Effect of Cilostazol and Aspirin During Hyperacute Stroke Phase in Rats: An Experimental Research Study
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Drug Delivery
2.3. Surgical Procedure
2.4. Neurological Examination
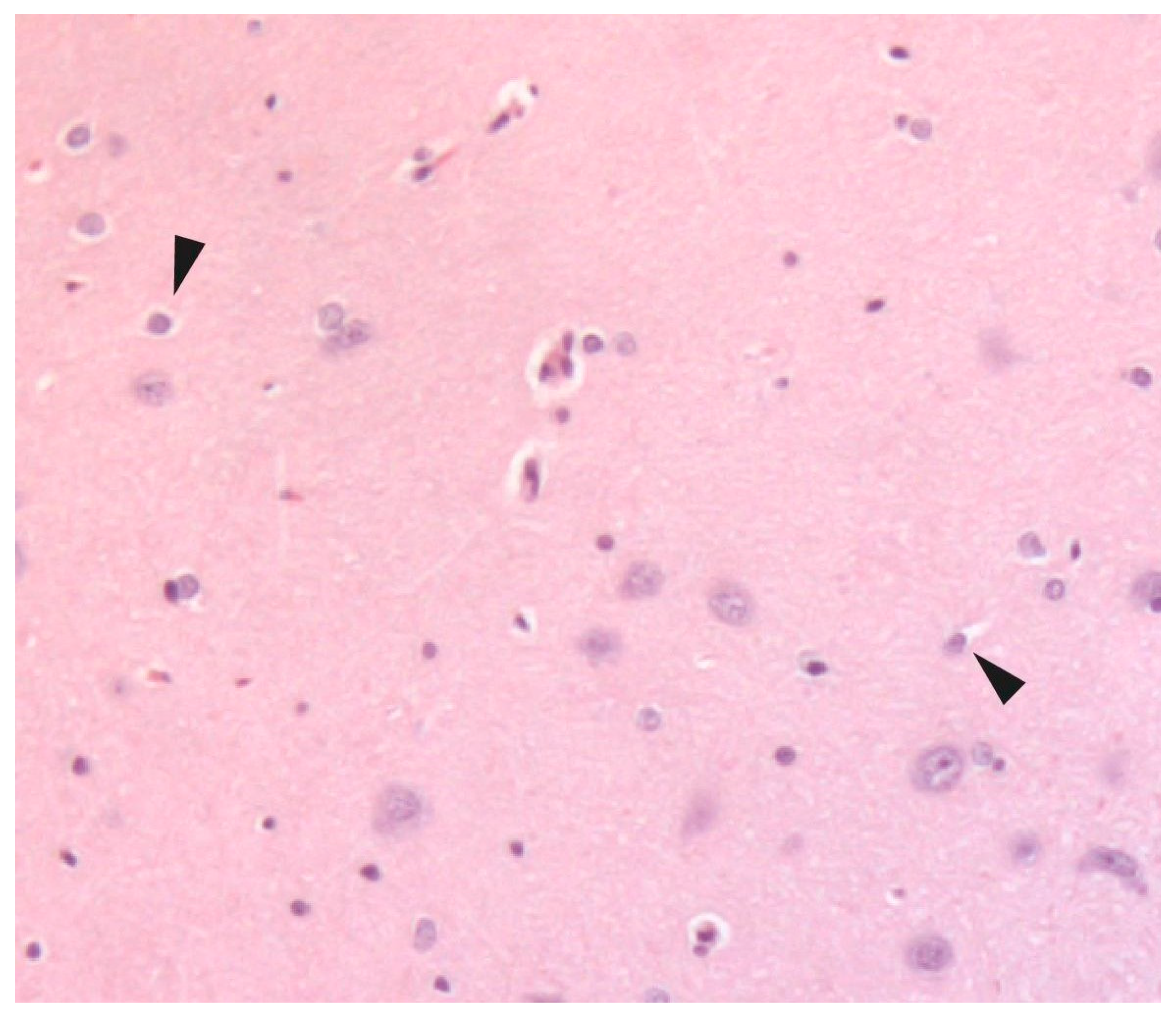
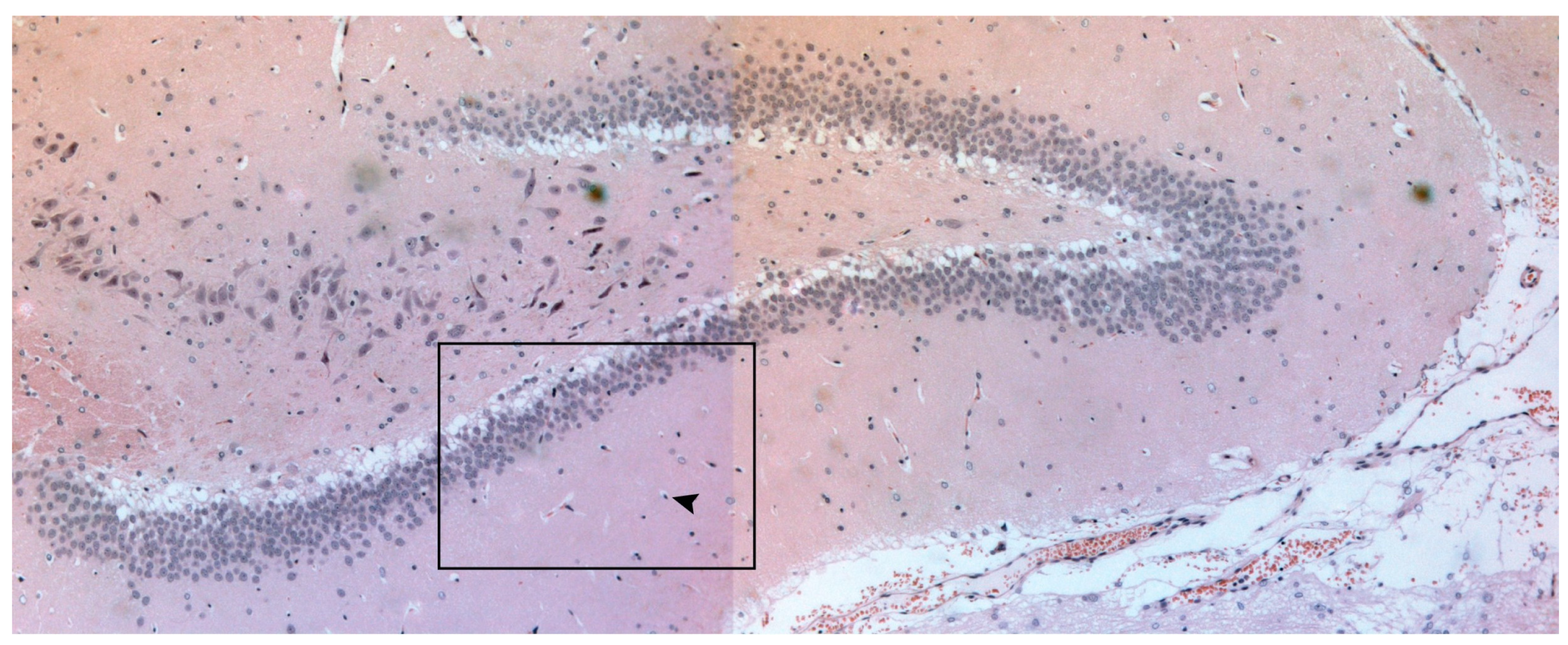
2.5. Histological Examination
2.6. Statistical Analysis
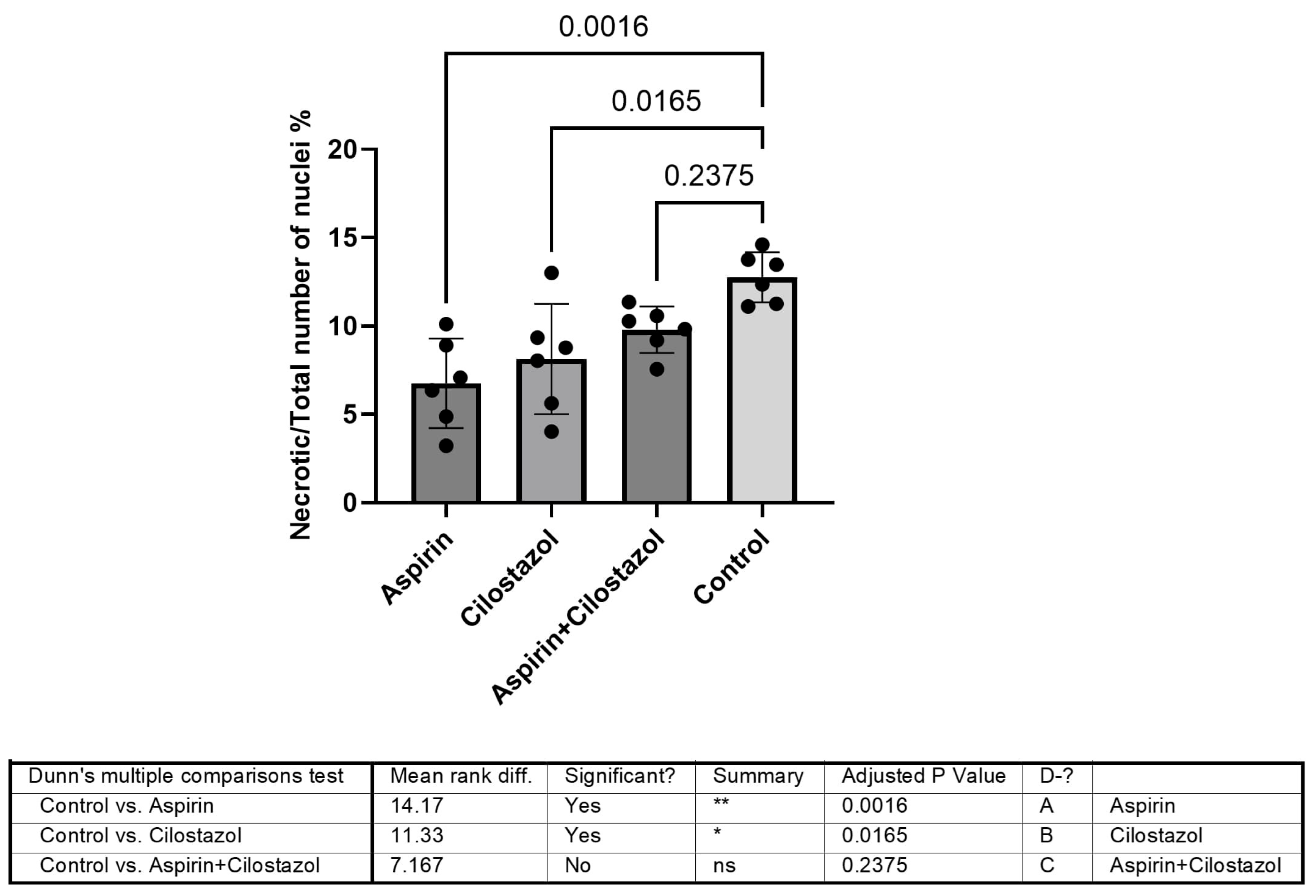
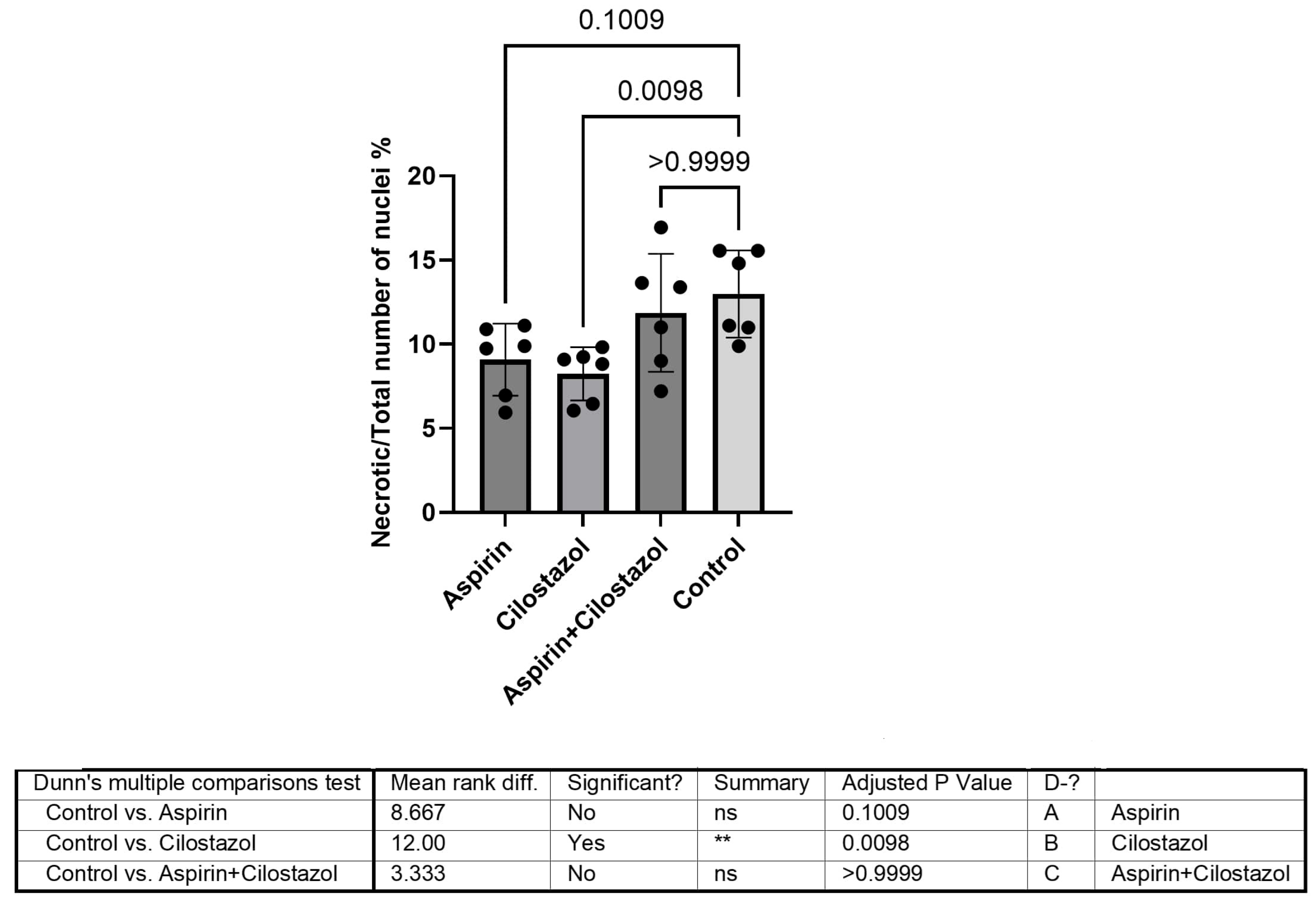
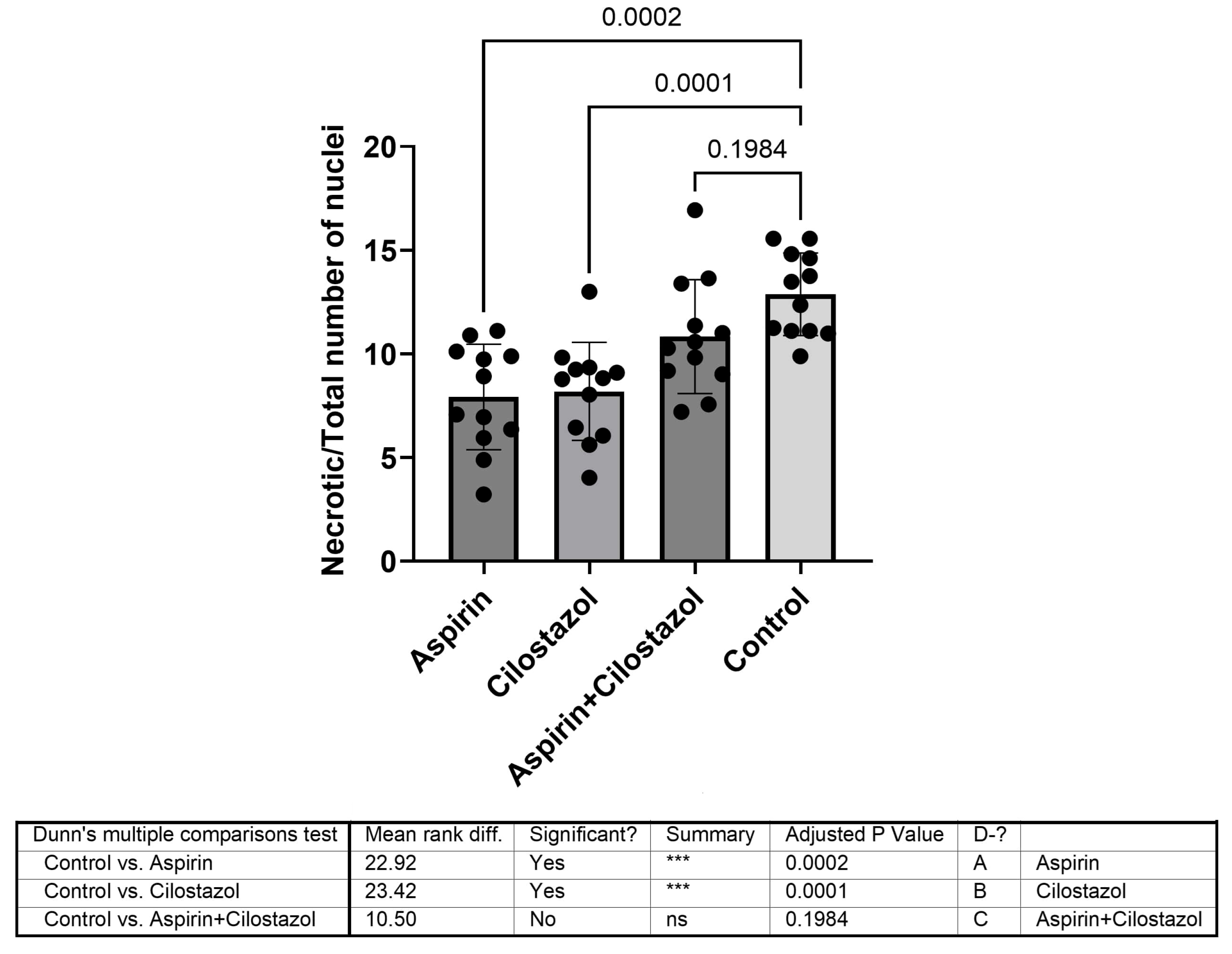
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. Wolters Kluwer Health 2021, 52, E364–E467. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, M.; Schneider, H.R.; Klingbeil, J.; Stockert, A.; Hartwigsen, G.; Weiller, C.; Saur, D. Resolution of diaschisis contributes to early recovery from post-stroke aphasia. Neuroimage 2022, 251, 119001. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.I.; Wang, H.C.; Tseng, K.Y.; Wang, Y.H.; Chang, C.Y.; Chen, Y.J.; Lai, C.S.; Chen, D.R.; Chang, L.L. Cilostazol Ameliorates Peripheral Neuropathic Pain in Streptozotocin-Induced Type I Diabetic Rats. Front. Pharmacol. 2022, 12, 771271. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, H.N.; Hong, K.W.; Shin, H.K.; Choi, B.T. Anti-depressant effects of phosphodiesterase 3 inhibitor cilostazol in chronic mild stress-treated mice after ischemic stroke. Psychopharmacology 2016, 233, 1055–1066. [Google Scholar]
