The Relationship Between Clinical Features of Ischemic Stroke and miRNA Expression in Stroke Patients: A Systematic Review
Abstract
1. Introduction
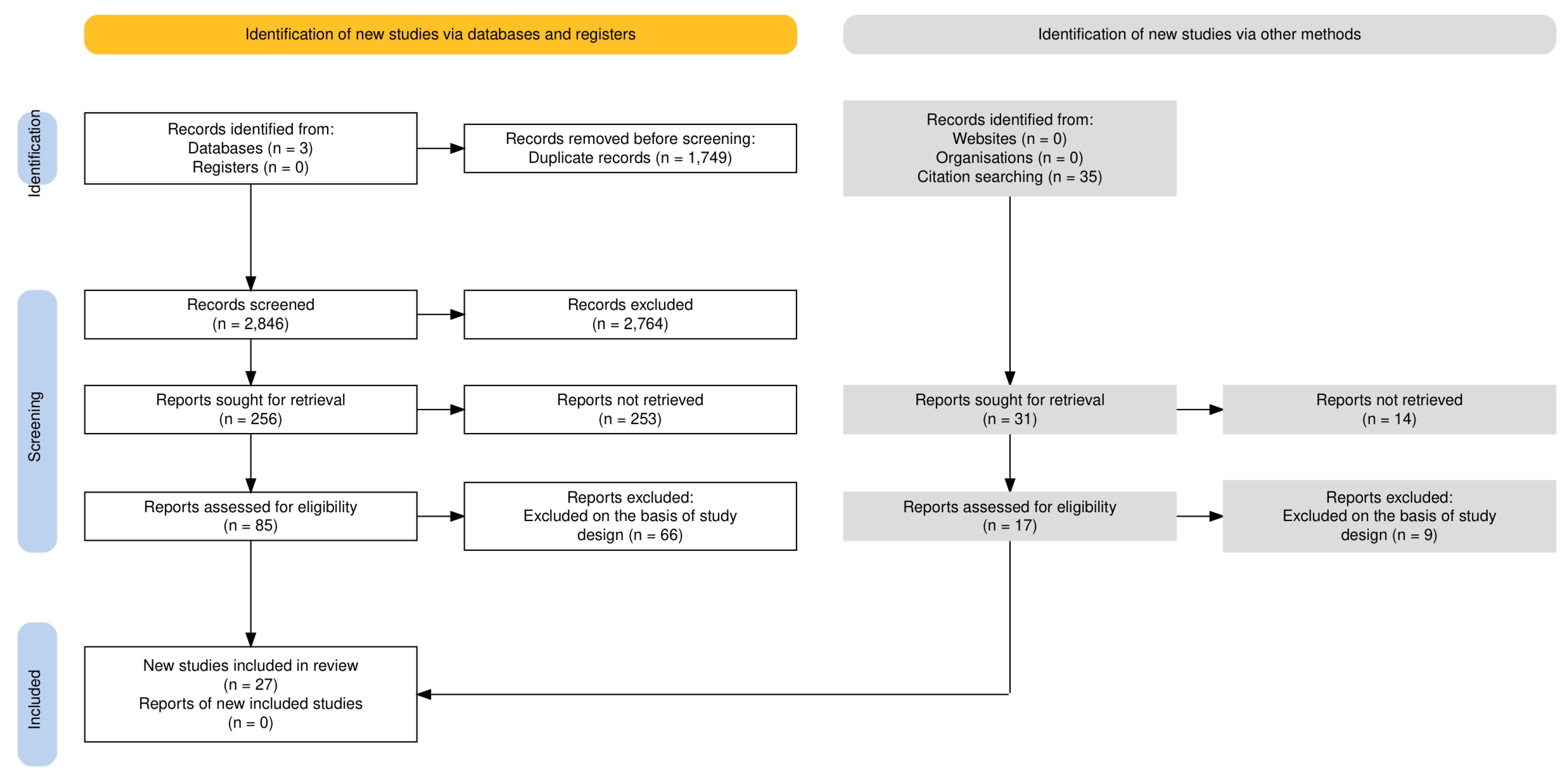
2. Materials and Methods
3. Results
3.1. The Relationship Between Clinical Severity of IS and miRNA Expression
3.2. The Relationship Between Infarct Volume at Admission and miRNA Expression
3.3. The Relationship Between Systemic Inflammatory Markers and miRNA Expression
3.4. The Relationship Between Prognosis and miRNA Expression
3.5. The Relationship Between Stroke Etiology Subtype and miRNA Expression
3.6. The Relationship Between the Risk of Stroke Recurrence and miRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. MicroRNA: An Emerging Predictive, Diagnostic, Prognostic and Therapeutic Strategy in Ischaemic Stroke. Cell Mol. Neurobiol. 2022, 42, 1301–1319. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Ni, J.; Cheng, J.; Jia, J.; Zhen, X. miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death Dis. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gao, L.; Zheng, J.; Li, T.; Shao, A.; Reis, C.; Chen, S.; Zhang, J. The Roles of MicroRNAs in Stroke: Possible Therapeutic Targets. Cell Transplant. 2018, 27, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Winkler, L.; Blasig, R.; Breitkreuz-Korff, O.; Berndt, P.; Dithmer, S.; Helms, H.C.; Puchkov, D.; Devraj, K.; Kaya, M.; Qin, Z.; et al. Tight junctions in the blood-brain barrier promote edema formation and infarct size in stroke—Ambivalent effects of sealing proteins. J. Cereb. Blood Flow. Metab. 2021, 41, 132–145. [Google Scholar] [CrossRef]
- Roitbak, T. MicroRNAs and Regeneration in Animal Models of CNS Disorders. Neurochem. Res. 2020, 45, 188–203. [Google Scholar] [CrossRef]
- Burlacu, C.C.; Ciobanu, D.; Badulescu, A.V.; Chelaru, V.-F.; Mitre, A.-O.; Capitanescu, B.; Hermann, D.M.; Popa-Wagner, A. Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 24, 251. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vijayan, M.; Reddy, P.H. Peripheral biomarkers of stroke: Focus on circulatory microRNAs. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 1984–1993. [Google Scholar] [CrossRef]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow. Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef]
- Toyoda, K.; Yoshimura, S.; Nakai, M.; Koga, M.; Sasahara, Y.; Sonoda, K.; Kamiyama, K.; Yazawa, Y.; Kawada, S.; Sasaki, M.; et al. Twenty-Year Change in Severity and Outcome of Ischemic and Hemorrhagic Strokes. JAMA Neurol. 2022, 79, 61–69. [Google Scholar] [CrossRef]
- Prus, K.; Bilotta, F.; Akça, B. Relationship Between the Expression of Specific Types of miRNA in Acute Ischemic Stroke Anatomic and Clinical Stroke Severity (Acute and Long Term). PROSPERO 2023 CRD42023472594. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023472594 (accessed on 30 November 2024).
- Hammer, B.; Virgili, E.; Bilotta, F. Evidence-based literature review: De-duplication a cornerstone for quality. World J. Methodol. 2023, 13, 390–398. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.-Y. Increased Circulating Exosomal miRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Huang, J.; Zheng, G.; Lv, Y.; Luo, N.; Liang, M.; Huang, L. Upregulated Serum MiR-146b Serves as a Biomarker for Acute Ischemic Stroke. Cell. Physiol. Biochem. 2018, 45, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- Jin, F.; Xing, J. Circulating miR-126 and miR-130a levels correlate with lower disease risk, disease severity, and reduced inflammatory cytokine levels in acute ischemic stroke patients. Neurol. Sci. 2018, 39, 1757–1765. [Google Scholar] [CrossRef]
