Blood–Brain Barrier Dysfunction, Edema Formation and Functional Recovery in Ischemic and Hemorrhagic Stroke: A Retrospective Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Participants
2.2. Clinical Variables and Definitions
2.3. Imaging Parameters
2.4. Outcome Parameters
2.5. Statistical Analysis
3. Results
3.1. Study Population
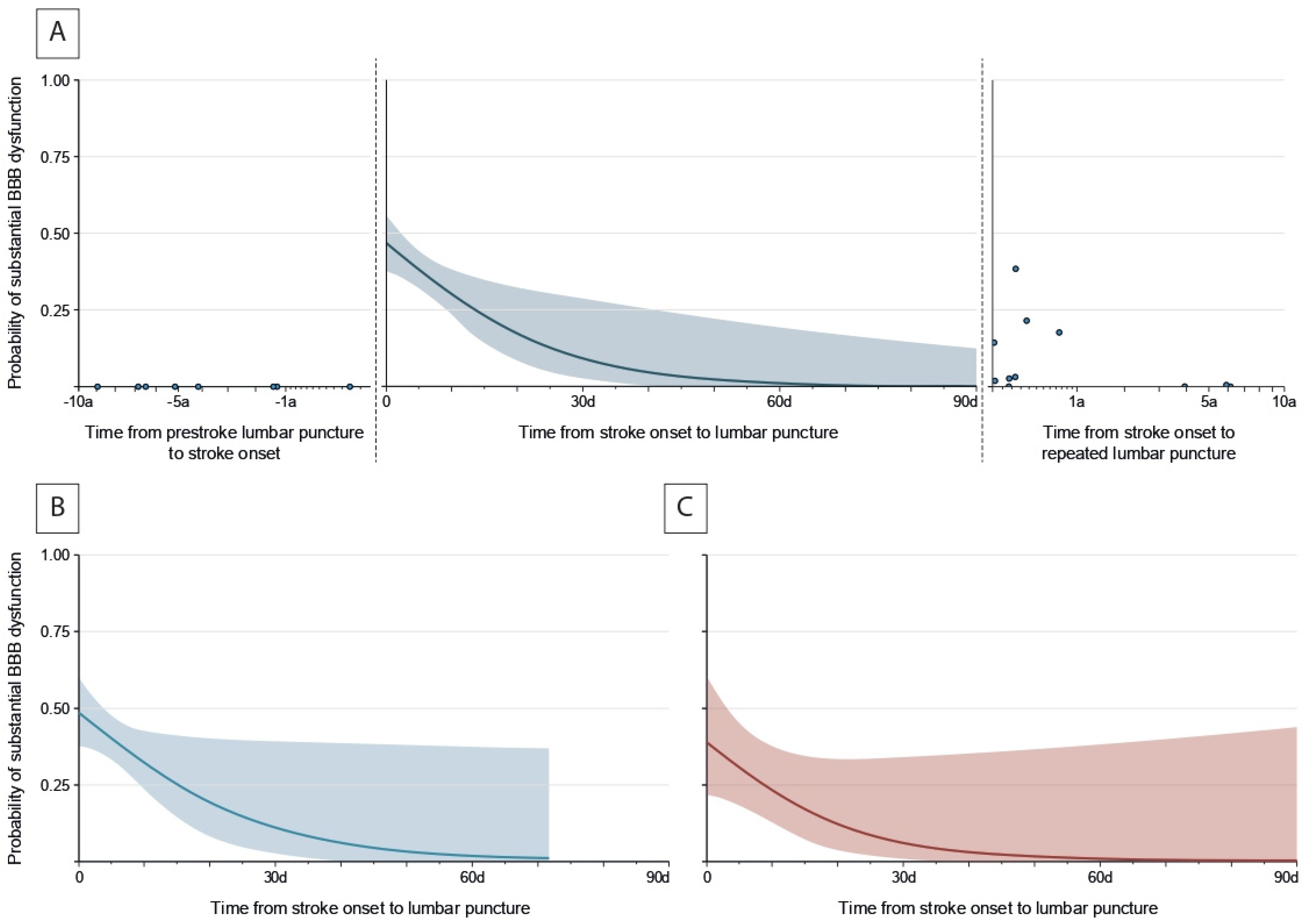
3.2. Temporal Patterns of Substantial BBB Dysfunction
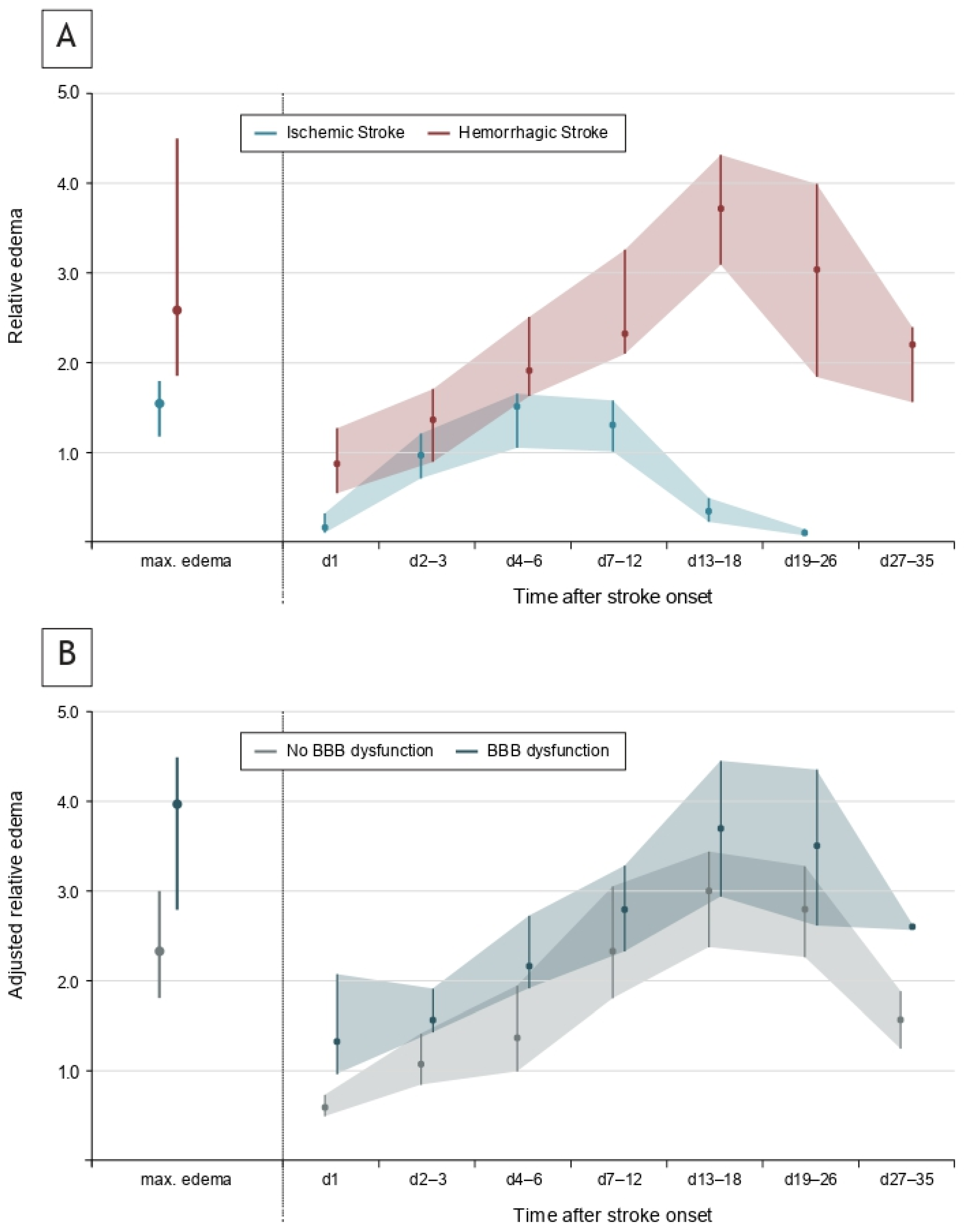
3.3. Temporal Evolution of Edema Formation
3.4. Association Between BBB Dysfunction, Edema and Functional Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Pillai, D.R.; Dittmar, M.S.; Baldaranov, D.; Heidemann, R.M.; Henning, E.C.; Schuierer, G.; Bogdahn, U.; Schlachetzki, F. Cerebral ischemia-reperfusion injury in rats--a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2009, 29, 1846–1855. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamaguchi, T.; Kirino, T.; Orzi, F.; Klatzo, I. The effects of 5-minute ischemia in Mongolian gerbils: I. Blood-brain barrier, cerebral blood flow, and local cerebral glucose utilization changes. Acta Neuropathol. 1983, 60, 207–216. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Sprügel, M.I.; Kuramatsu, J.B.; Volbers, B.; Gerner, S.T.; Sembill, J.A.; Madžar, D.; Bobinger, T.; Kölbl, K.; Hoelter, P.; Lücking, H.; et al. Perihemorrhagic edema: Revisiting hematoma volume, location, and surface. Neurology 2019, 93, e1159–e1170. [Google Scholar] [CrossRef]
- Staykov, D.; Wagner, I.; Volbers, B.; Hauer, E.M.; Doerfler, A.; Schwab, S.; Bardutzky, J. Natural course of perihemorrhagic edema after intracerebral hemorrhage. Stroke 2011, 42, 2625–2629. [Google Scholar] [CrossRef]
- Schwab, S.; Aschoff, A.; Spranger, M.; Albert, F.; Hacke, W. The value of intracranial pressure monitoring in acute hemispheric stroke. Neurology 1996, 47, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Battey, T.W.; Karki, M.; Singhal, A.B.; Wu, O.; Sadaghiani, S.; Campbell, B.C.; Davis, S.M.; Donnan, G.A.; Sheth, K.N.; Kimberly, W.T. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke 2014, 45, 3643–3648. [Google Scholar] [CrossRef]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Devinney, M.J.; Wong, M.K.; Wright, M.C.; Marcantonio, E.R.; Terrando, N.; Browndyke, J.N.; Whitson, H.E.; Cohen, H.J.; Nackley, A.G.; Klein, M.E.; et al. Role of Blood-Brain Barrier Dysfunction in Delirium following Non-cardiac Surgery in Older Adults. Ann. Neurol. 