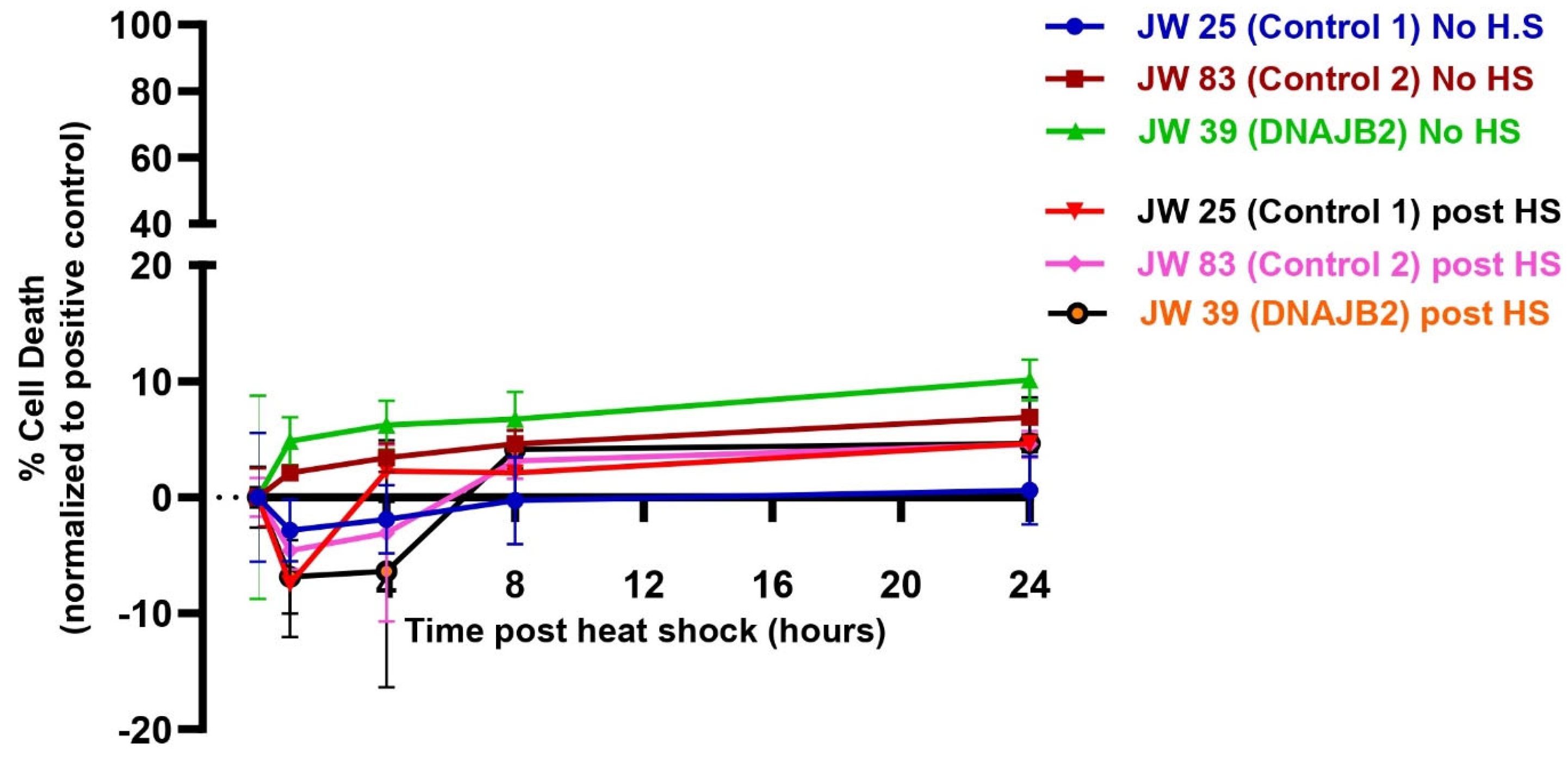
Despite the impaired cellular response to heat shock, the level of cell death was even between DNAJB2-mutated and control fibroblasts across all conditions (
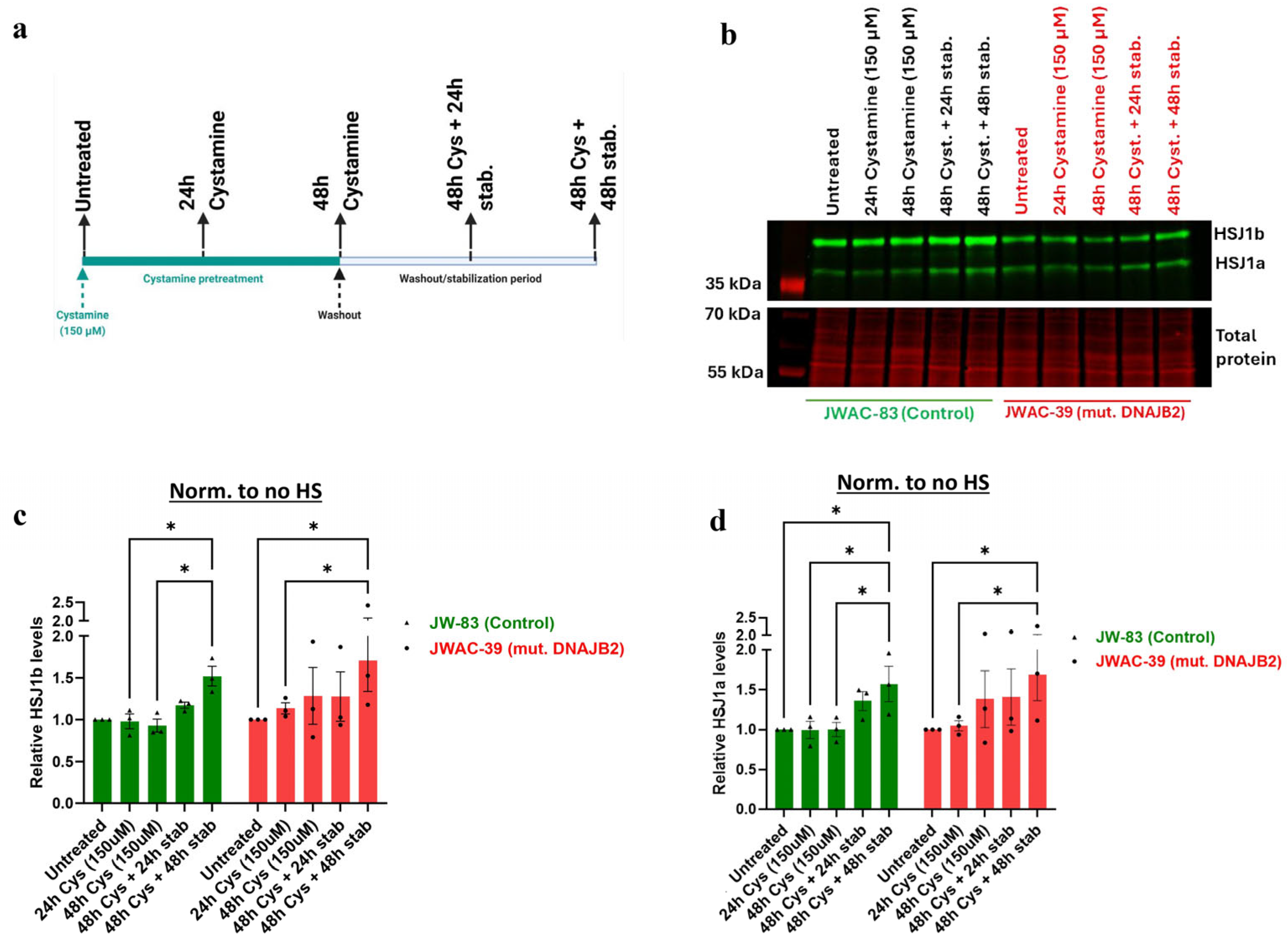
Figure 5). In the heat shock group, we observed an initial dip in fluorescence intensity immediately following heat shock, which stabilized after approximately 3 h. This fluctuation is technical, likely caused by the instability of the CellTox dye under heat shock conditions, rather than indicating actual changes in cell viability. While the mutation affected the expression of DNAJB2, it did not increase susceptibility to cell death under stress conditions. This indicates that the cellular dysfunction caused by the mutation is sufficiently balanced by the product of the normal allele. Next, we found that Cystamine (150 µM) pretreatment significantly increased DNAJB2 expression in both control and mut. DNAJB2 fibroblasts (
Figure 6b,c). This suggests that Cystamine may restore DNAJB2 expression, potentially offering a therapeutic strategy for enhancing the levels of this protein. Future studies investigating its precise role in BDNF up-regulation, caspase inhibition, and cysteine metabolism would provide deeper insights into its therapeutic potential in DNAJB2-related disorders.