- Jin, F.; Xing, J. Circulating pro-angiogenic and anti-angiogenic microRNA expressions in patients with acute ischemic stroke and their association with disease severity. Neurol. Sci. 2017, 38, 2015–2023. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z. Higher Peripheral Blood MiR-488 Level Predicts Poor Prognosis of Acute Ischemic Stroke. Clin. Lab. 2020, 66, 1391. [Google Scholar] [CrossRef]
- Liang, T.Y.; Lou, J.Y. Increased Expression of mir-34a-5p and Clinical Association in Acute Ischemic Stroke Patients and in a Rat Model. Med. Sci. Monit. 2016, 22, 2950–2955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Zhang, J.; Han, R.; Liu, H.; Sun, D.; Liu, X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J. Clin. Neurosci. 2015, 22, 291–295. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Li, Y.; Zhao, H. The relationship of long non-coding RNA maternally expressed gene 3 with microRNA-21 and their correlation with acute ischemic stroke risk, disease severity and recurrence risk. Clin. Neurol. Neurosurg. 2021, 210, 106940. [Google Scholar] [CrossRef]
- Liu, P.; Han, Z.; Ma, Q.; Liu, T.; Wang, R.; Tao, Z.; Li, G.; Li, F.; Zhang, S.; Li, L.; et al. Upregulation of MicroRNA-128 in the Peripheral Blood of Acute Ischemic Stroke Patients is Correlated with Stroke Severity Partially through Inhibition of Neuronal Cell Cycle Reentry. Cell Transplant. 2019, 28, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yun, H.J.; Elkin, K.; Guo, Y.; Ding, Y.; Li, G. MicroRNA-29b Suppresses Inflammation and Protects Blood-Brain Barrier Integrity in Ischemic Stroke. Mediat. Inflamm. 2022, 2022, 1755416. [Google Scholar] [CrossRef]
- Mostafa, S.; Al Masry, H.; Hussein, M.; Abd Elkareem, R.M.; Masoud, M.M. The potential role of micro-RNA 125b-5p level in predicting outcome from thrombolytic therapy in patients with acute ischemic stroke. J. Thromb. Thrombolysis 2023, 56, 275–282. [Google Scholar] [CrossRef]
- Rahmati, M.; Azarpazhooh, M.R.; Ehteram, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Ghannadan, H.; Mobarra, N. The elevation of S100B and downregulation of circulating miR-602 in the sera of ischemic stroke (IS) patients: The emergence of novel diagnostic and prognostic markers. Neurol. Sci. 2020, 41, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Sun, H.; Chen, H.; Wang, Y.; Zhang, Q. Decreased Serum Exosomal miR-152-3p Contributes to the Progression of Acute Ischemic Stroke. Clin. Lab. 2020, 66, 1615–1622. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Fu, F.; Liu, J.; Sun, W.; Chen, Y. Diagnostic and prognostic value of serum miR-9-5p and miR-128-3p levels in early-stage acute ischemic stroke. Clinics 2021, 76, e2958. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Ma, Y.; Tang, G.; Liu, Y.; Chen, X.; Zhang, Z.; Zeng, L.; Wang, Y.; Ouyang, Y.-B.; et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J. Cereb. Blood Flow. Metab. 2015, 35, 1977–1984. [Google Scholar] [CrossRef]
- Xiang, W.; Tian, C.; Lin, J.; Wu, X.; Pang, G.; Zhou, L.; Pan, S.; Deng, Z. Plasma let-7i and miR-15a expression are associated with the effect of recombinant tissue plasminogen activator treatment in acute ischemic stroke patients. Thromb. Res. 2017, 158, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, M.; Liu, D.; Zhu, Q.; Chen, H. Expression of miR-9 in the serum of patients with acute ischemic stroke and its effect on neuronal damage. Int. J. Clin. Exp. Pathol. 2018, 11, 5885–5892. [Google Scholar] [PubMed] [PubMed Central]
