Combined MR Volumetry and T2* Relaxometry Reveals the Olfactory System as an Iron-Dependent Structure Affected by Radiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Irradiation Procedure
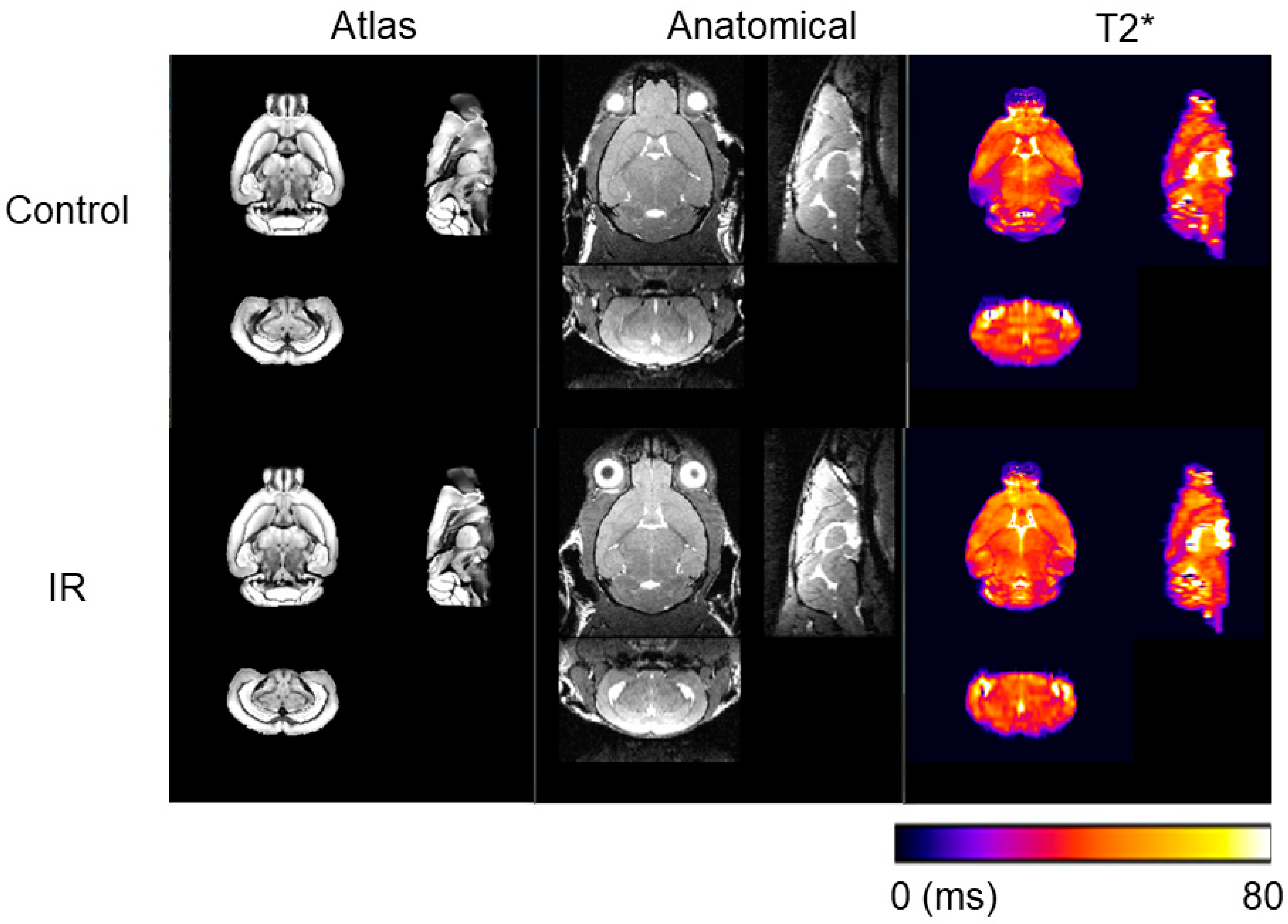
2.2. Magnetic Resonance Imaging (MRI) Data Acquisition and Processing
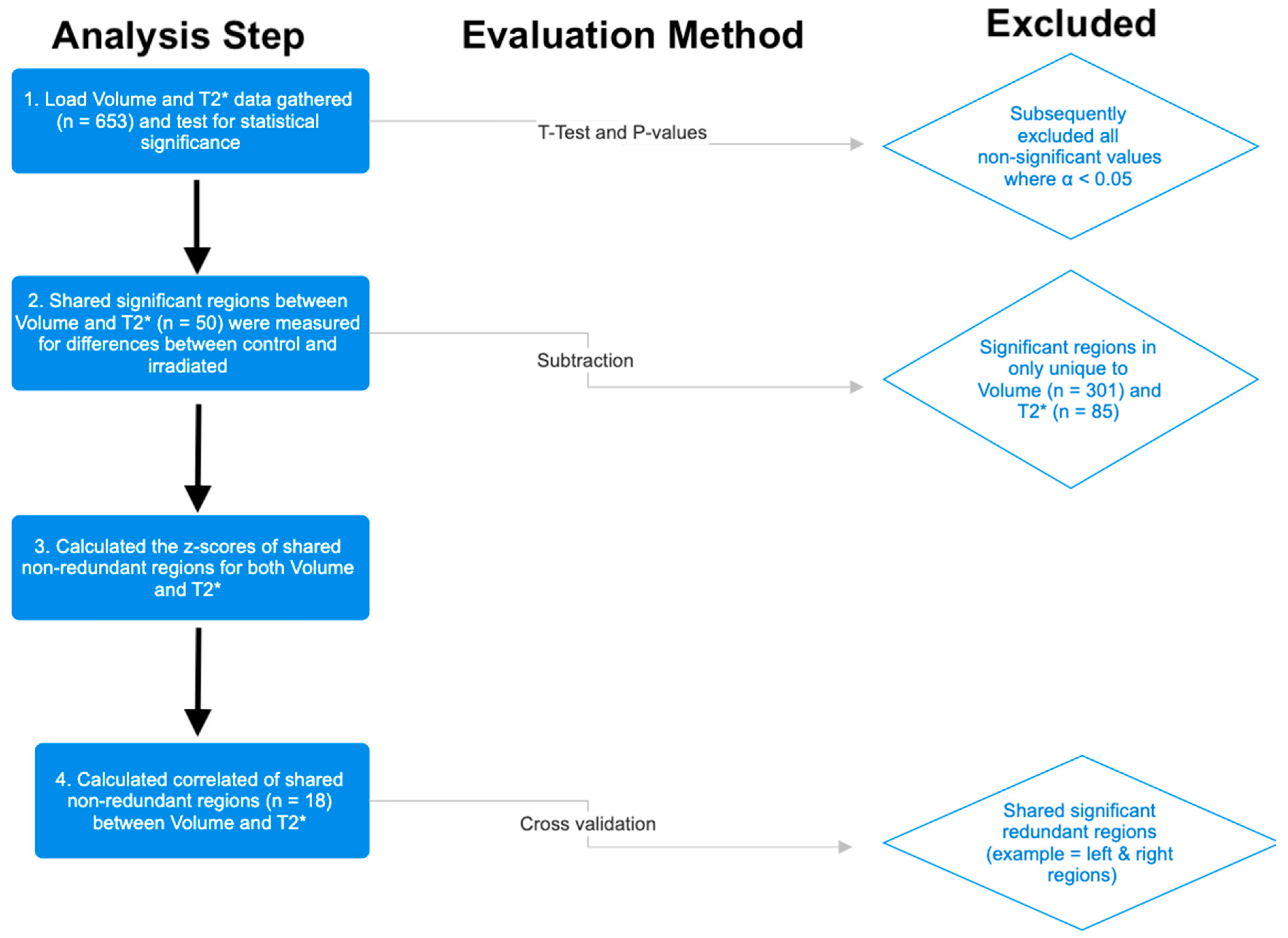
2.3. Statistical Analysis
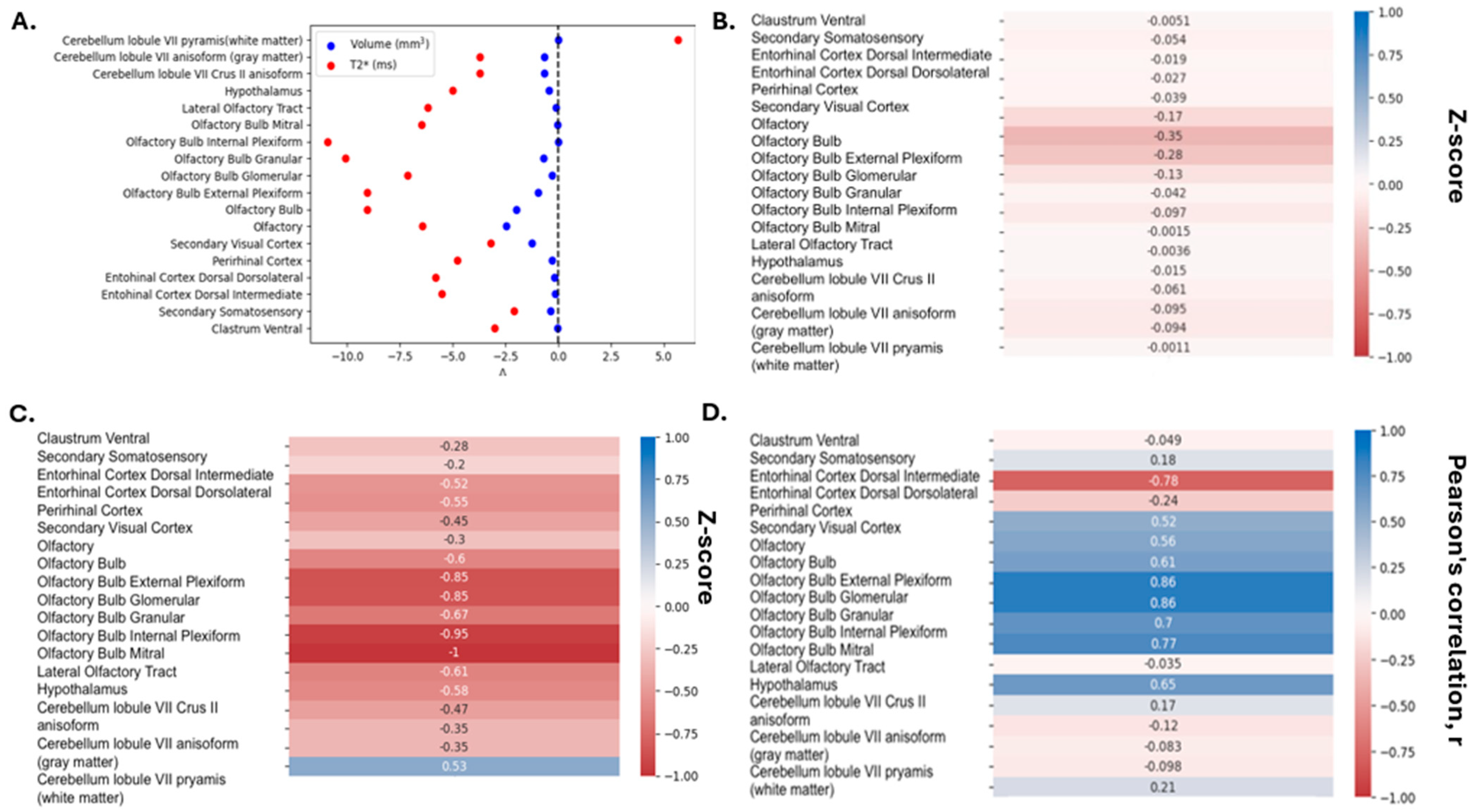
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehrer, E.J.; Jones, B.M.; Dickstein, D.R.; Green, S.; Germano, I.M.; Palmer, J.D.; Laack, N.; Brown, P.D.; Gondi, V.; Wefel, J.S.; et al. The Cognitive Effects of Radiotherapy for Brain Metastases. Front. Oncol. 2022, 12, 893264. [Google Scholar] [CrossRef]
- Meyers, C.A.; Smith, J.A.; Bezjak, A.; Mehta, M.P.; Liebmann, J.; Illidge, T.; Kunkler, I.; Caudrelier, J.-M.; Eisenberg, P.D.; Meerwaldt, J.; et al. Neurocognitive Function and Progression in Patients with Brain Metastases Treated with Whole-Brain Radiation and Motexafin Gadolinium: Results of a Randomized Phase III Trial. J. Clin. Oncol. 2004, 22, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-Induced Brain Injury: Current Concepts and Therapeutic Strategies Targeting Neuroinflammation. Neurooncol Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef] [PubMed]
- Scampoli, C.; Cammelli, S.; Galietta, E.; Siepe, G.; Buwenge, M.; Macchia, G.; Deodato, F.; Cilla, S.; Strigari, L.; Chiesa, S.; et al. Memantine in the Prevention of Radiation-Induced Brain Damage: A Narrative Review. Cancers 2022, 14, 2736. