Abstract
Background/Objectives: Spindle cell oncocytomas (SCOs) of the pituitary gland are rare tumors often misdiagnosed for nonfunctioning pituitary macroadenomas. Although classified as grade 1, they are often challenging in terms of diagnosis and treatment. Pituitary SCOs harbor peculiar features such as hypervascularity and stronger adherence to surrounding structures, with increased risk of hemorrhage, partial resection, and significantly higher recurrence rate. Almost 100 cases have been reported so far. The role of surgery is still crucial for the decompression of the optic chiasm as well as for achieving diagnosis. However, given the higher tendency of recurrence, the role of postoperative radiotherapy has been investigated over the last few years. Case presentation: Here, we reported a case of a 48-year-old female with a pituitary SCO treated at our institution, in which we focused on diagnosis, treatment, and follow-up. Conclusions: This type of tumor presents a challenge related to its higher vascularity and strong adherence to the surrounding structures. Adjuvant radiotherapy is something that should be considered, especially when gross total resection is not achieved, and finally, SCOs require diligent follow-up to monitor for any signs of disease recurrence or progression.
1. Introduction
Spindle cell oncocytomas (SCOs) are rare non-neuroendocrine tumors arising from the pituicytes of the posterior pituitary gland. They were included in the World Health Organization (WHO) classification of central nervous system tumors in 2007 after being first described by Roncaroli et al. in 2002 [1,2].
SCOs were previously thought to stem from the adenohypophysis; however, more recent research identified that SCOs are positive for thyroid transcription factor 1 (TTF1) which is only found in pituicytes and not in the folliculostellate cells of the adenohypophysis [3]. In our search from 2002 to 2024, there were only 98 cases published in the literature that highlight the rarity of this type of tumor.
SCOs are often misdiagnosed as pituitary adenomas; nevertheless, they are important to distinguish as they usually have higher vascularity as well as an increased tendency to invade the surrounding structures [4]. This results in a higher recurrence rate, thus requiring more frequent follow-ups. If gross total resection (GTR) is not achieved, 50% of tumors show significant progression requiring extra treatment by two years, recurrence occurs in 20% of people, and even distant metastasis has been reported [4].
Despite these factors, the WHO considers pituitary SCOs a grade 1 tumor with benign behavior [5]. Moreover, although this tumor is considered grade 1, it is important to understand these critical features as it guides treatment and follow-up and will improve patient outcomes.
Due to the low incidence of this neoplasm, the definitive knowledge of the unique clinical signs and symptoms, diagnostic imaging (DI), immunohistochemistry (IHC), and treatment is lacking. In this report and literature review, we aimed to provide further knowledge on these factors to improve the identification and treatment of individuals with SCO. In addition, the role of adjuvant radiotherapy is not fully understood in the treatment of pituitary SCO. However, recent literature demonstrated radiotherapy to be safe and effective for residual SCO after surgery [6]. Radiotherapy will be further assessed in our review of the literature to also help establish a comprehensive understanding of its potential role in the treatment of SCO.
2. Case Presentation
We present the case of a 48-year-old female with a two-month history of persistent headaches, mental and physical fatigue, and light sensitivity. Her past medical history includes chronic migraines, fibromyalgia, psoriasis, anxiety, obesity, and obstructive sleep apnea. When looking back, the patient realized that her headache symptoms had changed from her normal migraine symptoms to more frequent albeit less severe frontal pain and aching in the previous months.
Given the persistence of the above-mentioned symptoms, she was investigated with a head computed tomography (CT) scan, which showed a pituitary lesion with sellar and suprasellar extension. For further investigation, she underwent a brain magnetic resonance imaging (MRI) scan that demonstrated a homogenous mass in the pituitary gland (Figure 1). The MRI also showed that the mass was compressing the optic chiasm and was encasing both internal carotid arteries. These findings were thought to be compatible with pituitary macroadenoma.
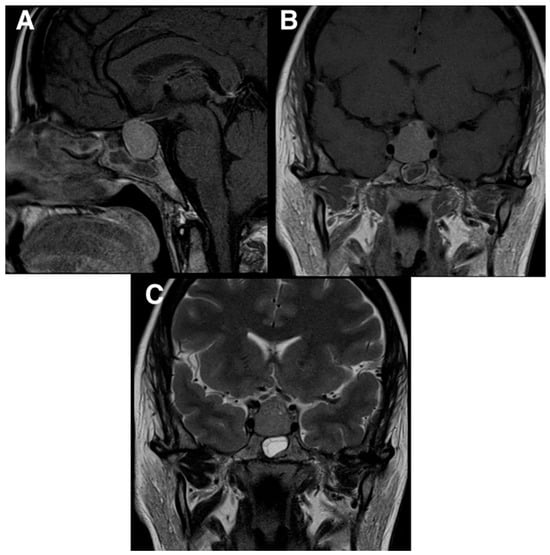
Figure 1.
