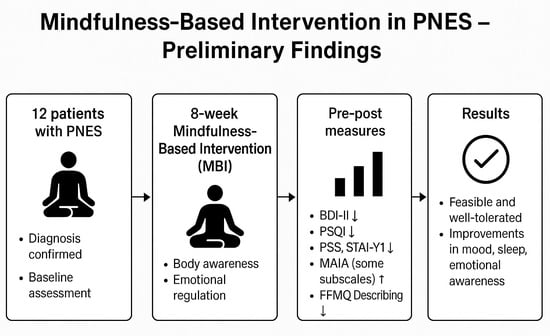
Preliminary Findings from a Mindfulness-Based Intervention in Patients with Psychogenic Non-Epileptic Seizures
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims and Context
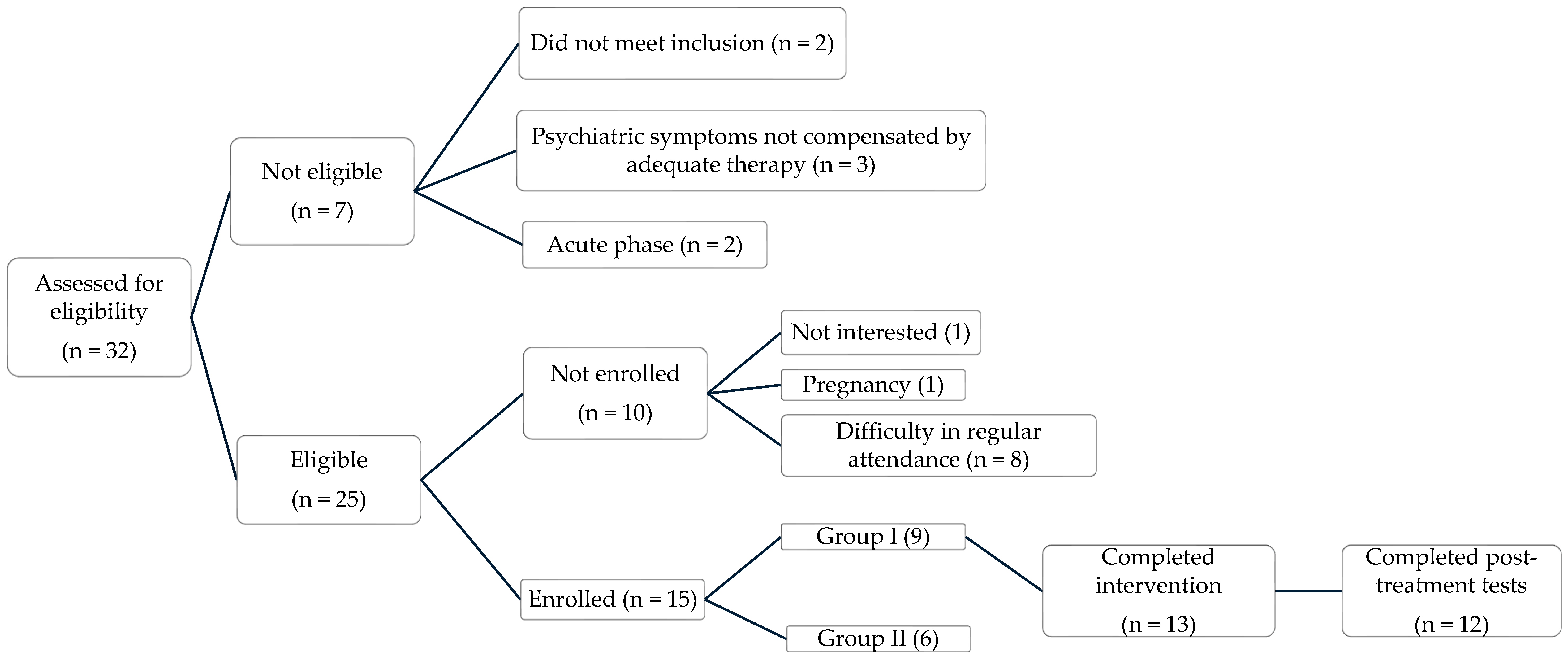
2.2. Study Design, Recruitment and Procedures
2.3. Intervention
2.4. Measures
- Five Facet Mindfulness Questionnaire (FFMQ) [28]. FFMQ is a 39-item scale measuring five facets of mindfulness: observing, describing, acting with awareness, nonjudging, and nonreactivity. The Italian version of the FFMQ has demonstrated satisfactory psychometric properties in both clinical and non-clinical populations. In particular, the validation study confirmed the original five-factor structure through confirmatory factor analysis and reported good internal consistency (Cronbach’s α ranging from 0.72 to 0.86 across subscales) [29].