- Qi, D.S.; Tao, J.H.; Zhang, L.Q.; Li, M.; Wang, M.; Qu, R.; Zhang, S.C.; Liu, P.; Liu, F.; Miu, J.C.; et al. Neuroprotection of Cilostazol against ischemia/reperfusion-induced cognitive deficits through inhibiting JNK3/caspase-3 by enhancing Akt1. Brain Res. 2016, 1653, 67–74. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Liu, X.; Wu, X.; Jose, P.A.; Liu, M.; Yang, Z. Low-Dose Aspirin Treatment Attenuates Male Rat Salt-Sensitive Hypertension via Platelet Cyclooxygenase 1 and Complement Cascade Pathway. J. Am. Heart Assoc. 2020, 9, e013470. [Google Scholar] [CrossRef]
- Mărgăritescu, O.; Mogoantă, L.; Pirici, I.; Pirici, D.; Cernea, D.; Mărgăritescu, C. Histopathological changes in acute ischemic stroke. Rom. J. Morphol. Embryol. 2009, 50, 327–339. [Google Scholar]
- Lopez, M.S.; Vemuganti, R. Modeling transient focal ischemic stroke in rodents by intraluminal filament method of middle cerebral artery occlusion. In Traumatic and Ischemic Injury: Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 101–113. [Google Scholar] [CrossRef]
- Sommer, C. Histology and infarct volume determination in rodent models of stroke. In Rodent Models of Stroke; Dirnagl, U., Ed.; Humana New York: New York, NY, USA, 2016; pp. 263–277. [Google Scholar]