Preoperative MRI scans demonstrating a homogenous mass in the pituitary gland. The MRI also showed that the mass was compressing the optic chiasm and was encasing the carotid arteries. (A) T1 MRI sagittal view; (B) T1 MRI coronal view; (C) T2 MRI coronal view.
On physical examination, the patient had no cranial nerve deficits, and her pupils were equal, round, and reactive to light and accommodation. She did not have any focal motor or sensory deficits. She also underwent an ophthalmological assessment that ruled-out papilledema and confirmed normal bilateral visual fields. Lastly, a thorough endocrinological assessment was completed, including a full hormonal panel that demonstrated only mild hyperprolactinemia.
Given the clinical and radiological findings, an elective surgical treatment was recommended and carried out using an endoscopic endonasal transsphenoidal approach. Intraoperatively, the tumor had the usual soft consistency, but it presented stronger adherence with what was thought to be the normal pituitary gland and, therefore, required extra work to separate it. Although no major bleeding was identified, an unusual constant tumor oozing made the procedure more challenging. An apparent GTR of the tumor was achieved and confirmed by the direct visualization of the suprasellar cistern coming down into the sella turcica. For this reason, no adjuvant radiotherapy was performed.
The patient had an uneventful postoperative period. She spent two days in the intensive care unit and was then transferred to the floor, where low levels of cortisol and mild diabetes insipidus were diagnosed and treated medically. Post-operative MRI ruled out intraoperative complications and confirmed the decompression of the optic chiasm (Figure 2). She was discharged home on postoperative day seven, neurologically intact.
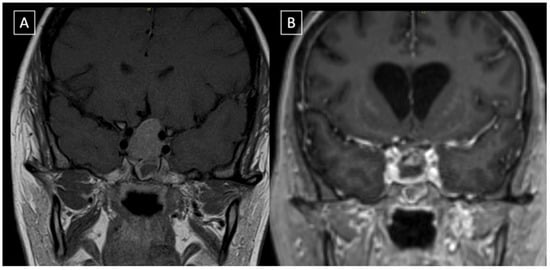
Figure 2.
Coronal reconstruction of the pre-op (A) and post-op (B) post gadolinium T1-weighted images. The post-operative MRI confirms that optic chiasm has been decompressed and gross total resection obtained. There is evidence of minimally enhancing intrasellar material that is likely gel foam used for skull base reconstruction.
She was reassessed in the follow-up four weeks after surgery, and no obvious focal deficits were identified. Her headache and light sensitivity had notably improved. A repeat MRI was completed three months postoperatively, and it confirmed the GTR of the SCO; the patient scheduled their next follow-up with repeat MRI at the sixth month mark.
The microscopy examination of the specimen demonstrated fascicles of spindle-cell tumor cells with eosinophilic cytoplasm and elongated nuclei showing moderate pleomorphism (Figure 3). Other areas of tumor cells showed more eosinophilic cytoplasm with clear borders and contained round to oval nuclei with mild pleomorphism. Granular cytoplasm was not seen; however, mitotic figures were present. No tumor necrosis was noted. The tumor cells were diffusely positive for TTF-1, S100, CD56, and synaptophysin (Figure 4). They were also focally positive for epithelial membrane antigen (EMA), glial fibrillary acidic protein (GFAP), CD68, and beta crystallin. There was no immunostaining for pituitary-specific transcription factor-1 (PIT-1) or steroidogenic factor-1 (SF-1).
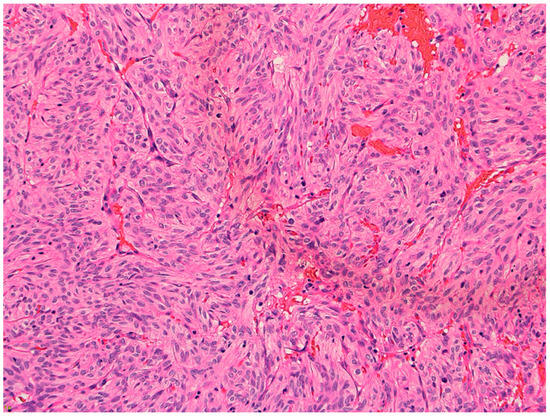
Figure 3.