- Perceived Stress Scale (PSS-10) [30]. The PSS-10 is a widely used 10-item self-report questionnaire designed to measure the degree to which individuals perceive their lives as unpredictable, uncontrollable, and overloaded. The Italian version (IPSS-10) supported a two-factor structure—Perceived Helplessness and Perceived Self-Efficacy—and demonstrated good internal consistency (Cronbach’s α = 0.87). Convergent validity was confirmed through significant correlations with anxiety and depression measures, supporting the scale’s suitability for use in both research and clinical settings [31].
- Beck Depression Inventory-II (BDI-II) [32,33]. The BDI-II is a 21-item self-report inventory developed to assess the presence and severity of depressive symptoms over the past two weeks, in accordance with DSM-IV criteria. The Italian adaptation confirmed the robust psychometric properties of the instrument in Italian samples; high internal consistency was reported (Cronbach’s α = 0.80–0.91) across both clinical and non-clinical populations, as well as good convergent validity with other measures of depression and anxiety. The questionnaire demonstrated satisfactory discriminant validity, effectively distinguishing between clinical and non-clinical groups [33].
- State-Trait Anxiety Inventory—State version (STAI-Y) [34]. The STAI-Y is a 40-item self-report questionnaire designed to assess the intensity of anxiety experienced in the present moment and in general. The Italian version has shown good psychometric properties [35]. The STAI-Y1 was used for this study (state anxiety).
- Pittsburgh Sleep Quality Index (PSQI) [36]. The PSQI is a widely used 19-item self-report questionnaire designed to assess subjective sleep quality and disturbances over a one-month interval. It has a global score derived from seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The Italian validation confirmed the original structure and psychometric robustness of the scale, reporting good internal consistency (Cronbach’s α = 0.83). Notably, the study supported the established cut-off score of 5 to distinguish good sleepers from poor sleepers [37].
- Dissociative Experiences Scale—II (DES-II) [38]. The DES-II is a 28-item self-report instrument to assess the frequency of dissociative phenomena in everyday life, including amnesia, depersonalization, derealization, absorption, and identity confusion. Though not diagnostic, the scale serves as a screening tool for clinically relevant dissociative symptoms and is commonly used to identify individuals who may require further evaluation (e.g., via the SCID-D). The Italian version has been used in both clinical and non-clinical populations, particularly in studies involving trauma, functional neurological disorders, and personality pathology, confirming the scale’s sensitivity and clinical relevance [39].
- Multidimensional Assessment of Interoceptive Awareness (MAIA) [40]. The MAIA is a 32-item self-report instrument designed to assess interoceptive awareness across eight distinct dimensions: Noticing, Not-Distracting, Not-Worrying, Attention Regulation, Emotional Awareness, Self-Regulation, Body Listening, and Trusting. The MAIA was specifically developed for use in mind–body practices and has been widely applied in mindfulness-based research. The Italian version of the MAIA has shown good psychometric properties [41].
- Meteoropathy Questionnaire (METEO-Q) [42] was administered at baseline to assess meteorosensitivity and meteoropathy. It is a validated self-report tool composed of 11 core items (5 quantitative, 6 qualitative) and a checklist of 21 symptoms rated on a 0–4 Likert scale. The validation study confirmed satisfactory psychometric characteristics and provided percentile-based cut-offs distinguishing different levels of meteorosensitivity and meteoropathy [42].
