Neonatal Perforator Stroke: Timing, Risk Factors, and Neurological Outcome from a Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
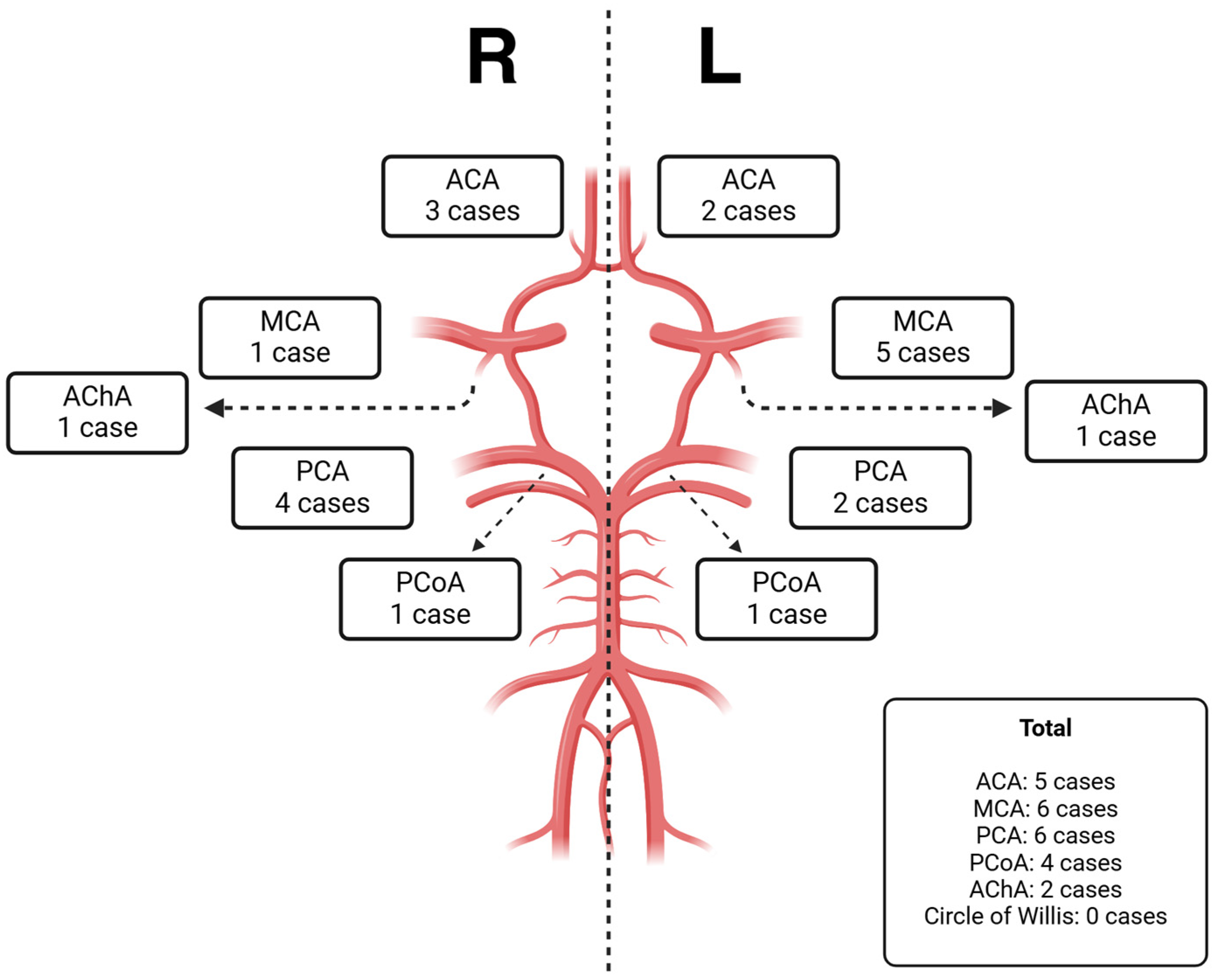
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PS | Perforator stroke |
| PAIS | Perinatal arterial ischemic stroke |
| cUS | Cranial ultrasound |
| MRI | Magnetic Resonance Imaging |
| NICU | Neonatal Intensive Care Unit |
| VLBW | Very low birth weight |
| PDA | Patent ductus arteriosus |
| hsPDA | Hemodynamically significant patent ductus arteriosus |
| UVC | Umbilical venous catheter |
| ECC | Epicutaneous-caval catheter |
| FVM | Fetal vascular malperfusion |
| FSE | Fast spin-echo |
| SWI | Susceptibility-weighted imaging |
| DWI | Diffusion-weighted imaging |
| pCALS | Pseudo-continuous arterial spin labeling |
| SGA | Small for gestational age |
| IUGR | Intrauterine growth restriction |
| CS | Cesarean section |
| IVH | Intraventricular hemorrhage |
| PCA | Posterior cerebral artery |
| MCA | Middle cerebral artery |
| ACA | Anterior cerebral artery |
| PCoA | Posterior communicating artery |
| AChA | Anterior choroidal artery |
| PLIC | Posterior limb of the internal capsule |
| HIE | Hypoxic-ischemic encephalopathy |
| CBH | Cerebellar hemorrhage |
| n.a. | Not available |
| IQR | Interquartile range |
| (p)NAIS | (Preterm) neonatal arterial ischemic stroke |
References
- Govaert, P. Sonographic stroke templates. Semin. Fetal Neonatal Med. 2009, 14, 284–298. [Google Scholar] [CrossRef]
- Baak, L.M.; van der Aa, N.E.; Verhagen, A.A.E.; Dudink, J.; Groenendaal, F.; Nijboer, C.H.A.; Benders, M.J.N.L.; Wagenaar, N. Early predictors of neurodevelopment after perinatal arterial ischemic stroke: A systematic review and meta-analysis. Pediatr. Res. 2023, 94, 20–33. [Google Scholar] [CrossRef]
- Jiang, W.J.; Srivastava, T.; Gao, F.; Du, B.; Dong, K.H.; Xu, X.T. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology 2006, 66, 1868–1872. [Google Scholar] [CrossRef]
- Derdeyn, C.P.; Fiorella, D.; Lynn, M.J.; Rumboldt, Z.; Cloft, H.J.; Gibson, D.; Turan, T.N.; Lane, B.F.; Janis, L.S.; Chimowitz, M.I. Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery 2013, 72, 777–795. [Google Scholar] [CrossRef]
- Lee, J. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA 2005, 293, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Benders, M.; Groenendaal, F.; De Vries, L. Preterm arterial ischemic stroke. Semin. Fetal Neonatal Med. 2009, 14, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Govaert, P.; Ramenghi, L.; Taal, R.; Dudink, J.; Lequin, M. Diagnosis of perinatal stroke II: Mechanisms and clinical phenotypes. Acta Paediatr. 2009, 98, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Ecury-Goossen, G.M.; Raets, M.M.; Lequin, M.; Feijen-Roon, M.; Govaert, P.; Dudink, J. Risk factors, clinical presentation, and neuroimaging findings of neonatal perforator stroke. Stroke 2013, 44, 2115–2120. [Google Scholar] [CrossRef]