- Zhong, C.; Yin, C.; Niu, G.; Ning, L.; Pan, J. MicroRNA miR-497 is closely associated with poor prognosis in patients with cerebral ischemic stroke. Bioengineered 2021, 12, 2851–2862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, J.; Chen, L.; Chen, B.; Huang, S.; Zeng, C.; Wu, H.; Chen, C.; Long, F. Increased serum exosomal miR-134 expression in the acute ischemic stroke patients. BMC Neurol. 2018, 18, 198. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, L. miR-124 Is Downregulated in Serum of Acute Cerebral Infarct Patients and Shows Diagnostic and Prognostic Value. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211035446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Ding, J.; Wang, B.; Wang, J.; Xu, M. Circular RNA DLGAP4 is down-regulated and negatively correlates with severity, inflammatory cytokine expression and pro-inflammatory gene miR-143 expression in acute ischemic stroke patients. Int. J. Clin. Exp. Pathol. 2019, 12, 941–948. [Google Scholar] [PubMed] [PubMed Central]
- Guo, C.; Zhong, C.; Li, Q.; Gao, Y.; Li, W.; Ou, Y. Expressions and neural function prognostic evaluation of serum microRNA-24 and microRNA-29b in elderly patients with acute ischemic stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 78–82. [Google Scholar] [PubMed]
- He, X.W.; Shi, Y.H.; Liu, Y.S.; Li, G.-F.; Zhao, R.; Hu, Y.; Lin, C.-C.; Zhuang, M.-T.; Su, J.-J.; Liu, J.-R. Increased plasma levels of miR-124-3p, miR-125b-5p and miR-192-5p are associated with outcomes in acute ischaemic stroke patients receiving thrombolysis. Atherosclerosis 2019, 289, 36–43. [Google Scholar] [CrossRef]
- He, X.W.; Shi, Y.H.; Zhao, R.; Liu, Y.-S.; Li, G.-F.; Hu, Y.; Chen, W.; Cui, G.-H.; Su, J.-J.; Liu, J.-R. Plasma Levels of miR-125b-5p and miR-206 in Acute Ischemic Stroke Patients After Recanalization Treatment: A Prospective Observational Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 1654–1661. [Google Scholar] [CrossRef]
- Ma, Q.; Li, G.; Tao, Z.; Wang, J.; Wang, R.; Liu, P.; Luo, Y.; Zhao, H. Blood microRNA-93 as an indicator for diagnosis and prediction of functional recovery of acute stroke patients. J. Clin. Neurosci. 2019, 62, 121–127. [Google Scholar] [CrossRef]
- Rainer, T.H.; Leung, L.Y.; Chan, C.P.Y.; Leung, Y.K.; Abrigo, J.M.; Wang, D.; Graham, C.A. Plasma miR-124-3p and miR-16 concentrations as prognostic markers in acute stroke. Clin. Biochem. 2016, 49, 663–668. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qu, M.; Li, Y.; Wang, L.; Zhang, L.; Wang, Y.; Tang, Y.; Tian, H.-L.; Zhang, Z.; Yang, G.-Y. MicroRNA-126-3p/-5p Overexpression Attenuates Blood-Brain Barrier Disruption in a Mouse Model of Middle Cerebral Artery Occlusion. Stroke 2020, 51, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, Z.; Yang, Z.; Huang, Q.; Liu, J.; Hong, B. Plasma MicroRNA-16 Is a Biomarker for Diagnosis, Stratification, and Prognosis of Hyperacute Cerebral Infarction. PLoS ONE. 2016, 11, e0166688. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, L.; Simats, A.; García-Berrocoso, T.; Montaner, J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418789340. [Google Scholar] [CrossRef]
- Fang, Z.; He, Q.W.; Li, Q.; Chen, X.L.; Baral, S.; Jin, H.J.; Zhu, Y.Y.; Li, M.; Xia, Y.P.; Mao, L.; et al. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J. 2016, 30, 2097–2107. [Google Scholar] [CrossRef]