- Popp, A.; Jaenisch, N.; Witte, O.W.; Frahm, C. Identification of ischemic regions in a rat model of stroke. PLoS ONE 2009, 4, e4764. [Google Scholar]
- Sommer, C.J. Ischemic stroke: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.L.; Li, W.H.; Liu, M.; Wang, Y.H.; Du, G.H. Cerebral ischemia-reperfusion aggravated cerebral infarction injury and possible differential genes identified by RNA-Seq in rats. Brain Res. Bull. 2020, 156, 33–42. [Google Scholar] [CrossRef]
- Yanai, S.; Semba, Y.; Ito, H.; Endo, S. Cilostazol improves hippocampus-dependent long-term memory in mice. Psychopharmacology 2014, 231, 2681–2693. [Google Scholar] [CrossRef]
- Yanai, S.; Ito, H.; Endo, S. Long-term cilostazol administration prevents age-related decline of hippocampus-dependent memory in mice. Neuropharmacology 2018, 129, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Galyfos, G.; Sianou, A. Cilostazol for Secondary Prevention of Stroke: Should the Guidelines Perhaps Be Extended? Vasc. Spec. Int. 2017, 33, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.M.; Hasso, A.N.; Handwerker, J.; Farid, H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 2012, 32, 1285–1297. [Google Scholar] [CrossRef]