2023, 94, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H.; Iggena, D.; Baldinger, T.; Prinz, V.; Meisel, A.; Endres, M.; Dirnagl, U.; Schwab, J.M. Evidence of intrathecal immunoglobulin synthesis in stroke: A cohort study. Arch. Neurol. 2012, 69, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Sprügel, M.I.; Sembill, J.A.; Kremer, S.; Gerner, S.T.; Knott, M.; Hock, S.; Engelhorn, T.; Dörfler, A.; Huttner, H.B.; Schwab, S. Evaluation of Functional Recovery Following Thrombectomy in Patients With Large Vessel Occlusion and Prestroke Disability. JAMA Netw. Open 2022, 5, e2227139. [Google Scholar] [CrossRef]
- Macha, K.; Hoelter, P.; Siedler, G.; Knott, M.; Schwab, S.; Doerfler, A.; Kallmünzer, B.; Engelhorn, T. Multimodal CT or MRI for IV thrombolysis in ischemic stroke with unknown time of onset. Neurology 2020, 95, e2954–e2964. [Google Scholar] [CrossRef]
- Siedler, G.; Sommer, K.; Macha, K.; Marsch, A.; Breuer, L.; Stoll, S.; Engelhorn, T.; Dörfler, A.; Arnold, M.; Schwab, S.; et al. Heart Failure in Ischemic Stroke: Relevance for Acute Care and Outcome. Stroke 2019, 50, 3051–3056. [Google Scholar] [CrossRef]
- Haupenthal, D.; Kuramatsu, J.B.; Volbers, B.; Sembill, J.A.; Mrochen, A.; Balk, S.; Hoelter, P.; Lücking, H.; Engelhorn, T.; Dörfler, A.; et al. Disability-Adjusted Life-Years Associated With Intracerebral Hemorrhage and Secondary Injury. JAMA Netw. Open 2021, 4, e2115859. [Google Scholar] [CrossRef]
- Rühl, L.; Kuramatsu, J.B.; Sembill, J.A.; Kallmünzer, B.; Madzar, D.; Gerner, S.T.; Giede-Jeppe, A.; Balk, S.; Mueller, T.; Jäger, J.; et al. Amantadine treatment is associated with improved consciousness in patients with non-traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2022, 93, 582–587. [Google Scholar] [CrossRef]
- Welte, T.M.; Steidl, J.; Stritzelberger, J.; Gollwitzer, S.; Lang, J.D.; Reindl, C.; Rampp, S.; Maslarova, A.; Brandner, S.; Hock, S.; et al. Surgical hematoma evacuation of cortical intracerebral hemorrhage ≥10 mL reduces risk of subsequent epilepsy by more than 70%: A retrospective monocenter study. Eur. J. Neurol. 2023, 30, 2099–2105. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Meretoja, A.; Strbian, D.; Putaala, J.; Curtze, S.; Haapaniemi, E.; Mustanoja, S.; Sairanen, T.; Satopää, J.; Silvennoinen, H.; Niemelä, M.; et al. SMASH-U: A proposal for etiologic classification of intracerebral hemorrhage. Stroke 2012, 43, 2592–2597. [Google Scholar] [CrossRef]