- Caballero-Garrido, E.; Pena-Philippides, J.C.; Lordkipanidze, T.; Bragin, D.; Yang, Y.; Erhardt, E.B.; Roitbak, T. In Vivo Inhibition of miR-155 Promotes Recovery after Experimental Mouse Stroke. J. Neurosci. 2015, 35, 12446–12464. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.D.; Xia, Y.P.; Gao, Y.; Zhu, Y.Y.; Chen, S.C.; Mao, L.; He, Q.W.; Yue, Z.Y.; Hu, B. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J. 2018, 32, 935–944. [Google Scholar] [CrossRef]
- Rakkar, K.; Bayraktutan, U. Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochim. Biophys. Acta. 2016, 1862, 56–71. [Google Scholar] [CrossRef]
- Dewdney, B.; Trollope, A.; Moxon, J.; Thomas Manapurathe, D.; Biros, E.; Golledge, J. Circulating MicroRNAs as Biomarkers for Acute Ischemic Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 522–530. [Google Scholar] [CrossRef]
- Edwardson, M.A.; Zhong, X.; Fiandaca, M.S.; Federoff, H.J.; Cheema, A.K.; Dromerick, A.W. Plasma MicroRNA Markers of Upper Limb Recovery Following Human Stroke. Sci. Rep. 2018, 8, 12558. [Google Scholar] [CrossRef] [PubMed]
- Čivrný, J.; Tomáš, D.; Černá, M. MRI of cerebral oedema in ischaemic stroke and its current use in routine clinical practice. Neuroradiology 2024, 66, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Salinas, C.; Wintermark, M. Imaging of acute ischemic stroke. Neuroimaging Clin. N. Am. 2010, 20, 455–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagaraja, N.; Forder, J.R.; Warach, S.; Merino, J.G. Reversible diffusion-weighted imaging lesions in acute ischemic stroke: A systematic review. Neurology 2020, 94, 571–587. [Google Scholar] [CrossRef]
- Gauriau, R.; Bizzo, B.C.; Comeau, D.S.; Hillis, J.M.; Bridge, C.P.; Chin, J.K.; Pawar, J.; Pourvaziri, A.; Sesic, I.; Sharaf, E.; et al. Head CT deep learning model is highly accurate for early infarct estimation. Sci. Rep. 2023, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, H.B.; Claus, S.P.; Bär, P.R.; Ramos, L.M.P.; Algra, A.; van Gijn, J.; Kappelle, L.J. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke 2001, 32, 424–430. [Google Scholar] [CrossRef]
- Neag, M.A.; Mitre, A.O.; Burlacu, C.C.; Inceu, A.-I.; Mihu, C.; Melincovici, C.-S.; Bichescu, M.; Buzoianu, A.-D. miRNA Involvement in Cerebral Ischemia-Reperfusion Injury. Front. Neurosci. 2022, 16, 901360. [Google Scholar] [CrossRef]
- Gendosz de Carrillo, D.; Kocikowska, O.; Rak, M.; Krzan, A.; Student, S.; Jędrzejowska-Szypułka, H.; Pawletko, K.; Lasek-Bal, A. The Relevance of Reperfusion Stroke Therapy for miR-9-3p and miR-9-5p Expression in Acute Stroke-A Preliminary Study. Int. J. Mol. Sci. 2024, 25, 2766. [Google Scholar] [CrossRef]

| Lp. | Author, Year | Study Design | Study Group (n) | Patient Characteristics | miRNA Studied | Primary Outcome | Secondary Outcome | Results | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Chen, 2017 [16] | Case control, prospective | 83 | 50 subjects, 33 controls; AIS patients <72 h from stroke onset | miR-223 Exosomes | NIHSS, infarct volume | TOAST classification, OCSP | - No difference in miR-223 level among TOAST subtypes; - A positive correlation was found between miR-223 and NIHSS; - No correlation between miR-223 and infarct volume. | 72 h from onset; no further follow up |