- Geraldo, A.; Parodi, A.; Bertamino, M.; Buffelli, F.; Uccella, S.; Tortora, D.; Moretti, P.; Ramenghi, L.; Fulcheri, E.; Rossi, A.; et al. Perinatal Arterial Ischemic Stroke in Fetal Vascular Malperfusion: A Case Series and Literature Review. Am. J. Neuroradiol. 2020, 41, 2377–2383. [Google Scholar] [CrossRef]
- van Wezel-Meijler, G. Neonatal Cranial Ultrasonography; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Kirton, A. Filling a lacune in perinatal stroke outcomes. Dev. Med. Child Neurol. 2016, 58, 8–9. [Google Scholar] [CrossRef]
- Benders, M.J.; Groenendaal, F.; Uiterwaal, C.S.; Nikkels, P.G.; Bruinse, H.W.; Nievelstein, R.A.; de Vries, L.S. Maternal and Infant Characteristics Associated With Perinatal Arterial Stroke in the Preterm Infant. Stroke 2007, 38, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N.; Nelson, K.B.; Ferriero, D.; Lynch, J.K.; Participants, T.N.-N.P.S.W. Ischemic Perinatal Stroke: Summary of a Workshop Sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007, 120, 609–616. [Google Scholar] [CrossRef]
- Sorg, A.-L.; von Kries, R.; Klemme, M.; Gerstl, L.; Felderhoff-Müser, U.; Dzietko, M. Incidence Estimates of Perinatal Arterial Ischemic Stroke in Preterm- and Term-Born Infants: A National Capture-Recapture Calculation Corrected Surveillance Study. Neonatology 2021, 118, 727–733. [Google Scholar] [CrossRef]
- van der Aa, N.E.; Benders, M.J.; Nikkels, P.G.; Groenendaal, F.; de Vries, L.S. Cortical Sparing in Preterm Ischemic Arterial Stroke. Stroke 2016, 47, 869–871. [Google Scholar] [CrossRef]
- Vogels, V.; Dammers, R.; van Bilsen, M.; Volovici, V. Deep Cerebral Perforators: Anatomical Distribution and Clinical Symptoms: An Overview. Stroke 2021, 52, E660–E674. [Google Scholar] [CrossRef]
- Djulejić, V.; Marinković, S.; Milić, V.; Georgievski, B.; Rašić, M.; Aksić, M.; Puškaš, L. Common features of the cerebral perforating arteries and their clinical significance. Acta Neurochir. 2015, 157, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Rzeplin´ski, R.; Tarka, S.; Tomaszewski, M.; Kucewicz, M.; Acewicz, A.; Małachowski, J.; Ciszek, B. Narrowings of the Deep Cerebral Perforating Arteries Ostia: Geometry, Structure, and Clinical Implications. J. Stroke 2025, 27, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Arts, T.; Siero, J.C.; Biessels, G.J.; Zwanenburg, J.J. Automated Assessment of Cerebral Arterial Perforator Function on 7T MRI. J. Magn. Reson. Imaging 2021, 53, 234–241. [Google Scholar] [CrossRef]