Hematoxylin and eosin (H&E) 20× showing spindled cells with eosinophilic cytoplasm arranged in nests and short fascicles.
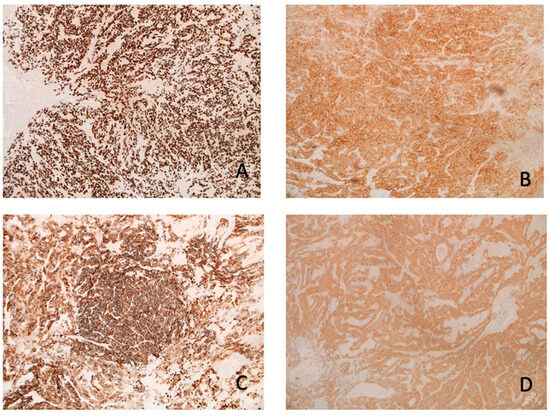
Figure 4.
IHC: The neoplastic cells are positive for (A) TTF1 (10×), (B) synaptophysin (10×), (C) CD56 (10×), and (D) S100 (10×).
3. Discussion
SCOs are very rare tumors of the posterior pituitary gland that originate from the pituicytes located in the neurohypophysis. They are called SCOs because their cells are spindle shaped under microscopic examination and contain many mitochondria in their cytoplasm [7]. Oncocytomas can occur in many different areas of the body, including kidney, breast, prostate gland, thyroid, and salivary glands [7]. However, they are often only called oncocytomas in these locations as they lack spindle-shaped cells, which are characteristic of the pituitary SCO [7,8].
Unfortunately, pituitary SCOs appear very similar to non-functioning pituitary adenomas in clinical presentation and diagnostic imaging. Yet, they require unique care because of two specific features: increased vascularity and increased fibrotic adherence to surrounding structures.
Additionally, SCOs’ increased vascularity has the potential to cause significant bleeding during surgery. Borges et al. described a case of recurring subclinical tumor bleeding that happened in a recurrent SCO [9,10]. In the same manuscript, Borges reported that nearly one-third of assessed cases showed significant intraoperative bleeding, and almost half of these cases demonstrated that the tumor was highly vascular [9,10]. Cases of spontaneous tumor hemorrhage were also described.
Careful tumor dissection and the strategic use of cottonoids with hemostatic agents is crucial when dealing with any tumor of the posterior pituitary gland, especially SCO. Also, SCOs have an elevated risk of progression or recurrence because of the adherent characteristics of the tumor. In a study by Hasegawa et al., it was found that GTR was only achieved in 24% of cases mainly because of those tumor features [5]. These characteristics make the recurrence of SCOs very likely, with 50% of tumors showing significant progression if GTR is not attained and radiotherapy is not added [5].
There are also case reports of SCOs managed transcranially. In fact, in cases with extensive suprasellar extension, this approach can allow for more intraoperative maneuverability to deal with significant bleeding and provide better accessibility to all areas of the tumor [10].
Oftentimes, partial resection is all that can be accomplished, leading to the growth of any residual tumor. In cases like this and with general tumor recurrence, interdisciplinary support should be pursued and thought given to both reoperation as well as adjuvant radiotherapy [10].
The role of preoperative radiotherapy is still being debated; Hasegawa et al. found in their meta-analysis that preoperative radiotherapy did not have an impact on those who achieved GTR [5]. There was also no statistical difference between the non-GTR group that received preoperative radiotherapy and the non-GTR group that did not receive preoperative radiotherapy. However, in patients who do not achieve GTR, postoperative radiotherapy should be considered as it has shown promising results in controlling tumor progression [11].
In our review of the literature (Table 1), we performed a search from 2002 to 2024 using databases such as PubMed, Google Scholar, and ScienceDirect to identify all published articles reporting pituitary SCO, which totaled 98 cases. Primary search terms included pituitary spindle cell oncocytoma, pituitary tumors, and posterior pituitary tumors. Case reports, case series, and original articles were included, whereas articles not presenting new cases or unpublished material were excluded. Article references were also hand searched to ensure no reports were missed. Each case was then analyzed for specific data points, including age, sex, clinical presentation, diagnostic imaging, IHC, surgical approach, use of adjuvant radiotherapy, and general outcomes. The data were then summarized into a structured table which was used to draw conclusions on significant topics like recurrence rates and the effectiveness of radiotherapy, as well as to present all cases reported in the literature thus far. All articles were screened for relevance by the authors, and any discrepancies were resolved through collaborative discussion.

Table 1.
Review of the literature (2002–2024).