- Sohn, M.; Lim, S. The Role of Cilostazol, a Phosphodiesterase-3 Inhibitor, in the Development of Atherosclerosis and Vascular Biology: A Review with Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 2593. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Mikhailidis, D.P.; Manolis, A.S. Update on Cilostazol: A Critical Review of Its Antithrombotic and Cardiovascular Actions and Its Clinical Applications. J. Clin. Pharmacol. 2022, 62, 320–358. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kimura, K.; Otsuka, T.; Toyoda, K.; Uchiyama, S.; Hoshino, H.; Sakai, N.; Okada, Y.; Origasa, H.; Naritomi, H.; et al. Dual Antiplatelet Therapy with Cilostazol for Secondary Prevention in Lacunar Stroke: Subanalysis of the CSPS.com Trial. Stroke 2023, 54, 697–705. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, J.M.; Kim, B.J.; Lee, J.S.; Kwon, S.U. Cilostazol mono and combination treatments in ischemic stroke an updated systematic review and meta-analysis. Stroke 2019, 50, 3503–3511. [Google Scholar] [CrossRef]
- Aoki, J.; Kimura, K. Cilostazol addition to aspirin may worsen the short-term outcome in patients with large artery disease: ADS Subanalysis. J. Neurol. Sci. 2024, 456, 122854. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, J.H.; Chi, N.F.; Chen, Y.C. Cilostazol plus Aspirin vs. Clopidogrel plus Aspirin in Acute Minor Stroke or Transient Ischemic Attack. J. Atheroscler. Thromb. 2024, 31, 904–916. [Google Scholar] [CrossRef]