4. Materials and Methods
4.1. Recruitment of Patients
The patient (JWAC-39) was clinically evaluated by a Neuromuscular Neurologist (Dr. Hristelina Ilieva) at Saint Luke’s Hospital, Kansas City (
Table 1) and diagnosed with severe sensory motor polyneuropathy. Genetic neuropathy panel revealed a
DNAJB2 c.823+6C>T mutation. The skin punch of the patient was obtained by the Weinberg ALS Center, Thomas Jefferson University and fibroblast lines were established. Fibroblast cultures were also obtained from two age-matched healthy controls (JWAC-25 and JWAC-83) for comparison.
4.2. Sanger Sequencing
Total RNA was extracted from patient fibroblasts using TRIzol (Invitrogen, Carlsbad, California, Catalog: 15596018) and stored at −80 °C till further analysis. The extracted samples were transported on dry ice for commercial Sanger sequencing to Azenta, South Plainfield, NJ, USA (formerly GENEWIZ), which included PCR clean-up and bidirectional sequencing of both the isoforms of DNAJB2. The primers were designed to cover the mutation site of both the isoforms from both the directions. All primers were synthesized by Genewiz and are listed in
Supplementary Table S2. Chromatograms were analyzed using Chromas version 2.6.6 to identify potential splicing events, such as exon or the intron retention of the transcript, caused by the variant.
4.3. Collection and Culturing of Fibroblasts
After obtaining informed consent from the patient/healthy volunteers, a 3 mm skin punch biopsy was performed on each participant after local anesthesia. The collected skin biopsies were seeded in a 10 cm dish containing Dulbecco’s Modified Eagle Medium (DMEM), high glucose (Cytiva, Marlborough, MA, USA, Cat. SH30243.01) supplemented with 20% fetal bovine serum (FBS) (Gibco, Jenks, OK, USA, Catalog: 10437028), and 1% penicillin-streptomycin (HyClone, Catalog: SV30010). The dishes were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Fibroblasts were allowed to grow, and media was changed every 2–3 days until the growing fibroblast cultures reached confluence. The cells were frozen in liquid nitrogen, and the subsequent passages from 3 to 6 were used for the experiments.
4.4. Heat Shock Treatment
To induce a heat shock response, fibroblast cultures were exposed to 42 °C for 1 h in a CO
2 incubator, followed by a recovery period at 37 °C at various time points (3, 8, and 24 h post heat shock). The expressions were compared to the cells without undergoing heat shock treatment. This temperature (42 °C) has been reported to be effective in inducing heat shock response, including the upregulation of heat shock proteins such as HSP40 and HSP70, without causing immediate cell death [
27,
28,
29]. The levels of DNAJB2 were assessed via Western blot at these time points to determine the trajectory of the heat shock response. The optimal time points for subsequent treatments were selected based on the maximal expression levels of the protein.
4.5. Cystamine Treatment
For Cystamine treatment, fibroblasts were incubated with 150 µM Cystamine for 24 and 48 h. This dose was based on a previous study that used 100 µM in mouse immortalized neuronal cells demonstrating its effectiveness in increasing HSJ1b levels [
11]. In our preliminary experiments, a 150 µM dose was found to be more effective in eliciting the desired response. To allow sufficient time for DNAJB2 protein production, cells were harvested after a 48 h incubation period following treatment (
Figure S4a,b).
4.6. CellTox Assay
To determine cell viability post heat shock, the CellTox™ Green Cytotoxicity Assay was employed. Initially, the CellTox™ Green Dye was completely thawed in a 37 °C water bath, mixed using a vortex mixer for homogeneity, and briefly centrifuged to collect the dye at the bottom of the tube. Cells were harvested and adjusted to the desired density, specifically 7500 cells per 100 µL of fresh cell culture medium. Subsequently, 10 µL of the CellTox™ Green Dye was added to each 5 mL of cell suspension, mixed by inversion or gentle vortexing to ensure even distribution of the dye. The cell–dye mixture was then pipetted into a sterile multiwell plate at 100 µL per well for a 96-well format and allowed to adhere overnight.