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Han, C.; Tian, L.; Wang, Y.; Han, B. Ferroptosis in Radiation-Induced Brain Injury: Roles and Clinical Implications. Biomed. Eng. Online 2024, 23, 93. [Google Scholar] [CrossRef]
- Feng, Z.; Min, L.; Chen, H.; Deng, W.; Tan, M.; Liu, H.; Hou, J. Iron Overload in the Motor Cortex Induces Neuronal Ferroptosis Following Spinal Cord Injury. Redox Biol. 2021, 43, 101984. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron Imbalance in Neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef]
- Petronek, M.S.; St-Aubin, J.J.; Lee, C.Y.; Spitz, D.R.; Gillan, E.G.; Allen, B.G.; Magnotta, V.A. Quantum Chemical Insight into the Effects of the Local Electron Environment on T2*-Based MRI. Sci. Rep. 2021, 11, 20817. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, Techniques, and Applications of T2*-Based MR Imaging and Its Special Applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Ben Bashat, D.; Thaler, A.; Lerman Shacham, H.; Even-Sapir, E.; Hutchison, M.; Evans, K.C.; Orr-Urterger, A.; Cedarbaum, J.M.; Droby, A.; Giladi, N.; et al. Neuromelanin and T2*-MRI for the Assessment of Genetically at-Risk, Prodromal, and Symptomatic Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 139. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Thedens, D.R.; Lullmann, O.; Steinbach, E.J.; Tamplin, M.R.; Petronek, M.S.; Grumbach, I.M.; Allen, B.G.; Harshman, L.A.; Magnotta, V.A. An Improved Postprocessing Method to Mitigate the Macroscopic Cross-Slice B0 