| 2. | Chen, 2018 [17] | Case control, prospective | 230 | 128 subjects, 102 control; AIS patients <24 h from onset | miR-146b | NIHSS, infarct volume | correlation between miR-146b and CRP and IL-6 levels | - Positive correlation between miR-146b and CRP and IL-6; - miR-146b positively correlated with both infarct volume and NIHSS. | 24 from onset |
| 3. | Ji, 2016 [18] | Case control, prospective | 131 | 65 subjects, 66 controls; AIS patients <24 h from onset | miR-9 miR-124 | Level of miR-9 and miR-124 expression | Correlation of miR and NiHSS, infarct volume, and IL-6 | - Both positively correlated to NIHSS, infarct volume, and IL-6. | 24 from onset |
| 4. | Jin, 2018 [19] | Case control, prospective | 296 | 148 subjects, 148 controls; AIS patients | miR-126 miR-130a miR-222 miR-218 miR-185 | Predicting AIS risk | Correlation of miR with NIHSS, TNF alfa, IL1beta, Il-6, and Il-10 | - Levels of miR-222, -218, and -185 were elevated in AIS; - miR-126 and miR-130 were negatively correlated to NIHSS; - miR-218 was positively correlated to NIHSS; - miR-126 and miR-130 were negatively correlated to TNF alfa, IL1beta, and Il-6; - miR-126 and miR-130 correlate with lower disease risk. | No follow up |
| 5. | Jin, 2017 [20] | Case control, prospective | 216 | 106 subjects, 110 controls; AIS within 24 of stroke onset | miR-126 miR-130a miR-378 miR-222 miR-218 miR-185 miR-206 miR-101 | miR expression | Correlation with NIHSS | - miRs 126, 130a, 222, 218, and 185 may serve as biomarkers for AIS; - miRs 126, 130a, and 378 declined; - miRs 222, 218, and 185 elevated; - miRs 126, 378, 101, 222, 218, and 206 were correlated with NIHSS. | 24 from onset |
| 6. | Liang, 2020 [21] | Case control, prospective | 136 | 76 subjects, 60 controls; AIS patients | miR-448 | miR expression | Correlation between miR and NIHSS/ stroke type | - miR-488 was not significantly different among groups of different etiology; - miR-448 expression was elevated in the AIS group; - Significant positive correlation between miR-488 and NIHSS. | No follow up |
| 7. | Liang, 2016 [22] | Case control, prospective | 199 | 102 subjects, 97 controls; AIS patients <72 h from stroke onset | miR-34a-5p | Expression of miR-34a-5p | Correlation between miR expression and NIHSS and infarct volume | - Overexpression of miRNA-34a-5p in acute ischemic stroke patients; - Negative association between miRNA-34a-5p and NIHSS and infarct volume. | 72 h |
| 8. | Liu, 2015 [23] | Case control, prospective | 42 | 31 subjects, 11 controls; AIS within 24 of stroke onset | miR-9 miR-124 miR-219 | miR expression | Correlation between miR expression and NIHSS, stroke volume and inflammatory parameters | - miR-124 was significantly decreased, miR-9 was decreased in patients with larger stroke, and there were no significant changes in serum miR-219; - miR-124 and miR-9 were negatively correlated with infarct volume and CRP; - All miRs correlated negatively with MMP9. | 24 h |
| 9. | Liu, 2021 [24] | Case control, prospective | 270 | 170 subjects, 100 controls; AIS patients | miR-21 | expression of lnc-MEG3 and miR-21 | Correlation between miR-21 and Il-6, Il-17a, TNFalfa, NIHSS, and accumulating recurrence rate | - miR-21 was reduced in AIS; - miR-21 was negatively correlated with TNF-α, IL-17A, NIHSS, and accumulating recurrence rate in AIS. | No follow up |
| 10. | Liu, 2019 [25] | Case control, prospective | 65 | 40 subjects, 25 controls; AIS patients | miR-128 | Expression of miR-128 | Correlation between miR and NIHSS at 7 days, mRS at 90 days, and infarct volume | - miR128 expression was higher in AIS; - miR-128 levels in circulating lymphocytes were positively correlated with the infarction volume, NIHSS scores at 7 days, and mRS at 90 days; - miR-128 levels in the circulating neutrophils and plasma were not correlated with the infarction volume and NIHSS. | 90 days |