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Baseline Clinical Characteristics
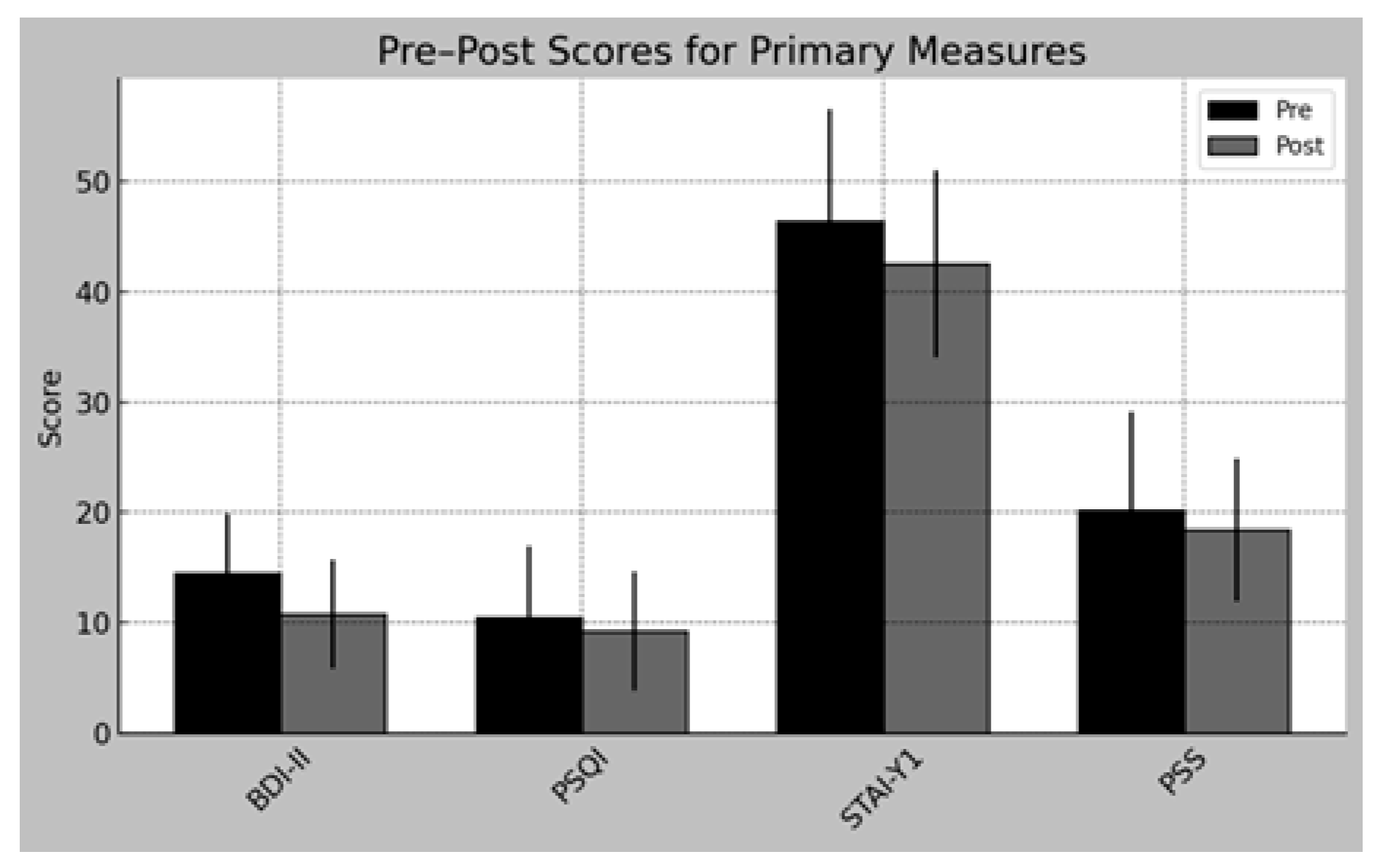
3.3. Pre–Post Comparisons
3.4. Change in Clinical Classifications
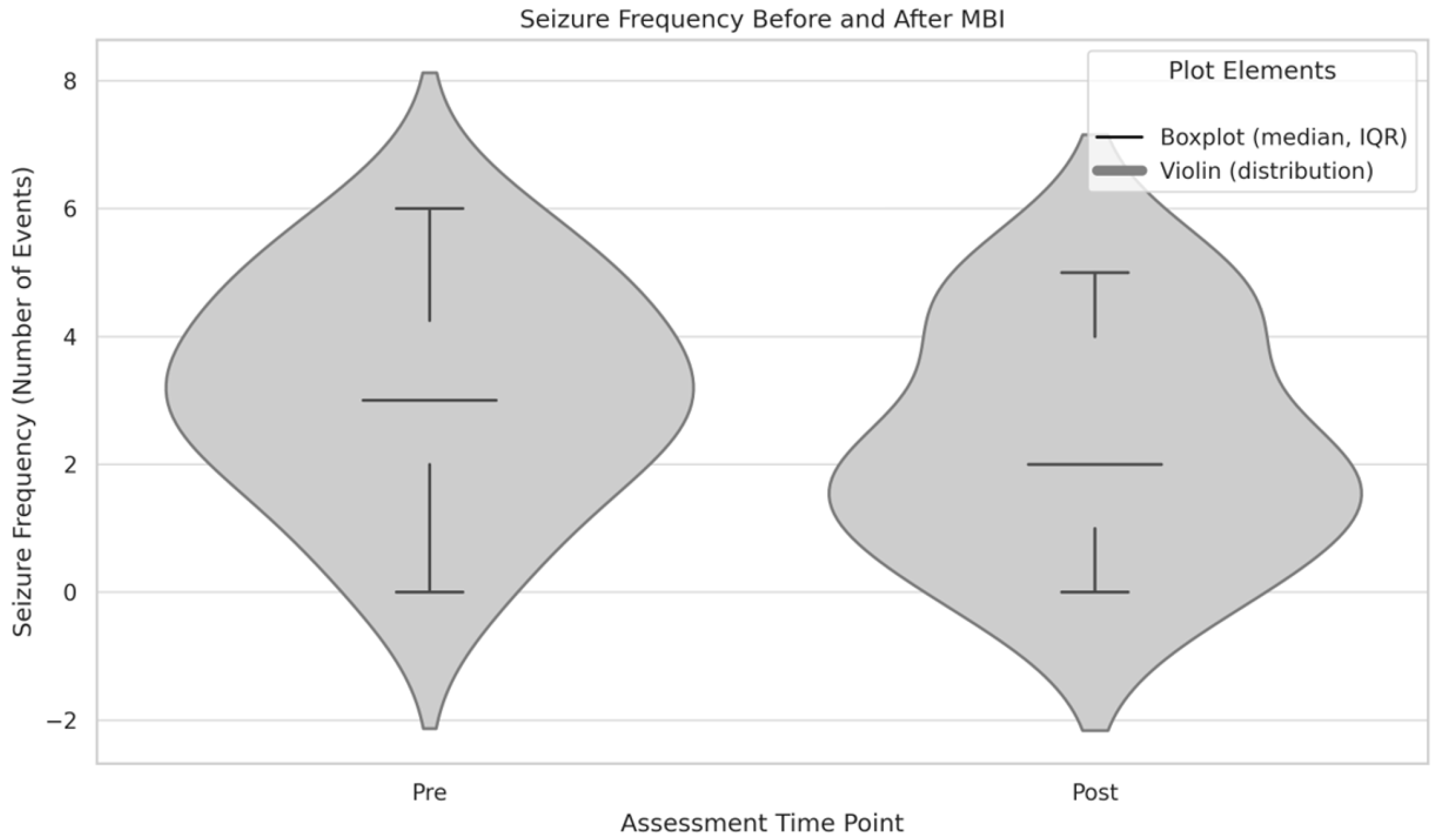
3.5. Seizure Frequency (Exploratory Analysis)
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNES | Psychogenic Non-Epileptic Seizures |
| ICM | Integrative Cognitive Model |
| MBSR | Mindfulness-Based Stress Reduction |
| MBI | Mindfulness-Based Intervention |
| BDI-II | Beck Depression Inventory-II |
| PSQI | Pittsburgh Sleep Quality Index |
| PSS-10 | Perceived Stress Scale—10 items |
| STAI-Y | State-Trait Anxiety Inventory—Form Y |
| FFMQ | Five-Facet Mindfulness Questionnaire |
| MAIA | Multidimensional Assessment of Interoceptive Awareness |
| DES-II | Dissociative Experiences Scale—II |
| METEO-Q | Meteoropathy Questionnaire |
| AOUP | Azienda Ospedaliero-Universitaria Pisana |
Appendix A
- Seizure History
- At what age did your seizures begin?