Field Effect on R2* Measurements in the Mouse Brain at 7T. Tomography 2024, 10, 1074–1088. [Google Scholar] [CrossRef]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High Resolution Three-Dimensional Brain Atlas Using an Average Magnetic Resonance Image of 40 Adult C57Bl/6J Mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Oguz, I.; Zhang, H.; Rumple, A.; Sonka, M. RATS: Rapid Automatic Tissue Segmentation in Rodent Brain MRI. J. Neurosci. Methods 2014, 221, 175–182. [Google Scholar] [CrossRef]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. Neuroimage 2011, 54, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Elster, A.D. Gradient-Echo MR Imaging: Techniques and Acronyms. Radiology 1993, 186, 1–8. [Google Scholar] [CrossRef]
- Langkammer, C.; Schweser, F.; Krebs, N.; Deistung, A.; Goessler, W.; Scheurer, E.; Sommer, K.; Reishofer, G.; Yen, K.; Fazekas, F.; et al. Quantitative Susceptibility Mapping (QSM) as a Means to Measure Brain Iron? A Post Mortem Validation Study. NeuroImage 2012, 62, 1593–1599. [Google Scholar] [CrossRef]
- Liu, C.; Wei, H.; Gong, N.-J.; Cronin, M.; Dibb, R.; Decker, K. Quantitative Susceptibility Mapping: Contrast Mechanisms and Clinical Applications. Tomography 2015, 1, 3–17. [Google Scholar] [CrossRef]
- He, N.; Ling, H.; Ding, B.; Huang, J.; Zhang, Y.; Zhang, Z.; Liu, C.; Chen, K.; Yan, F. Region-Specific Disturbed Iron Distribution in Early Idiopathic Parkinson’s Disease Measured by Quantitative Susceptibility Mapping. Hum. Brain Mapp. 2015, 36, 4407–4420. [Google Scholar] [CrossRef]