The mean age of all the patients with pituitary SCO was found to be fifty-seven years. Regarding any difference between sex, forty-seven patients were female, and fifty-one patients were male. Of particular importance, we found that there was recurrence or tumor growth in 31% of patients with pituitary SCO who did not receive adjuvant radiotherapy. Meanwhile, only 18% of patients who did receive adjuvant radiotherapy experienced tumor recurrence or progression. This information on adjuvant radiotherapy shows that it has the potential to be very useful in achieving tumor stability and decreases the chances of progression and need for further operations. Akyoldas et al. reported five cases that utilized radiotherapy, all of which showed the tumors to be stable at follow-up. Also, in this study, gamma knife radiosurgery was used each time and reported a median tumor margin dose of 12Gy and a median maximal dose of 24Gy [6].
These rates of recurrence/progression underscore the importance of continued surveillance with these tumors. Many of the reports noted how partial resection was all that could be achieved due to the highly vascular nature of the tumor. However, recurrence was even found in cases that appeared to achieve GTR.
Our case specifically highlights the importance of being aware of the high vascularity of these tumors due to their increased risk of bleeding during surgical resection. If a SCO is suspected/identified, measures can be put into place to prepare for increased bleeding, such as careful tumor dissection and strategic use of cottonoids with hemostatic agents in addition to blood products typed and matched if they are needed. Special consideration is also needed regarding the follow-up plan for patients with SCO due to their increased rate of recurrence.
4. Conclusions
SCOs are rare tumors of the posterior pituitary gland that have many unique features that require specific treatment and follow-up. Although this tumor presents similarly to a pituitary adenoma, there are marked differences in the physical appearance of the tumor, IHC, and follow-up required. From a surgical perspective, the challenge is related to higher vascularity and stronger adherence to the surrounding structures. This makes the surgery itself more difficult and a GTR less likely, with a higher recurrence rate.
To date, the use of radiotherapy was not well established. However, our review does provide encouraging results that post-operative radiotherapy has the potential to minimize tumor progression and increase tumor stability. Only 18% of the patients who had a pituitary SCO and received adjuvant radiotherapy developed recurrence/progression compared to 31% of patients who did not receive adjuvant radiotherapy.
In our opinion, radiotherapy should definitely be considered, especially when GTR is not achieved. In our case, a GTR was achieved; thus, we decided to proceed without radiotherapy and with closer follow-up appointments to identify any early sign of recurrence. Closer follow-up appointments are also very necessary as many patients develop recurrence or tumor progression, sometimes despite GTR. These high rates of tumor recurrence/progression underscore the need for careful and frequent monitoring.
Author Contributions
Conceptualization, S.M.P. and J.H.; Data Curation, J.H.; Writing—Original Draft Preparation, J.H. and Z.G.; Writing—Review and Editing, S.M.P. and B.M.; Visualization, J.H. and B.M.; Supervision, S.M.P.; Project Administration, S.M.P. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This case report was conducted in accordance with the Declaration of Helsinki. Institutional Review Board (IRB) approval was not required for this case report, as it involves a single patient and does not include any experimental procedures.
Informed Consent Statement
The authors confirm that patient consent is not applicable to this article. This is a retrospective case report using de-identified data.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IHC | Immunohistochemistry |