- Can you describe your very first seizure?
- How often do your seizures occur (per week/month)?
- How long does a typical seizure last?
- Do you experience any warning signs before the seizure?
- Are the episodes stereotyped (i.e., do they always present in the same way)?
- During episodes, what symptoms occur (e.g., motor activity, loss of consciousness, sensory changes, loss of urine, tongue biting/morsus)?
- Do you have the feeling that you can control your seizure in some way?
- What usually happens during the seizure (movements, sensations, loss of consciousness)?
- What do bystanders or family members observe during your seizures?
- What happens after the seizure (confusion, fatigue, rapid recovery)?
- Have you ever injured yourself during a seizure?
- Context and Triggers
- In what situations do seizures mostly occur (alone, in public, at home)?
- Are your seizures more frequent during particular times (e.g., day/night, stressful periods)?
- Do you notice any relation with your sleep, medication use, or physical activity?
- Medical and Neurological History
- Do you have a family history of epilepsy?
- Did you have previous neuroimaging (CT, MRI) performed to exclude organic causes?
- Have you been diagnosed with epilepsy or any other neurological condition in the past?
- Have you undergone EEG or video-EEG monitoring? If yes, what were the results?
- What treatments have you received for your seizures (medications, psychotherapy, hospitalization)?
- Any significant head injuries, neurological illnesses, or known medical conditions?
- Psychological and Psychiatric History
- Have you ever experienced psychological trauma (abuse, accidents, losses)?
- Have you ever been diagnosed with a mental health condition (anxiety, depression, PTSD)?
- How do you usually cope with stress?
- Do you have a history of self-harm or suicidal thoughts?
- Personal and Social History
- Tell me about your current living situation and social support.
- How has your education and work life been affected by the seizures?
- How do your seizures impact your daily functioning and quality of life?
- How do people around you (family, colleagues) react to your seizures?
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Huff, J.S.; Lui, F.; Murr, N.I. Psychogenic nonepileptic seizures. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Reuber, M.; Brown, R.J. Understanding psychogenic nonepileptic seizures—Phenomenology, semiology and the Integrative Cognitive Model. Seizure 2017, 44, 199–205. [Google Scholar] [CrossRef]
- Rosenbaum, M. Psychogenic seizures. Psychosomatics 2000, 41, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Beimer, N.J.; LaFrance, W.C. Evaluation and treatment of psychogenic nonepileptic seizures. Neurol. Clin. 2022, 40, 799–820. [Google Scholar] [CrossRef]
- Lanzillotti, A.I.; Sarudiansky, M.; Lombardi, N.R.; Korman, G.P.; D´Alessio, L. Updated review on the diagnosis and primary management of psychogenic nonepileptic seizure disorders. Neuropsychiatr. Dis. Treat. 2021, 17, 1825–1838. [Google Scholar] [CrossRef]
- van der Kruijs, S.J.M.; Bodde, N.M.G.; Vaessen, M.J.; Lazeron, R.H.C.; Vonck, K.; Boon, P.; Hofman, P.A.M.; Backes, W.H.; Aldenkamp, A.P.; Jansen, J.F.A. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J. Neurol. Neurosurg. Psychiatry 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Kerr, W.T.; Tatekawa, H.; Lee, J.K.; Karimi, A.H.; Sreenivasan, S.S.; O’Neill, J.; Smith, J.M.; Hickman, L.B.; Savic, I.; Nasrullah, N.; et al. Clinical MRI morphological analysis of functional seizures compared to seizure-naïve and psychiatric controls. Epilepsy Behav. 2022, 134, 108858. [Google Scholar] [CrossRef]