- Carrera, E.; Tononi, G. Diaschisis: Past, present, future. Brain 2014, 137, 2408–2422. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Clarkson, J.; Jackson, D.M.; Sammut, I.A. Mitochondrial involvement in transhemispheric diaschisis following hypoxia-ischemia: Clomethiazole-Mediated Amelioration. Neuroscience 2007, 144, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Haller, E.; Williams, S.N.; Haim, E.D.; Tajiri, N.; Hernandez-Ontiveros, D.G.; Frisina-Deyo, A.; Boffeli, S.M.; Sanberg, P.R.; Borlongan, C.V. Compromised blood-brain barrier competence in remote brain areas in ischemic stroke rats at the chronic stage. J. Comp. Neurol. 2014, 522, 3120–3137. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Dargahi, L.; Khodabakhsh, P.; Kadivar, M.; Farahmandfar, M. Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke. CNS Neurosci. Ther. 2022, 28, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Mundugaru, R.; Sivanesan, S.; Popa-Wagner, A.; Udaykumar, P.; Kirubagaran, R.; KP, G.; Vidyadhara, D.J. Pluchea lanceolata protects hippocampal neurons from endothelin-1 induced ischemic injury to ameliorate cognitive deficits. J. Chem. Neuroanat. 2018, 94, 75–85. [Google Scholar] [CrossRef]