- Rundblad, L.I.S.; Iversen, H.K.; West, A.S. Pleocytosis in cerebrospinal fluid attributed to ischemic stroke: A review of the literature. J. Neurol. Sci. 2023, 449, 120664. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Auer, M.; Zeileis, A.; Deisenhammer, F. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: Implications for increased clinical specificity. Clin. Chem. Lab. Med. 2016, 54, 285–292. [Google Scholar] [CrossRef]
- Sprügel, M.I.; Kuramatsu, J.B.; Volbers, B.; Saam, J.I.; Sembill, J.A.; Gerner, S.T.; Balk, S.; Hamer, H.M.; Lücking, H.; Hölter, P.; et al. Impact of Statins on Hematoma, Edema, Seizures, Vascular Events, and Functional Recovery After Intracerebral Hemorrhage. Stroke 2021, 52, 975–984. [Google Scholar] [CrossRef]
- Muehlen, I.; Sprügel, M.; Hoelter, P.; Hock, S.; Knott, M.; Huttner, H.B.; Schwab, S.; Kallmünzer, B.; Doerfler, A. Comparison of Two Automated Computed Tomography Perfusion Applications to Predict the Final Infarct Volume After Thrombolysis in Cerebral Infarction 3 Recanalization. Stroke 2022, 53, 1657–1664. [Google Scholar] [CrossRef]
- Volbers, B.; Giede-Jeppe, A.; Gerner, S.T.; Sembill, J.A.; Kuramatsu, J.B.; Lang, S.; Lücking, H.; Staykov, D.; Huttner, H.B. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology 2018, 90, e1005–e1012. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Kimberly, W.T.; Dutra, B.G.; Boers, A.M.M.; Alves, H.; Berkhemer, O.A.; van den Berg, L.; Sheth, K.N.; Roos, Y.; van der Lugt, A.; Beenen, L.F.M.; et al. Association of Reperfusion With Brain Edema in Patients With Acute Ischemic Stroke: A Secondary Analysis of the MR CLEAN Trial. JAMA Neurol. 2018, 75, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahi Salman, R.; Frantzias, J.; Lee, R.J.; Lyden, P.D.; Battey, T.W.K.; Ayres, A.M.; Goldstein, J.N.; Mayer, S.A.; Steiner, T.; Wang, X.; et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: A systematic review and meta-analysis of individual patient data. Lancet. Neurol. 2018, 17, 885–894. [Google Scholar] [CrossRef]
- Sheth, K.N. Spontaneous Intracerebral Hemorrhage. N. Engl. J. Med. 2022, 387, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, N.A.; Casolla, B.; Leung, T.W.; de Leeuw, F.E. Stroke. Lancet 2024, 403, 2820–2836. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Katsanos, A.H.; Sandset, E.C.; Turc, G.; Nguyen, T.N.; Bivard, A.; Fischer, U.; Khatri, P. Thrombolysis for acute ischaemic stroke: Current status and future perspectives. Lancet. Neurol. 2023, 22, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocritical Care 2020, 32, 647–666. [Google Scholar] [CrossRef]