The following day, cells were subjected to a 1 h heat shock at 42 °C. For positive control wells, Lysis Solution was added at a ratio of 1:25 (4 µL per 100 µL of cells) to represent the maximum signal obtainable from the lysed cells. Fluorescence intensity was measured at intervals between 0 and 24 h post-heat shock using an excitation wavelength of 485–500 nm and an emission wavelength of 520–530 nm. Prior to each reading, the plate was shaken for one minute at 700–900 rpm to ensure homogeneity and was returned to the incubator between readings. The fluorescence readings (RFU) were plotted against time to assess the cytotoxic effects of the heat shock treatment on the cells.
4.7. RNA Isolation and PCR Analysis
Cells were collected directly in TRIzol (Invitrogen, Carlsbad, CA, USA, Catalog: 15596018) and total RNA was isolated following the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized from the isolated RNA using the QuantiTect Reverse Transcription Kit (Qiagen, Germantown, MD, USA, Catalog: 205311). Quantitative PCR (qPCR) was performed using the PowerUp™ SYBR™ Green Master Mix (Thermo Fisher, Waltham, MA, USA, Catalog: A25742) according to the manufacturer’s instructions.
We used splice junction primers that could clearly distinguish isoform a from b. HS1a composed of exons 2 to 10 whereas HS1b composed of exons 2 to 9 (missing exon 10). Therefore, HS1a primers amplify the junction between exon 9 and exon 10 whereas HS1b primers amplify the extended region of exon 9 that is not present in HSJ1a.
For each qPCR reaction, 30 ng of cDNA was used. The experiments were performed in duplicates across 5 independent RT-qPCR experiments using cell lines from different passages of both patient and control samples. The purified RNA samples were first incubated with gDNA Wipeout Buffer to effectively eliminate DNA contamination. Moreover, we performed no template control (NTC) of the primers to ensure no contamination and primer-dimer formation. The amplification cycles of specific transcripts were normalized to the amplification cycles of the housekeeping gene, GAPDH. The cycle threshold (Ct) values were analyzed using the ThermoFisher Connect Platform to determine the relative fold change in gene expression. The primers used for all the transcripts are listed in
Table S1 [
5].
4.8. Protein Extraction and Western Blot Analysis
Following heat shock and/or treatment with Cystamine, cells from each well of a 6-well plate were scraped using 50 µL of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl pH 8.0, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 150 mM NaCl). The RIPA buffer was supplemented with 1x Halt™ Protease Inhibitor Cocktail (EDTA-Free, 100X, Cat. 87785, Thermo Fisher Scientific, Waltham, MA, USA) and 1x Halt™ Phosphatase Inhibitor Cocktail (100X, Cat. 78420, Thermo Fisher Scientific, Waltham, MA, USA).
Cells were incubated on ice for 5 min, followed by sonication at 4 °C for 10 cycles (30 s on, 30 s off) using the Diagenode Pico Bioruptor Sonication System with MiniChiller 300. The sonicated extracts were then centrifuged at 21,000× g for 10 min at 4 °C. The supernatant was collected and used for subsequent experiments. Protein concentration of the extracts was determined using the Pierce™ BCA Protein Assay Kit (Catalog: A55865, Thermo Fisher Scientific, Waltham, MA, USA).
For Western blot analysis, 20 µg of protein were loaded and separated by 12% SDS-PAGE, followed by transfer to nitrocellulose membrane with the Trans-Blot Turbo system (Bio-Rad, Hercules, CA, USA). Total protein was stained with the Revert 700 Total Protein Stain (Li-Cor Biosciences, Lincoln, NE, USA) and scanned with the Odyssey scanner (Li-Cor Systems, Lincoln, NE, USA). The membranes were then blocked with 5% non-fat dry milk (Fisher Scientific, Waltham, MA, USA, Catalog: NC9871209). The blots were then probed with the following primary antibodies: DNAJB2 Polyclonal antibody (Proteintech, Rosemont, IL, USA, Catalog: 10838-1-AP, RRID: AB_2277491). Secondary antibodies: Goat anti-Rabbit IgG (H+L) Alexa Fluor™ Plus 800 ((Thermo Fisher Scientific, Waltham, MA, USA, Catalog: A32735, RRID: AB_2633284). Both the primary and secondary antibodies were diluted in 3% Bovine serum albumin and 0.2% Tween. Band intensities were measured using Image Studio software version 2.1.15 (Li-Cor Systems, Lincoln, NE, USA). Bands of interest were captured with intensity measured, and comparably sized adjacent regions were captured to reflect mean levels of background intensity for background normalization. Normalized values, with respect to total protein, were used to determine the relative upregulation or downregulation of target proteins. Each experiment was repeated at least three times to ensure reproducibility. Fold changes in protein expression in treated fibroblasts were calculated relative to untreated controls for the Cystamine experiment.