| 11. | Ma, 2022 [26] | Case control, prospective (+animal) | 100 | 60 subjects, 40 controls; AIS 6 h after stroke onset | miR-29b | Expression of miR-29b | Correlation with. NIHSS | - miR-29b in NEU is downregulated in IS; - Negative correlation between NEU miR and NIHSS score at admission. | 6 h |
| 12. | Mostafa, 2023 [27] | Case control, prospective | 80 | 40 subjects, 40 controls, AIS treated with rtPA, assessed on admission and daily throughout 7 days and after 3 months | miR-125b-5p | miR expression | Correlation with stroke type, NIHSS, infarct volume and mRS | - No difference in miR expression in different stroke types; - Positive correlation between miR expression and NIHSS and infarct volume at day 7; - Higher miR expression in patients with complications after rtPA and with poor outcome. | 3 months |
| 13. | Rahmati, 2020 [28] | Case control, prospective | 104 | 52 subjects, 52 controls; AIS patients | miR-602 | Expression of S100B and miR-602 | Correlation between miR expression and NIHSS and survival at 3 months | - Lower miR-602 in AIS patients; - miR-602 was elevated in patients with a higher NIHSS score. | 3 months |
| 14. | Song, 2020 [29] | Case control, prospective | Unknown number of patients and controls | AIS patients | miR-152-3p | miR expression | Correlation between miR expression and NIHSS, stroke type, and stroke phase | - miR-152-3p in patients with AIS was significantly lower; - A decrease in exosome miR-152-3p level is significantly related to the severity of endothelial injury; - The lowest level of exosome miR-152-3p was found in large- artery atherosclerosis; - miR-152-3p level was significantly lower in the acute phase than in the chronic phase. | No follow up |
| 15. | Wang, 2021 [30] | Case control, prospective | 176 | 88 subjects, 88 controls; AIS patients | miR-9-5p miR-128-3p | miR expression | Correlation of miR and mRS and patient comorbidity | - Expression of the miRs was elevated in AIS patients; - miRNAs were positively correlated with the prognostic MRS scores; - Levels of miR-9-5p and miR-128-3p were correlated with BP, BMI, LDL levels, hypertension, and hyperlipidemia. | No follow up |
| 16. | Wand, 2015 [31] | Case control, prospective (+animal) | 117l | 58 subjects, 59 controls; AIS patients, sampule taken < 72 h after onset | miR-29b | miR expression in white blood cells | Correlation between miR29b and NIHSS, mRS, and infarct volume | - miR-29b was significantly downregulated in stroke patients; - miR-29b negatively correlated with NIHSS and infarct volume. | 3 months |
| 17. | Xiang, 2017 [32] | Case control, prospective | 125 | 46 subjects without rtPA, 40 subjects with rtPA, 39 controls; AIS patients 24 h post onset | let-7i miR-15a | miR expression | Correlation between miR and NIHSS | - miR-15a expression decreased by 0.5-fold in the rt-PA group compared to that in the non-rt-PA group; - No significant correlation between miR15a and NIHSS. | No follow up |
| 18. | Sue, 2018 [33] | Case control, prospective | 120 | 65 subjects, 55 controls; AIS patients within 24 h of AIS | miR-9 | miR-9 expression | Correlation between miR09 and NIHSS, inflammatory factors, OGD- induced neuronal injury | - miR-9 was highly expressed in the serum of patients with AIS; - miR-9 was positively correlated with NIHSS; - serum miR-9 in AIS patients had a positive correlation with serum Il-1beta, TNFalfa, and IL-8. | No follow up |