- Demartini, B.; Volpe, R.; Mattavelli, G.; Goeta, D.; D’Agostino, A.; Gambini, O. The neuromodulatory effect of tDCS in patients affected by functional motor symptoms: An exploratory study. Neurol. Sci. 2019, 40, 1821–1827. [Google Scholar] [CrossRef]
- O’Brien, F.M.; Fortune, G.M.; Dicker, P.; O’Hanlon, E.; Cassidy, E.; Delanty, N.; Garavan, H.; Murphy, K.C. Psychiatric and neuropsychological profiles of people with psychogenic nonepileptic seizures. Epilepsy Behav. 2015, 43, 39–45. [Google Scholar] [CrossRef]
- Nahab, F.B.; Kundu, P.; Maurer, C.; Shen, Q.; Hallett, M. Impaired sense of agency in functional movement disorders: An fMRI study. PLoS ONE 2017, 12, e0172502. [Google Scholar] [CrossRef] [PubMed]
- Baslet, G. Psychogenic non-epileptic seizures: A model of their pathogenic mechanism. Seizure 2011, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.S.; Coons, P.M. The differential diagnosis of epilepsy, pseudoseizures, dissociative identity disorder, and dissociative disorder not otherwise specified. Bull. Menn. Clin. 2000, 64, 164. [Google Scholar]
- Cobb, S.J.; Vaughn, B.V.; Sagherian, K. Nonpharmacologic interventions and seizure frequency in patients with psychogenic nonepileptic seizures: An integrative review. J. Am. Psychiatr. Nurses Assoc. 2023, 29, 290–306. [Google Scholar] [CrossRef]
- Roemer, L.; Williston, S.K.; Rollins, L.G. Mindfulness and emotion regulation. Curr. Opin. Psychol. 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Jha, A.P.; Krompinger, J.; Baime, M.J. Mindfulness training modifies subsystems of attention. Cogn. Affect. Behav. Neurosci. 2007, 7, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.D.; Lindsay, E.K. How does mindfulness training affect health? A mindfulness stress buffering account. Curr. Dir. Psychol. Sci. 2014, 23, 401–407. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Mindfulness-based stress reduction (MBSR). Constr. Hum. Sci. 2003, 8, 73. [Google Scholar]
- Kabat-Zinn, J. Some reflections on the origins of MBSR, skillful means, and the trouble with maps. In Mindfulness; Routledge: London, UK, 2013; pp. 281–306. [Google Scholar] [CrossRef]
- Baslet, G.; Ridlon, R.; Raynor, G.; Gonsalvez, I.; Dworetzky, B.A. Sustained improvement with mindfulness-based therapy for psychogenic nonepileptic seizures. Epilepsy Behav. 2022, 126, 108478. [Google Scholar] [CrossRef]
- Baslet, G.; Ehlert, A.; Oser, M.; Dworetzky, B.A. Mindfulness-based therapy for psychogenic nonepileptic seizures. Epilepsy Behav. 2020, 103 Pt A, 106534. [Google Scholar] [CrossRef]
- Baslet, G.; Dworetzky, B.; Perez, D.L.; Oser, M. Treatment of Psychogenic Nonepileptic Seizures: Updated Review and Findings From a Mindfulness-Based Intervention Case Series. Clin. EEG Neurosci. 2015, 46, 54–64. [Google Scholar] [CrossRef]
- Cummins, D.D.; Schulman, Z.; Maher, C.; Tortolero, L.; Saad, A.; Martinez, L.N.; Davidson, R.J.; Marcuse, L.V.; Saez, I.; Panov, F. Influence of mindfulness meditation on intracranial EEG parameters in epileptic and non-epileptic brain areas. Epilepsy Behav. 2024, 161, 110150. [Google Scholar] [CrossRef]
- Khoury, B.; Lecomte, T.; Fortin, G.; Masse, M.; Therien, P.; Bouchard, V.; Chapleau, M.-A.; Paquin, K.; Hofmann, S.G. Mindfulness-based therapy: A comprehensive meta-analysis. Clin. Psychol. Rev. 2013, 33, 763–771. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Mantzios, M.; Giannou, K. Group vs. single mindfulness meditation: Exploring avoidance, impulsivity, and weight management in two separate mindfulness meditation settings. Appl. Psychol. Health Well-Being 2014, 6, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Forms. Microsoft Corporation. Available online: https://forms.office.com (accessed on 26 June 2024).