In the densitometric analysis of the two DNAJB2 protein isoforms, we have separately quantified isoform a and the doublet of isoform b. Due to limitations in doublet resolution, it was not possible to quantify the individual components of the HSJ1b isoform.
4.9. SiRNA Knockdown of DNAJB2 in Fibroblasts
To ensure the specificity of the bands detected in Western blot analysis, the DNAJB2 gene was knocked down in fibroblasts using small interfering RNAs (siRNAs). The siRNA transfection process involved using specific siRNA sequences targeting exon 3 and 4 of DNAJB2 gene (DNAJB2 Silencer™ Select Pre-Designed siRNA, Thermofisher, Waltham, MA, USA, assay ID: s6959) using P2 Primary Cell 4D X Kit L (24 RCT) (Lonza Bioscience, Walkersville, MD, USA, Catalog: V4XP-2024) following the manufacturers protocol, the siRNA was incubated for 48 h to ensure for efficient downregulation.
The validation of the knockdown was assessed through qPCR, measuring the expression levels of the two DNAJB2 gene isoforms. Similarly, the effects of the knockdown at the protein level were analyzed using Western blotting.
4.10. Statistical Analysis
All the values of protein expression in control and mutant DNAJB2 (mut. DNAJB2) fibroblasts were normalized to the basal expression levels of the respective proteins in the mut. DNAJB2 fibroblasts to allow for comparison of the basal differences between the lines and the level of expression under different experimental conditions.
Data were analyzed using GraphPad Prism 10.2.3 version software. To compare within the group, the heat-shock response after heat shock was normalized to its respective baseline expression in both RNA and protein experiments. To compare the fold change in RNA/protein expression to the heat shock response across groups, the baseline and treatment conditions for all lines were normalized to the baseline expression of the mutant fibroblasts. Statistical comparisons between groups to assess baseline protein expression were performed using the Friedman test with uncorrected Dunn’s test. For comparisons evaluating the effect of heat shock, a mixed-effects model with uncorrected Fisher’s LSD was used for experiments with missing values at some time-points, while repeated measures two-way ANOVA with uncorrected Fisher’s LSD was applied for experiments with no missing values. Results were considered statistically significant at p * = <0.05, ** = <0.01, *** = <0.001, **** = <0.0001. Data in the graphs are presented as mean ± SEM.
5. Conclusions
Our study demonstrates that the c.823+6C>T variant in the
DNAJB2 gene is associated with impaired expression of DNAJB2 in fibroblasts under stress. As this mutation is heterozygous, it has affected only one DNAJB2 allele, resulting in partial disruption of DNAJB2 expression and function. DNAJB2-mutated cells demonstrated lower HSJ1b expression at baseline. While they mounted a robust stress response upon stimulation via heat shock, their response did not reach the levels observed in control cells. While the effects in fibroblasts are modest, a cell like the neuron that lives for many years in humans accumulates a significant amount of changed proteins and needs to continuously fold them appropriately. Thus, even small alterations in protein folding capacity could be detrimental. For example, when exposed to stressors like injury or oxidative stress, both sensory and motor neurons can upregulate HSPs as a protective mechanism, but the magnitude of this response is higher in sensory neurons [
30]. Thus, a mutation in the heat shock protein makes sensory neurons potentially more vulnerable to stress-induced damage, potentially contributing to the disease’s pathological processes. While our findings suggest a potential therapeutic role for Cystamine in enhancing DNAJB2 levels, further investigation is necessary to explore its mechanism and optimize its efficacy, particularly under stress conditions.
Future studies should explore the underlying mechanisms of impaired upregulation of DNAJB2. Additionally, the impact of the mutation on specific client proteins should be investigated using a technique that identifies protein interactions, like Proximity Ligation assays combined with sequencing. Identifying novel therapeutic targets to restore protein homeostasis in DNAJB2-mutated cells remains a crucial area of research.