- Fakhari, N.; Aguet, J.; Nguyen, M.B.; Zhang, N.; Mertens, L.; Jain, A.; Sled, J.G.; Villemain, O.; Baranger, J. Automated classification of cerebral arteries and veins in the neonate using ultrafast doppler spectrogram. Phys. Med. Biol. 2024, 69, 245006. [Google Scholar] [CrossRef]
- Tortora, D.; Severino, M.; Malova, M.; Parodi, A.; Morana, G.; Sedlacik, J.; Govaert, P.; Volpe, J.J.; Rossi, A.; Ramenghi, L.A. Differences in subependymal vein anatomy may predispose preterm infants to GMH–IVH. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F59–F65. [Google Scholar] [CrossRef]
- Tortora, D.; Severino, M.; Malova, M.; Parodi, A.; Morana, G.; Ramenghi, L.; Rossi, A. Variability of Cerebral Deep Venous System in Preterm and Term Neonates Evaluated on MR SWI Venography. Am. J. Neuroradiol. 2016, 37, 2144–2149. [Google Scholar] [CrossRef]
- Nold, M.F.; Veldman, A.; Michel-Behnke, I. Thrombosis in the critically ill neonate: Incidence, diagnosis, and management. Vasc. Health Risk Manag. 2008, 4, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Pimentel, S.; Pinto, F.; Nona, J. Perinatal ischemic stroke: A five-year retrospective study in a level-III maternity. Einstein-Sao Paulo 2015, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, A.J.; Saling, M.; Rogerson, S.; Anderson, P.; Cheong, J.; Solms, M. Cerebral Arterial Asymmetries in the Neonate: Insight into the Pathogenesis of Stroke. Symmetry 2022, 14, 456. [Google Scholar] [CrossRef]
- Benders, M.J.; Groenendaal, F.; Uiterwaal, C.S.; de Vries, L.S. Perinatal Arterial Stroke in the Preterm Infant. Semin. Perinatol. 2008, 32, 344–349. [Google Scholar] [CrossRef]
- Abels, L.; Lequin, M.; Govaert, P. Sonographic templates of newborn perforator stroke. Pediatr. Radiol. 2006, 36, 663–669. [Google Scholar] [CrossRef]
- Steggerda, S.; de Vries, L. Neonatal stroke in premature neonates. Semin. Perinatol. 2021, 45, 151471. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Miller, S.P.; Barkovich, A.J.; Partridge, J.C.; Ferriero, D.M. Perinatal stroke in term infants with neonatal encephalopathy. Neurology 2004, 62, 2088–2091. [Google Scholar] [CrossRef]
- Rutherford, M.A.; Ramenghi, L.A.; Cowan, F.M. Neonatal stroke. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F377–F384. [Google Scholar] [CrossRef]
- Nelson, K.B.; Lynch, J.K. Stroke in newborn infants. Lancet Neurol. 2004, 3, 150–158. [Google Scholar] [CrossRef]
- Fisher, C.M. Transient Ischemic Attacks. N. Engl. J. Med. 2002, 347, 1642–1643. [Google Scholar] [CrossRef]