| SCO | Spindle-cell oncocytoma |
| CT | Computed Tomography |
| MRI | Magnetic resonance imaging |
| TTF-1 | Thyroid Transcription Factor-1 |
| EMA | Epithelial Membrane Antigen |
| NVD | Nausea, vomiting, and diarrhea |
| F/U | Follow-up |
| VS | Vision |
| VD | Visual defect |
| PIT-1 | Pituitary-specific positive transcription factor-1 |
| PTTG-1 | Pituitary Tumor Transforming Gene-1 |
| GFAP | Glial Fibrillary Acidic Protein |
| Gal3 | Galectin-3 |
| Bcl2 | B-cell lymphoma 2 |
| TSR | Transsphenoidal resection |
| GK | Gamma Knife |
| Gy | Gray |
| ND | Not described |
| AMA | Anti-mitochondrial Ab |
References
- Roncaroli, F.; Scheithauer, B.W.; Cenacchi, G.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Abell-Aleff, P.; Santi, M.; Yates, A.J. Spindle cell oncocytoma of the adenohypophysis: A tumor of folliculostellate cells? Am. J. Pathol. 2002, 26, 1048–1055. [Google Scholar] [CrossRef]
- Joshi, S.; Krishnamurthy, B.; McKelvie, P.; Dhillon, R. Case report of pituitary spindle cell oncocytoma concurrent to growth-hormone secreting pituitary adenoma. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2024, 36, 101971. [Google Scholar] [CrossRef]
- Guerrero-Pérez, F.; Vidal, N.; Marengo, A.P.; Del Pozo, C.; Blanco, C.; Rivero-Celada, D.; Díez, J.J.; Iglesias, P.; Picó, A. Posterior pituitary tumours: The spectrum of a unique entity. Endocrine 2019, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, J.; Inoue, A.; Miyake, T.; Ohno, T.; Kitazawa, R.; Kunieda, T. Clinicopathological features and endoscopic findings of spindle cell oncocytoma: A case report and review of the literature. Int. J. Surg. Case Rep. 2023, 109, 108536. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Van Gompel, J.J.; Oushy, S.H.; Pollock, B.E.; Link, M.J.; Meyer, F.B.; Bancos, I.; Erickson, D.; Davidge-Pitts, C.J.; Little, J.T.; et al. A Comprehensive Study of Spindle Cell Oncocytoma of the Pituitary Gland: Series of 6 Cases and Meta-Analysis of 85 Cases. World Neurosurg. 2021, 149, E197–E216. [Google Scholar] [CrossRef]
- Akyoldaş, G.; Hergünsel, B.; Özdemir, I.E.; Şengöz, M.; Peker, S. Gamma knife radiosurgery for pituitary spindle cell oncocytomas. Clin. Neurol. Neurosurg. 2019, 187, 105560. [Google Scholar] [CrossRef]
- Hodzic, Z.; Rowan, N.R.; Kashiwazaki, R.; Willson, T.J.; Wang, E.W.; Lee, S.E. A systematic review of sinonasal oncocytomas and oncocytic carcinomas: Diagnosis, management, and technical considerations. Int. Forum Allergy Rhinol. 2017, 7, 514–524. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Chien, S.-C.; Tsai, H.-C.; Wei, K.-C.; Chuang, C.-C.; Jung, S.-M. Pituitary spindle cell oncocytoma: Two cases report and literature review. Int. J. Surg. Case Rep. 2024, 124, 110328. [Google Scholar] [CrossRef]
- Borges, M.T.; Lillehei, K.O.; Kleinschmidt-DeMasters, B.K. Spindle cell oncocytoma with late recurrence and unique neuroimaging characteristics due to recurrent subclinical intratumoral bleeding. J. Neuro-Oncol. 2011, 101, 145–154. [Google Scholar] [CrossRef]
- Larsen, A.M.G.; Cote, D.J.; Zaidi, H.A.; Bi, W.L.; Schmitt, P.J.; Iorgulescu, J.B.; Miller, M.B.; Smith, T.R.; Lopes, M.B.; Jane, J.A.; et al. Spindle cell oncocytoma of the pituitary gland. J. Neurosurg. 2018, 131, 517–525. [Google Scholar] [CrossRef]
- Chang, C.N.; Shen, C.C. Spindle cell oncocytoma of the pituitary tumor: A rare case report and literature reviews. Front. Surg. 2023, 9, 1021680. [Google Scholar] [CrossRef]
- Shimizu, A.; Nonami, Y.; Kanamuro, T.; Masui, K.; Yamamoto, T.; Amano, K.; Kawamata, T.; Ichihara, A.; Nagashima, Y. Pituicytoma with pleomorphism: A case report with cytological findings. Diagn. Cytopathol. 2022, 51, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Tena-Suck, M.L.; Hernández-Pacheco, A.A.; Rocandio-Hernández, D.; Salinas-Lara, C.; Sánchez-Garibay, C. Spindle Cells Oncocytoma and Rathke’s Cyst; A Collision Sellar Tumor– Brief Communication & Case Report. Med Clin. Case Rep. 2022, 2, 1022. [Google Scholar] [CrossRef]