- Álvarez-Camacho, M.; Gonella, S.; Campbell, S.; Scrimger, R.A.; Wismer, W.V. A Systematic Review of Smell Alterations after Radiotherapy for Head and Neck Cancer. Cancer Treat. Rev. 2017, 54, 110–121. [Google Scholar] [CrossRef]
- Ophir, D.; Guterman, A.; Gross-Isseroff, R. Changes in Smell Acuity Induced by Radiation Exposure of the Olfactory Mucosa. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 853–855. [Google Scholar] [CrossRef]
- Kim, J.-M.; Jeong, M.S.; Shin, D.-H.; Seol, J.-H.; Hong, S.-C.; Cho, J.H.; Kim, J.K. Olfactory Identification Test Using Familiar Distracters for Koreans. Clin. Exp. Otorhinolaryngol. 2014, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.C.; Rodgers, S.P.; Inoue, T.; Pedersen, S.E.; Leasure, J.L.; Gaber, M.W. Olfactory Memory Impairment Differs by Sex in a Rodent Model of Pediatric Radiotherapy. Front. Behav. Neurosci. 2018, 12, 158. [Google Scholar] [CrossRef]
- Haehner, A.; Hummel, T.; Reichmann, H. Olfactory Loss in Parkinson’s Disease. Park. Dis. 2011, 2011, 450939. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.A.; Kurtenbach, S.; Sargi, Z.B.; Harbour, J.W.; Choi, R.; Kurtenbach, S.; Goss, G.M.; Matsunami, H.; Goldstein, B.J. Single-Cell Analysis of Olfactory Neurogenesis and Differentiation in Adult Humans. Nat. Neurosci. 2020, 23, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, A.; Gambardella, C.; Belluzzi, O. Neurogenesis in the Adult Olfactory Bulb. Neural Regen. Res. 2011, 6, 575–600. [Google Scholar]