- Helgadottir, H.; Tropea, T.; Gizurarson, S.; Meiri, H.; Mandalà, M. Aspirin causes endothelium-dependent vasodilation of resistance arteries from non-gravid and gravid rats. Pregnancy Hypertens. 2019, 15, 141–145. [Google Scholar] [CrossRef]
- Helgadóttir, H.; Tropea, T.; Gizurarson, S.; Mandalà, M. Endothelium-derived hyperpolarizing factor (Edhf) mediates acetylsalicylic acid (aspirin) vasodilation of pregnant rat mesenteric arteries. Int. J. Mol. Sci. 2021, 22, 10162. [Google Scholar] [CrossRef] [PubMed]
| Parameter Estimates | Variable | Estimate | Standard Error | 95% CI (Asymptotic) | |t| | p-Value | p-Value Summary |
|---|---|---|---|---|---|---|---|
| β0 | Intercept | 11.77 | 1.128 | 9.494 to 14.05 | 10.44 | <0.0001 | **** |
| β1 | Neurologic Examination [4] | 0.5444 | 0.9294 | −1.333 to 2.421 | 0.5858 | 0.5612 | ns |
| β2 | Neurologic Examination [3] | 1.659 | 1.079 | −0.5198 to 3.837 | 1.538 | 0.1318 | ns |
| β3 | Neurologic Examination [1] | −0.4031 | 1.401 | −3.233 to 2.427 | 0.2877 | 0.7750 | ns |
| β4 | Team [1] | −4.306 | 1.095 | −6.517 to −2.094 | 3.932 | 0.0003 | *** |
| β5 | Team [2] | −4.157 | 1.080 | −6.337 to −1.976 | 3.850 | 0.0004 | *** |
| β6 | Team [3] | −1.054 | 1.166 | −3.408 to 1.301 | 0.9037 | 0.3714 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiadou, C.; Papapetrou, A.; Galyfos, G.; Vekrellis, K.; Katafygiotis, P.; Lazaris, A.; Geroulakos, G.; Megalopoulos, A.; Liapis, C.; Kostomitsopoulos, N.; et al. Effect of Cilostazol and Aspirin During Hyperacute Stroke Phase in Rats: An Experimental Research Study. Neurol. Int. 2025, 17, 69. https://doi.org/10.3390/neurolint17050069