- Baer, R.A.; Smith, G.T.; Hopkins, J.; Krietemeyer, J.; Toney, L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006, 13, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Giromini, L.; Bonalume, L.; Tagini, A.; Lang, M.; Amadei, G. The Italian five facet mindfulness questionnaire: A contribution to its validity and reliability. J. Psychopathol. Behav. Assess. 2014, 36, 415–423. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Mondo, M.; Sechi, C.; Cabras, C. Psychometric evaluation of three versions of the Italian Perceived Stress Scale. Curr. Psychol. 2021, 40, 1884–1892. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck depression inventory–II. Psychol. Assess. 1996. [Google Scholar] [CrossRef]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; NOVA Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory (STAI); Consulting Psychologists Press: Palo Alto, CA, USA, 1982. [Google Scholar]
- Pedrabissi, L.; Santinello, M. Verifica Della Validità Dello STAI Forma Y di Spielberger; Giunti Organizzazioni Speciali: Florence, Italy, 1989. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh sleep quality index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Carlson, E.B.; Putnam, F.W. The dissociative experiences scale (DES-II). Psychoanal. Inq. 2000, 20, 361–366. [Google Scholar]
- Saggino, A.; Molinengo, G.; Rogier, G.; Garofalo, C.; Loera, B.; Tommasi, M.; Velotti, P. Improving the psychometric properties of the dissociative experiences scale (DES-II): A Rasch validation study. BMC Psychiatry 2020, 20, 8. [Google Scholar] [CrossRef]
- Mehling, W.E.; Price, C.; Daubenmier, J.J.; Acree, M.; Bartmess, E.; Stewart, A. The multidimensional assessment of interoceptive awareness (MAIA). PLoS ONE 2012, 7, e48230. [Google Scholar] [CrossRef] [PubMed]
- Calì, G.; Ambrosini, E.; Picconi, L.; Mehling, W.E.; Committeri, G. Investigating the relationship between interoceptive accuracy, interoceptive awareness, and emotional susceptibility. Front. Psychol. 2015, 6, 1202. [Google Scholar] [CrossRef]
- Mazza, M.; Di Nicola, M.; Catalano, V.; Callea, A.; Martinotti, G.; Harnic, D.; Bruschi, A.; Battaglia, C.; Janiri, L. Description and validation of a questionnaire for the detection of meteoropathy and meteorosensitivity: The METEO-Q. Compr. Psychiatry 2012, 53, 103–106. [Google Scholar] [CrossRef]
- JASP, Version 0.95; JASP Team: Amsterdam, The Netherlands, 2025. Available online: https://jasp-stats.org/how-to-use-jasp (accessed on 26 June 2024).