- Beera, K.G.; Li, Y.-Q.; Dazai, J.; Stewart, J.; Egan, S.; Ahmed, M.; Wong, C.S.; Jaffray, D.A.; Nieman, B.J. Altered Brain Morphology after Focal Radiation Reveals Impact of Off-Target Effects: Implications for White Matter Development and Neurogenesis. Neuro-Oncology 2018, 20, 788–798. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamau, N.R.; Tamplin, M.R.; Lee, C.-Y.; Axelson, E.D.; Grumbach, I.M.; Petronek, M.S. Combined MR Volumetry and T2* Relaxometry Reveals the Olfactory System as an Iron-Dependent Structure Affected by Radiation. Neurol. Int. 2025, 17, 53. https://doi.org/10.3390/neurolint17040053
Kamau NR, Tamplin MR, Lee C-Y, Axelson ED, Grumbach IM, Petronek MS. Combined MR Volumetry and T2* Relaxometry Reveals the Olfactory System as an Iron-Dependent Structure Affected by Radiation. Neurology International. 2025; 17(4):53. https://doi.org/10.3390/neurolint17040053
Chicago/Turabian StyleKamau, Njenga R., Michelle R. Tamplin, Chu-Yu Lee, Eric D. Axelson, Isabella M. Grumbach, and Michael S. Petronek. 2025. "Combined MR Volumetry and T2* Relaxometry Reveals the Olfactory System as an Iron-Dependent Structure Affected by Radiation" Neurology International 17, no. 4: 53. https://doi.org/10.3390/neurolint17040053
APA StyleKamau, N. R., Tamplin, M. R., Lee, C.-Y., Axelson, E. D., Grumbach, I. M., & Petronek, M. S. (2025). Combined MR Volumetry and T2* Relaxometry Reveals the Olfactory System as an Iron-Dependent Structure Affected by Radiation. Neurology International, 17(4), 53. https://doi.org/10.3390/neurolint17040053