Anastasiadou C, Papapetrou A, Galyfos G, Vekrellis K, Katafygiotis P, Lazaris A, Geroulakos G, Megalopoulos A, Liapis C, Kostomitsopoulos N, et al. Effect of Cilostazol and Aspirin During Hyperacute Stroke Phase in Rats: An Experimental Research Study. Neurology International. 2025; 17(5):69. https://doi.org/10.3390/neurolint17050069
Chicago/Turabian StyleAnastasiadou, Christiana, Anastasios Papapetrou, George Galyfos, Kostas Vekrellis, Patroklos Katafygiotis, Andreas Lazaris, George Geroulakos, Angelos Megalopoulos, Christos Liapis, Nikolaos Kostomitsopoulos, and et al. 2025. "Effect of Cilostazol and Aspirin During Hyperacute Stroke Phase in Rats: An Experimental Research Study" Neurology International 17, no. 5: 69. https://doi.org/10.3390/neurolint17050069
APA StyleAnastasiadou, C., Papapetrou, A., Galyfos, G., Vekrellis, K., Katafygiotis, P., Lazaris, A., Geroulakos, G., Megalopoulos, A., Liapis, C., Kostomitsopoulos, N., & Kakisis, J. (2025). Effect of Cilostazol and Aspirin During Hyperacute Stroke Phase in Rats: An Experimental Research Study. Neurology International, 17(5), 69. https://doi.org/10.3390/neurolint17050069