| 19. | Zhong, 2021 [34] | Case control, prospective (+animal) | 128 | 89 subjects, 39 controls; AIS patients | miR-497 | miR-497 expression | Correlation of miR with neurological function (NIHSS) and oxidative stress (SOD, MDA); association with prognosis of CIS | - miR-497 negatively correlated with the NIHSS and MDA concentration; - Positively related to SOD concentration; - Higher prognostic mortality in the low miR-497 group. | 3 years |
| 20. | Zhou, 2018 [35] | Case control, prospective | 100 | 50 subjects, 50 controls; AIS patients within 24 h of AIS | miR-134 | miR-134 expression in AIS | Correlation between miR expression and NIHSS, prognosis, infarct volume and inflammatory factors (IL-6, CRP) | - High expression of miR134 in AIS patients - miR134 was positively correlated with NIHSS, infarct volume and worse prognosis; - miR134 was positively correlated with IL-6 and cRP. | 3 months |
| 21. | Zhou, 2021 [36] | Case control, prospective | 216 | 108 subjects, 108 controls; AIS patients at 24 h, 48 h, and 72 h | miR-124 | miR-124 expression | Correlation between miR expression and inflammatory factors and prognosis in GOS | - miR-124 expression was poorly expressed in the serum of ACI patients; - miR expression was not correlated to infarct classification, infarct size, low-density lipoprotein level, and homocysteine level; - miR-124 expression was negatively correlated with IL-6, IL-8, and CRP; - Low expression of miR-124 was positively correlated with the poor prognosis. | 30 days |
| 22. | Zhu, 2019 [37] | Case control, prospective | 340 | 170 subjects, 170 controls; AIS within 24 h of onset | miR-143 | miR-143 and circ-DLGAP expression | Correlation between miR143 and NIHSS and inflammatory factors | - miR-143 was positively associated with NIHSS score, CRP, ESR, TNF-α, IL-1β, IL-6, IL-8, IL-17, and IL-22. | No follow up |
| 23. | Gus, 2020 [38] | Case control, prospective | 235 | 170 elderly AIS patients, 65 control AIS patients; divided into groups based on mRS and NIHSS score | miR-24 miR-29b | miR-24 and miR-29b expression | Correlation between miR expression and NIHSS and prognosis | - miR-24 and miR-29b in the AIS group were significantly lower than those in the healthy control group - miR-24 and miR-29b in the poor neural function prognosis group were significantly lower than those in the good neural function prognosis group - Expression levels of serum miR-24 and miR-29b in the severe group were significantly lower than those in the mild and moderate groups; - Expression levels of serum miR-24 and miR-29b were negatively correlated with NIHSS score. | No follow up |
| 24. | He, 2019 [39] | Prospective cohort study | 84 | 84 subjects; AIS patients that received rtPA | miR-124-3p miR-125b-5p miR-192-5p | miR expression | Correlation between miR expression and outcome and stroke severity | - miR-124-3p, miR-125b-5p, and miR-192-5p levels were higher in patients with unfavorable outcomes than in patients with favourable outcomes - miR-124-3p and miR-125b-5p were closely associated with stroke severity. | 3 months |