- Grände, P.O.; Romner, B. Osmotherapy in brain edema: A questionable therapy. J. Neurosurg. Anesthesiol. 2012, 24, 407–412. [Google Scholar] [CrossRef]
- Bereczki, D.; Fekete, I.; Prado, G.F.; Liu, M. Mannitol for acute stroke. Cochrane Database Syst. Rev. 2007, 2007, Cd001153. [Google Scholar] [CrossRef]
- Sandercock, P.A.; Soane, T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 2011, Cd000064. [Google Scholar] [CrossRef] [PubMed]
- Sprügel, M.I.; Kuramatsu, J.B.; Gerner, S.T.; Sembill, J.A.; Beuscher, V.D.; Hagen, M.; Roeder, S.S.; Lücking, H.; Struffert, T.; Dörfler, A.; et al. Antiplatelet Therapy in Primary Spontaneous and Oral Anticoagulation-Associated Intracerebral Hemorrhage. Stroke 2018, 49, 2621–2629. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Sembill, J.A.; Knott, M.; Xu, M.; Roeder, S.S.; Hagen, M.; Sprügel, M.I.; Mrochen, A.; Borutta, M.; Hoelter, P.; Engelhorn, T.; et al. Simplified Edinburgh CT Criteria for Identification of Lobar Intracerebral Hemorrhage Associated with Cerebral Amyloid Angiopathy. Neurology 2022, 98, e1997–e2004. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, A.K.; Rübsamen, N.; Grefkes, C.; Rost, N.S.; Berger, K.; Karch, A. Development and Validation of Prediction Models for Severe Complications After Acute Ischemic Stroke: A Study Based on the Stroke Registry of Northwestern Germany. J. Am. Heart Assoc. 2022, 11, e023175. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Messé, S.R.; Kleindorfer, D.; Smith, E.E.; Fonarow, G.C.; Xu, H.; Zhao, X.; Lytle, B.; Cigarroa, J.; Schwamm, L.H. Cryptogenic stroke: Contemporary trends, treatments, and outcomes in the United States. Neurol. Clin. Pract. 2020, 10, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Henschke, N.; Wolf, J.; Zimmer, K.; Safer, A.; Schröder, R.J.; Inselmann, G.; Brenke, C.; Becher, H.; Grau, A.J. Intracerebral haemorrhage in a population-based stroke registry (LuSSt): Incidence, aetiology, functional outcome and mortality. J. Neurol. 2013, 260, 2541–2550. [Google Scholar] [CrossRef] [PubMed]

| Overall Stroke Patients (n = 138) | |
|---|---|
| Age, years, median (IQR) | 58 (46–73) |
| Female sex, n (%) | 72 (52.2%) |
| Medical history, n (%) | |
| Atrial fibrillation | 16 (11.6%) |
| Anticoagulation therapy | 9 (6.5%) |
| Hypertension | 88 (63.8%) |
| Diabetes mellitus | 26 (18.8%) |
| Previous ischemic stroke or TIA | 27 (19.6%) |
| Previous hemorrhagic stroke | 5 (3.6%) |