- Abdulrazeq, H.; Anderson, M.; Poggi, J.; Sampath, S.; Kanach, C.; Dellale, I.; Sampath, P. Management of pituitary spindle cell oncocytomas: A case report and review of the literature. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2021, 23, 100972. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, S.; Kim, M.-S.; Hwang, J.-H.; Hahm, M.H. Spindle cell oncocytoma of the sella turcica with anaplastic features and rapid progression in short-term follow-up: A case report with proposal of distinctive radiologic features. J. Pathol. Transl. Med. 2021, 55, 225–229. [Google Scholar] [CrossRef]
- Kottangal, G.V.; Madhavan, L.; Kuruvilla, S.; Parameswaran, K.K.; Kollathodi, S.B. Spindle cell oncocytoma, a misdiagnosed rare entity of the pituitary—A case report with review of literature and special emphasis on the morphological differentials. Indian J. Pathol. Oncol. 2021, 8, 533–537. [Google Scholar] [CrossRef]
- Taka, T.M.; Yang, C.Y.; Limbo, J.N.; Chan, A.Y.; Davies, J.; Kuan, E.C.; Turner, S.G.; Hsu, F.P.K. Pituitary spindle cell oncocytoma: Illustrative case. J. Neurosurg. Case Lessons 2021, 2, 21356. [Google Scholar] [CrossRef]
- Leonardo, T.; Antonio, A.; Giorgio, F.; Giulio, B.; Giorgio, C.; Aldo, P.; Emanuele, F.; Giovanna, M.; Marco, L. Arterial Embolization and Second-Look in Spindle Cell Oncocytoma of the Pituitary Gland: Case Report and Review of Literature. World Neurosurg. 2020, 142, 87–92. [Google Scholar] [CrossRef]
- Samadian, M.; Mousavinejad, S.; Khoshsirat, S.; Dehghan, M.; Sharifi, G.; Ebrahimzadeh, K.; Rezaei, O. Trans-nasal trans-sphenoidal endoscopic resection of spindle cell oncocytoma of adenohypophysis: The first case report in a child and a review of literature. Asian J. Neurosurg. 2020, 15, 210–213. [Google Scholar] [CrossRef]
- Borg, A.; Jaunmuktane, Z.; Dorward, N. Tumours of the Neurohypophysis—One unit’s experience and literature review. World Neurosurg. 2020, 134, E968–E978. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.H.; Sheinberg, D.; Buttrick, S.; Levine, C.G.; Bhatia, R.G.; Lam, B.L.; Pasol, J.; Ayala, A.R.; Starke, R.M. Imaging Characteristics of a Hypervascular Pituitary Spindle Cell Oncocytoma on Magnetic Resonance Imaging and Digital Subtraction Angiography. World Neurosurg. 2020, 133, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Chainey, J.; Chan, V.K.-Y.; Au, K.; Das, S. Multiple recurrences of spindle cell oncocytoma: A case report and literature review. Clin. Neuropathol. 2020, 39, 32–39. [Google Scholar] [CrossRef]
- Sollfrank, L.; Lettmaier, S.; Erdmann, M.; Uslu, U. Panniculitis Under Successful Targeted Inhibition of the MAPK/ERK Signaling Pathway in a Patient with BRAF V600E-mutated Spindle Cell Oncocytoma of the Pituitary Gland. Anticancer. Res. 2019, 39, 3955–3959. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.-M.; Lee, H.-P.; Hsieh, P.-P. Pituitary spindle cell oncocytoma presented as pituitary apoplexy. J. Surg. Case Rep. 2019, 2019, rjz179. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.S.; Potla, S.; Sarris, C.E.; Przybylowski, C.J.; Baranoski, J.F.; Mooney, M.A.; Barranco, F.D.; White, W.L.; Eschbacher, J.M.; Little, A.S. Rare Thyroid Transcription Factor 1–Positive Tumors of the Sellar Region: Barrow Neurological Institute Retrospective Case Series. World Neurosurg. 2019, 129, e294–e302. [Google Scholar] [CrossRef]
- Witte, H.M.; Riecke, A.; Saeger, W.; Hackenbroch, C.; Mathieu, R.; Mauer, U.M.; Schulz, C. Spindle cell oncocytoma of the neurohypophysis with metastasis to the sphenoparietal sinus and immunohistochemical negativity for S100 and epithelial membrane antigen (EMA). Br. J. Neurosurg. 2018, 37, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Batra, V.V.; Gupta, R.K.; Singh, D.; Sharma, M.C.; Kumar, V. Is spindle cell oncocytoma a true entity or a variant of pituicytoma? A case report with review of literature. Neurol. India 2018, 66, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Toda, M.; Akiyama, T.; Takahashi, S.; Nishimoto, M.; Ozawa, H.; Ikari, Y.; Yoshida, K. Combined Endoscopic Endonasal and Video-microscopic Transcranial Approach with Preoperative Embolization for a Posterior Pituitary Tumor. World Neurosurg. 2018, 119, 201–208. [Google Scholar] [CrossRef]