- Kirton, A.; Deveber, G. Advances in Perinatal Ischemic Stroke. Pediatr. Neurol. 2009, 40, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ecury-Goossen, G.M.; van der Haer, M.; Smit, L.S.; Feijen-Roon, M.; Lequin, M.; de Jonge, R.C.J.; Govaert, P.; Dudink, J. Neurodevelopmental outcome after neonatal perforator stroke. Dev. Med. Child Neurol. 2016, 58, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, C.E.; el Bakkali, L.; Verhagen, E.A.; Steggerda, S.J.; Alderliesten, T.; Lequin, M.H.; van de Pol, L.A.; Benders, M.J.; van Bel, F.; Koopman-Esseboom, C.; et al. Brain MRI Injury Patterns across Gestational Age among Preterm Infants with Perinatal Asphyxia. Neonatology 2024, 121, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Brink, H.v.D.; Doubal, F.N.; Duering, M. Advanced MRI in cerebral small vessel disease. Int. J. Stroke 2023, 18, 28–35. [Google Scholar] [CrossRef]
- Brink, H.v.D.; Kopczak, A.; Arts, T.; Onkenhout, L.; Siero, J.C.; Zwanenburg, J.J.; Hein, S.; Hübner, M.; Gesierich, B.; Duering, M.; et al. CADASIL Affects Multiple Aspects of Cerebral Small Vessel Function on 7T-MRI. Ann. Neurol. 2023, 93, 29–39. [Google Scholar] [CrossRef]
- Markus, H.S.; de Leeuw, F.E. Cerebral small vessel disease: Recent advances and future directions. Int. J. Stroke 2023, 18, 4–14. [Google Scholar] [CrossRef]
- Ramenghi, L.A.; Fumagalli, M.; Groppo, M.; Consonni, D.; Gatti, L.; Bertazzi, P.A.; Mannucci, P.M.; Mosca, F. Germinal matrix hemorrhage: Intraventricular hemorrhage in very-low-birth-weight infants. Stroke 2011, 42, 1889–1893. [Google Scholar] [CrossRef]
- Ferriero, D.M.; Fullerton, H.J.; Bernard, T.J.; Billinghurst, L.; Daniels, S.R.; DeBaun, M.R.; Deveber, G.; Ichord, R.N.; Jordan, L.C.; Massicotte, P.; et al. Management of Stroke in Neonates and Children: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke 2019, 50, E51–E96. [Google Scholar] [CrossRef]
| Patients’ Characteristics | Preterm Babies (9) | Term Babies (10) |
|---|---|---|
| Sex | 5F (55%), 4M (45%) | 8M (80%), 2F (20%) |
| Gestational age (median) | 27 GW | 38 GW |
| Birth weight (mean ± SD) | 1060 ± 430 g | 3240 ± 520 g |
| BW percentile (median; 25th–75th pc) | 60; 32–86.5 | 52; 29.75–77 |
| Body length percentile (median; 25th–75th pc) | 46; 34.5–65 | 50; 34.5–65.5 |
| Cranial circumference percentile (median; 25th–75th pc) | 44; 22.5–62 | 40; 22.75–58.25 |
| SGA (N;%) | 1 (11%) | 1 (10%) |
| IUGR (N;%) | 2 (22%) | 0 |
| Twin gestation (N;%) | 2 (22%) | 0 |
| APGAR 1′ (median) | 6.5 | 5 |
| APGAR 5′ (median) | 8 | 7 |
| CS (N;%) | 6 (66%) | 5 (50%) |
| Risk factors | ||
| Prenatal | ||
| Placental malperfusion | 1 (11%) | 1 (10%) |
| Placental abruption | 3 (33%) | 1 (10%) |
| Pre-eclampsia | 1 (11%) | 2 (20%) |
| Perinatal | ||
| Asphyxia | 0 | 6 (60%) |
| Urgent CS | 5 of 6 CS (83%) | 5 of 5 CS (100%) |
| Postnatal | ||
| hsPDA | 6 (66%) | 0 |
| Central venous catheter | 9 (100%) | 10 (100%) |
| Altered coagulation (tot 7) | 1 (11%) | 0 |
| Sepsis | 7 (77%) | 0 |