| 25. | He, 2019 [40] | Prospective cohort study | 94 | 94 subjects; AIS 24 h after thrombolysis with or without endovascular treatment | miR-125b-5p miR-206 | Correlation between miR and stroke severity (NIHSS, infarct volume) and outcome | Correlation between miR expression and hemorrhagic transformation | - miR-125b-5p and miR-206 levels were correlated with NIHSS and infarction volumes; - miR-125b-5p levels were significantly higher in patients with an unfavorable outcome; - No association between miRNAs and ICH. | 90 days |
| 26. | Ma, 2019 [41] | Prospective cohort study | ? | AIS patients within 6 h from onset | miR-93 (plasma and neutrophil) | Expression of plasma and neutrophil miR-93 | Correlation between miR-93 and stroke severity and inflammation | - miR-93 levels in plasma and neutrophil detected by real-time PCR were evidently reduced in AIS patients; - miR-93 was not correlated with infarct volume and NIHSS; - miR-93 levels in plasma and neutrophils of AIS patients were negatively correlated with the expression of TNF-α and IL-10; - Neutrophil miR-93 was positively correlated with Barthel index 7 days post stroke. | No follow up |
| 27. | Rainer, 2016 [42] | Prospective cohort study | 84 | 84 AIS patients; AIS patients presenting to the ER within 24 from onset | miR124-3p miR16 | 3 month mortality | Post-stroke mRS | Plasma miR-124-3p concentrations were elevated in patients who died compared to patients who survived; - mir124 was higher in patients with a 3-month mRS > 2 compared to patients with mRS ≤ 2; - Higher miR-16 concentrations in patients who survived than in patients who died; - miR16 concentrations were lower in patients achieving mRS > 2 than in patients with mRS ≤ 2. | 3 months |
| Outcome | Relationship Between miRNA Expression and Selected Outcomes | ||
|---|---|---|---|
| Positive | Negative | No Association | |
| Clinical severity | miR-9* miR-101 miR-124* miR-125b-5p miR-128 miR-134 miR-143 miR-146b miR-185 miR-206 miR-218 miR-222 miR-223 miR-488 miR-602 | miR-9* miR-16 miR-21 miR-24 miR-29b miR-34a-5p miR-124* miR-126 miR-130 miR-152-3p miR-378 miR-497 | miR-9* miR-15a miR-93 miR124* miR-128* miR-219 |
| Infarct volume | miR-9* miR-124* miR-125b-5p miR-128* miR-134 miR-146b miR-206 | miR-9* miR-29b miR-34a-5p miR-124* | miR-93 miR-124* miR-128* miR-223 |
| Systemic inflammatory markers | miR-9* miR124* miR-134 miR-143 miR-146b miR-497 | miR-9* miR-21 miR-93 miR-124* miR-126 miR-130 | miR-9* miR-124* |
| Prognosis | miR-9 miR-24 miR-124 | miR-134 | |
| Etiology subtype | miR152-3p | miR-124 miR-223 miR-488 | |
| Risk of stroke recurrence | miR-21 miR-126 miR-130 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prus, K.; Rejdak, K.; Bilotta, F. The Relationship Between Clinical Features of Ischemic Stroke and miRNA Expression in Stroke Patients: A Systematic Review. Neurol. Int. 2025, 17, 55. https://doi.org/10.3390/neurolint17040055
Prus K, Rejdak K, Bilotta F. The Relationship Between Clinical Features of Ischemic Stroke and miRNA Expression in Stroke Patients: A Systematic Review. Neurology International. 2025; 17(4):55. https://doi.org/10.3390/neurolint17040055
Chicago/Turabian StylePrus, Katarzyna, Konrad Rejdak, and Federico Bilotta. 2025. "The Relationship Between Clinical Features of Ischemic Stroke and miRNA Expression in Stroke Patients: A Systematic Review" Neurology International 17, no. 4: 55. https://doi.org/10.3390/neurolint17040055
APA StylePrus, K., Rejdak, K., & Bilotta, F. (2025). The Relationship Between Clinical Features of Ischemic Stroke and miRNA Expression in Stroke Patients: A Systematic Review. Neurology International, 17(4), 55. https://doi.org/10.3390/neurolint17040055