- Nagata, Y.; Inoshita, N.; Fukuhara, N.; Yamaguchi-Okada, M.; Nishioka, H.; Yamada, S. Low-Grade Glioma of the Neurohypophysis: Clinical Characteristics and Surgical Outcomes. World Neurosurg. 2018, 114, e1225–e1231. [Google Scholar] [CrossRef]
- Sosa, S.; Danilowicz, K.; González Abbati, S.; Sevlever, G. Pituitary spindle cell oncocytoma: A case report with review of literature. Medicina 2018, 78, 33–36. [Google Scholar]
- Xie, J.; Silverman, J.F.; Pu, C.; Graner, S.; Storto, P.; Donangelo, I.; Jasnosz, K.M. Spindle cell oncocytoma of adenohypophysis: Cytogenetics and β-catenin findings with pathology differential diagnosis and review of the literature. Hum. Pathol. Case Rep. 2017, 9, 71–75. [Google Scholar] [CrossRef]
- Rafiq, N.M.; Kuniak, M.; Anichini, G.; Togersen, A.; Kamel, M.H. Spindle cell oncocytoma of the adenohypophysis: 2 case reports of unusual radiological and intra-operative findings. Interdiscip. Neurosurg. 2017, 10, 81–85. [Google Scholar] [CrossRef]
- Osman, M.; Wild, A. Spindle Cell Oncocytoma of the Anterior Pituitary Presenting with an Acute Clinical Course Due to Intraventricular Hemorrhage. A Case Report and Review of Literature. Am. J. Case Rep. 2017, 18, 894–901. [Google Scholar] [CrossRef]
- Aamodt, W.W.; Siegler, J.E.; Viaene, A.N.; Rubenstein, M.N. Late onset progressive multifocal leukoencephalopathy in Hodgkin lymphoma. J. Clin. Neurosci. 2017, 43, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Epari, S.; Tampi, C.; Goel, A. Spindle cell oncocytoma of adenohypophysis: Review of literature and report of another recurrent case. Neuropathology 2017, 37, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Billeci, D.; Marton, E.; Giordan, E.; Carraro, V.; Ronzon, M.; Rossi, S. Spindle cell oncocytoma: Report of two cases with massive bleeding and review of the literature. J. Clin. Neurosci. 2017, 39, 39–44. [Google Scholar] [CrossRef]
- Kong, X.; Li, D.; Kong, Y.; Zhong, D. Malignant adenohypophysis spindle cell oncocytoma with repeating recurrences and a high Ki-67 index. Medicine 2017, 96, e5657. [Google Scholar] [CrossRef] [PubMed]
- Hagel, C.; Buslei, R.; Buchfelder, M.; Fahlbusch, R.; Bergmann, M.; Giese, A.; Flitsch, J.; Lüdecke, D.K.; Glatzel, M.; Saeger, W. Immunoprofiling of glial tumours of the neurohypophysis suggests a common pituicytic origin of neoplastic cells. Pituitary 2017, 20, 211–217. [Google Scholar] [CrossRef]
- Custodio, P.J.P.; Jho, D.H.; Pu, C.; Gordon, M.B.; Donangelo, I. Spindle Cell Oncocytoma of the Pituitary Presenting with Severe Hyponatremia. AACE Clin. Case Rep. 2016, 2, e237–e243. [Google Scholar] [CrossRef]
- Hasiloglu, Z.; Ure, E.; Comunoglu, N.; Tanriover, N.; Oz, B.; Gazioglu, N.; Mihmanli, I. New radiological clues in the diagnosis of spindle cell oncocytoma of the adenohypophysis. Clin. Radiol. 2016, 71, 937.e5–937.e11. [Google Scholar] [CrossRef]
- Guadagno, E.; Cervasio, M.; Di Somma, A.; Califano, M.; Solari, D. Del Basso De Caro, M. Essential role of ultrastructural examination for spindle cell oncocytoma: Case report of a rare neoplasm and review of the literature. Ultrastruct. Pathol. 2016, 40, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Kondo, T.; Tran, T.M.; Oishi, N.; Nakazawa, T.; Mochizuki, K.; Inoue, T.; Kasai, K.; Tahara, I.; Jieying, W.; et al. Spindle cell oncocytoma of adenohypophysis: Report of a case and immunohistochemical review of literature. Pathol. -Res. Pract. 2015, 212, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Zygourakis, C.C.; Rolston, J.D.; Lee, H.S.; Partow, C.; Kunwar, S.; Aghi, M.K. Pituicytomas and spindle cell oncocytomas: Modern case series from the University of California, San Francisco. Pituitary 2015, 18, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Yu, J.; Qu, L.; Hu, X.; Gao, H.; Liu, P.; Zheng, X.; Sun, Y.; Huang, H. Spindle cell oncocytoma of the adenohypophysis: Two case reports and a review of the literature. Mol. Med. Rep. 2015, 12, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Rotman, J.A.; Kucharczyk, W.; Zadeh, G.; Kiehl, T.-R.; Al-Ahmadi, H. Spindle cell oncocytoma of the adenohypophysis: A case report illustrating its natural history with 8-year observation and a review of the literature. Clin. Imaging 2014, 38, 499–504. [Google Scholar] [CrossRef]