| GA | MRI Indication | PS Number | Vessel Interested | MRI Incidental Findings | Neurological Impairment | |
|---|---|---|---|---|---|---|
| 1 | 27 | Prematurity | 1 | PCA L | IVH | n.a. |
| 2 | 29 | Prematurity | 1 | pcOa R | IVH | 0 |
| 3 | 25 | Prematurity | 2 | PCOA + ACA R | 0 | 0 |
| 4 | 40 | HIE | 2 | PCOA + ACA R | 0 | 0 |
| 5 | 32 | Prematurity | 1 | ACA L | 0 | Attention deficit and hyperactivity |
| 6 | 39 | Hypoglycemia | 1 | PCA R | 0 | 0 |
| 7 | 33 | Prematurity | 1 | ACA R | Dilatation of the periencefalic spaces with incomplete Sylvian opercolarization | 0 |
| 8 | 37 | HIE | 1 | MCA L | PLIC’s alteration | Hemiplegia |
| 9 | 36 | HIE | 1 | PCA L | 0 | 0 |
| 10 | 40 | HIE | 2 | AChA + PCoA L | PLIC’s alteration | Hemiplegia |
| 11 | 25 | Prematurity | 2 | PCA + AChA R | IVH | Attention deficit and hyperactivity |
| 12 | 25 | Prematurity | 1 | MCA L | IVH | 0 |
| 13 | 29 | Prematurity | 1 | PCA R | 0 | 0 |
| 14 | 36 | HIE | 1 | MCA R | 0 | n.a. |
| 15 | 39 | Seizures | 1 | MCA L | Transverse sinus thrombosis | n.a. |
| 16 | 36 | Seizures | 1 | MCA L | 0 | n.a. |
| 17 | 37 | Seizures | 1 | MCA L | 0 | 0 |
| 18 | 40 | HIE | 1 | ACA L | CBH | n.a. |
| 19 | 23 | Prematurity | 1 | PCA L | CBH | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calandrino, A.; Cipresso, G.; Battaglini, M.; Caruggi, S.; Bonato, I.; Massirio, P.; Andreato, C.; Vinci, F.; Parodi, A.; Malova, M.; et al. Neonatal Perforator Stroke: Timing, Risk Factors, and Neurological Outcome from a Single-Center Experience. Neurol. Int. 2025, 17, 59. https://doi.org/10.3390/neurolint17040059
Calandrino A, Cipresso G, Battaglini M, Caruggi S, Bonato I, Massirio P, Andreato C, Vinci F, Parodi A, Malova M, et al. Neonatal Perforator Stroke: Timing, Risk Factors, and Neurological Outcome from a Single-Center Experience. Neurology International. 2025; 17(4):59. https://doi.org/10.3390/neurolint17040059
Chicago/Turabian StyleCalandrino, Andrea, Gaia Cipresso, Marcella Battaglini, Samuele Caruggi, Irene Bonato, Paolo Massirio, Chiara Andreato, Francesco Vinci, Alessandro Parodi, Mariya Malova, and et al. 2025. "Neonatal Perforator Stroke: Timing, Risk Factors, and Neurological Outcome from a Single-Center Experience" Neurology International 17, no. 4: 59. https://doi.org/10.3390/neurolint17040059
APA StyleCalandrino, A., Cipresso, G., Battaglini, M., Caruggi, S., Bonato, I., Massirio, P., Andreato, C., Vinci, F., Parodi, A., Malova, M., Bertamino, M., Amadori, E., Severino, M., Resaz, M., Rossi, A., Striano, P., & Ramenghi, L. A. (2025). Neonatal Perforator Stroke: Timing, Risk Factors, and Neurological Outcome from a Single-Center Experience. Neurology International, 17(4), 59. https://doi.org/10.3390/neurolint17040059