| NIHSS score at hospital admission, median (IQR) | 4 (2–9) |
| Treatment with intravenous alteplase, n (%) | 38/103 (27.5%) |
| Treatment with endovascular therapy, n (%) | 7/103 (5.1%) |
| Time from first observation of symptoms to hospital admission, hours, median (IQR) | 8.6 (2.6–48.8) |
| Diagnosis, n (%) | |
| Ischemic stroke | 103 (74.6%) |
| Hemorrhagic stroke | 35 (25.4%) |
| Ischemic stroke subtype | |
| Cardioembolic disease | 26/103 (25.2%) |
| Large-vessel disease | 24/103 (23.3%) |
| Small-vessel disease | 16/103 (15.5%) |
| Other cause a | 15/103 (14.6%) |
| Undetermined | 22/103 (21.4%) |
| Hemorrhagic stroke subtype | |
| Hypertension | 16/35 (45.7%) |
| Amyloid angiopathy | 11/35 (31.4%) |
| Anticoagulation | 5/35 (14.3%) |
| Undetermined | 3/35 (8.6%) |
| Imaging characteristics | |
| Stroke volume on day 2, mL, median (IQR) b | 5.6 (2.5–21.6) |
| Stroke location | |
| Deep, n (%) | 23 (16.7%) |
| Lobar, n (%) | 102 (73.9%) |
| Infratentorial, n (%) | 13 (9.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, C.A.; Sembill, J.A.; Kallmünzer, B.; Bailer, M.; Singer, L.; Engelhorn, T.; Dörfler, A.; Schwab, S.; Balk, S.; Sprügel, M.I. Blood–Brain Barrier Dysfunction, Edema Formation and Functional Recovery in Ischemic and Hemorrhagic Stroke: A Retrospective Study. Neurol. Int. 2025, 17, 177. https://doi.org/10.3390/neurolint17110177
Müller CA, Sembill JA, Kallmünzer B, Bailer M, Singer L, Engelhorn T, Dörfler A, Schwab S, Balk S, Sprügel MI. Blood–Brain Barrier Dysfunction, Edema Formation and Functional Recovery in Ischemic and Hemorrhagic Stroke: A Retrospective Study. Neurology International. 2025; 17(11):177. https://doi.org/10.3390/neurolint17110177
Chicago/Turabian StyleMüller, Christian A., Jochen A. Sembill, Bernd Kallmünzer, Maximilian Bailer, Ludwig Singer, Tobias Engelhorn, Arnd Dörfler, Stefan Schwab, Stefanie Balk, and Maximilian I. Sprügel. 2025. "Blood–Brain Barrier Dysfunction, Edema Formation and Functional Recovery in Ischemic and Hemorrhagic Stroke: A Retrospective Study" Neurology International 17, no. 11: 177. https://doi.org/10.3390/neurolint17110177
APA StyleMüller, C. A., Sembill, J. A., Kallmünzer, B., Bailer, M., Singer, L., Engelhorn, T., Dörfler, A., Schwab, S., Balk, S., & Sprügel, M. I. (2025). Blood–Brain Barrier Dysfunction, Edema Formation and Functional Recovery in Ischemic and Hemorrhagic Stroke: A Retrospective Study. Neurology International, 17(11), 177. https://doi.org/10.3390/neurolint17110177