- Fujisawa, H.; Tohma, Y.; Muramatsu, N.; Kida, S.; Kaizaki, Y.; Tamamura, H. Spindle cell oncocytoma of the ade-nohypophysis with marked hypervascularity. Neurol. Med. Chir. 2012, 52, 594–598. [Google Scholar] [CrossRef]
- Alexandrescu, S.; Brown, R.E.; Tandon, N.; Bhattacharjee, M.B. Neuron precursor features of spindle cell oncocy-toma of adenohypophysis. Ann. Clin. Lab. Sci. 2012, 42, 123–128. [Google Scholar]
- Singh, G.; Agarwal, S.; Sharma, M.C.; Suri, V.; Sarkar, C.; Garg, A.; Kale, S.S. Spindle cell oncocytoma of the adenohypophysis: Report of a rare case and review of literature. Clin. Neurol. Neurosurg. 2012, 114, 267–271. [Google Scholar] [CrossRef]
- Chandler, J.; Ogiwara, H.; Shafizadeh, S.; Dubner, S.; Raizer, J. Spindle cell oncocytoma of the pituitary and pituicytoma: Two tumors mimicking pituitary adenoma. Surg. Neurol. Int. 2011, 2, 116. [Google Scholar] [CrossRef]
- Romero-Rojas, A.E.; Diaz-Perez, J.A.; Amaro, D.; Lozano, J.M. Spindle cell oncocytoma of the adenohypophysis: A rare tumor mimicking pituitary aden. J. Of. Clinical. Pathol. 2011, 64, 823. [Google Scholar] [CrossRef]
- Vajtai, I.; Beck, J.; Kappeler, A.; Hewer, E. Spindle cell oncocytoma of the pituitary gland with follicle-like component: Organotypic differentiation to support its origin from folliculo-stellate cells. Acta Neuropathol. 2011, 122, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mlika, M.; Azouz, H.; Chelly, I.; Ben Saïd, I.; Jemel, H.; Haouet, S.; Zitouna, M.; Kchir, N. Spindle cell oncocytoma of the adenohypophysis in a woman: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 64. [Google Scholar] [CrossRef]
- Matyja, E.; Maksymowicz, M.; Grajkowska, W.; Olszewski, W.; Zieliński, G. Spindle cell oncocytoma of the adeno-hypophysis—A clinicopathological and ultrastructural study of two cases. Folia Neuropathol. 2010, 48, 175–184. [Google Scholar]
- Demssie, Y.N.; Joseph, J.; Dawson, T.; Roberts, G.; de Carpentier, J.; Howell, S. Recurrent spindle cell oncocytoma of the pituitary, a case report and review of literature. Pituitary 2011, 14, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Borota, O.C.; Scheithauer, B.; Fougner, S.L.; Hald, J.; Ramm-Pettersen, J.; Bollerslev, J. Spindle cell oncocytoma of the adenohypophysis: Report of a case with marked cellular atypia and recurrence despite adjuvant treatment. Clin. Neuropathol. 2009, 28, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Coiré, C.; Horvath, E.; Smyth, H.; Kovacs, K. Rapidly recurring folliculostellate cell tumor of the adenohypophysis with the morphology of a spindle cell oncocytoma: Case report with electron microscopic studies. Clin. Neuropathol. 2009, 28, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Bhatt, A.; Chang, H.T. Teaching NeuroImage: Spindle cell oncocytoma of the pituitary gland. Neurology 2008, 71, e3. [Google Scholar] [CrossRef]
- Vajtai, I.; Sahli, R.; Kappeler, A. Spindle cell oncocytoma of the adenohypophysis: Report of a case with a 16-year follow-up. Pathol. Res. Pr. 2006, 202, 745–750. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, C.; Hedley-Whyte, E.T.; Sharma, M.C.; Zervas, N.T.; Sridhar, E.; Louis, D.N. Spindle cell oncocytoma of the adenohypophysis: Report of two cases. Acta Neuropathol. 2005, 110, 97–99. [Google Scholar] [CrossRef]
- Kloub, O.; Perry, A.; Tu, P.-H.; Lipper, M.; Lopes, M.B. Spindle cell oncocytoma of the adenohypophysis: Report of two recurrent cases. Am. J. Surg. Pathol. 2005, 29, 247–253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).