- Martino, I.; Bruni, A.; Labate, A.; Vasta, R.; Cerasa, A.; Borzì, G.; De Fazio, P.; Gambardella, A. Psychopathological constellation in patients with PNES: A new hypothesis. Epilepsy Behav. 2018, 78, 297–301. [Google Scholar] [CrossRef]
- Araújo Filho, G.M.D.; Caboclo, L.O.S.F. Anxiety and mood disorders in psychogenic nonepileptic seizures. J. Epilepsy Clin. Neurophysiol. 2007, 13, 28–31. [Google Scholar] [CrossRef]
- Walsh, S.; Levita, L.; Reuber, M. Comorbid depression and associated factors in PNES versus epilepsy: Systematic review and meta-analysis. Seizure 2018, 60, 44–56. [Google Scholar] [CrossRef]
- Vanek, J.; Prasko, J.; Ociskova, M.; Genzor, S.; Holubova, M.; Hodny, F.; Nesnidal, V.; Slepecky, M.; Sova, M.; Minarikova, K. Sleep disturbances in patients with nonepileptic seizures. Nat. Sci. Sleep 2021, 13, 209–218. [Google Scholar] [CrossRef]
- Latreille, V.; Lambert, I.; Dauvilliers, Y.; Montplaisir, J. Sleep in psychogenic nonepileptic seizures: A review. Sleep Med. Rev. 2018, 37, 139–145. [Google Scholar] [CrossRef]
- Soler, J.; Valdepérez, A.; Feliu-Soler, A.; Pascual, J.C.; Portella, M.J.; Martín-Blanco, A.; Alvarez, E.; Pérez, V. Effects of the dialectical behavioral therapy-mindfulness module on attention in patients with borderline personality disorder. Behav. Res. Ther. 2012, 50, 150–157. [Google Scholar] [CrossRef]
- Bornemann, B.; Singer, T. Taking time to feel our body: Steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology 2017, 54, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Meer, S. Investigating Self-Perception of Emotion in Individuals with Non-Epileptic Seizures (NES). Ph.D. Thesis, University of Leeds, Leeds, UK, 2023. [Google Scholar]
- Millman, L.M.; Short, E.; Stanton, B.; Winston, J.S.; Nicholson, T.R.; Mehta, M.A.; Reinders, A.A.; Edwards, M.J.; Goldstein, L.H.; David, A.S.; et al. Interoception in functional motor symptoms and functional seizures: Preliminary evidence of intact accuracy alongside reduced insight and altered sensibility. Behav. Res. Ther. 2023, 168, 104379. [Google Scholar] [CrossRef]
- Edwards, E.; Shivaji, S.; Wupperman, P. The Emotion Mapping Activity: Preliminary evaluation of a mindfulness-informed exercise to improve emotion labeling in alexithymic persons. Scand. J. Psychol. 2018, 59, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.E.; Singer, T. Effects of contemplative dyads on engagement and perceived social connectedness over 9 months of mental training: A randomized clinical trial. JAMA Psychiatry 2017, 74, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Hooven, C. Interoceptive awareness skills for emotion regulation: Theory and approach of mindful awareness in body-oriented therapy (MABT). Front. Psychol. 2018, 9, 798. [Google Scholar] [CrossRef]
- Myers, L.; Trobliger, R.; Bortnik, K.; Zeng, R.; Saal, E.; Lancman, M. Psychological trauma, somatization, dissociation, and psychiatric comorbidities in patients with psychogenic nonepileptic seizures compared with those in patients with intractable partial epilepsy. Epilepsy Behav. 2019, 92, 108–113. [Google Scholar] [CrossRef]
- Roberts, N.A.; Reuber, M. Alterations of consciousness in psychogenic nonepileptic seizures: Emotion, emotion regulation and dissociation. Epilepsy Behav. 2014, 30, 43–49. [Google Scholar] [CrossRef]
| PNES Characteristics | Potential Target of Mindfulness Practice |
|---|---|
| Experiential avoidance | Non-judgmental acceptance and present-moment grounding |
| Altered sense of agency | Enhanced mind–body connection and interoceptive insight |
| Somatization | Increased emotional awareness and emotional regulation |
| Alexithymia | Improved recognition and labeling of emotions (observing) |
| Variable | Value |
|---|---|
| Gender | 8 Males, 4 Females |
| Age (mean ± SD) | 54 ± 6.6 years |
| Age range | 42–63 years |
| Domain (Measure) | Pre (M) | Post (M) | Dir. | Z | p | Effect Size (r) | 95% CI for r | Significance |
|---|---|---|---|---|---|---|---|---|
| Depression (BDI-II) | 14.50 ± 5.40 | 10.83 ± 4.93 | ↓ | 17 | 0.092 | 0.564 | [−0.002, 0.856] | Trend |
| Sleep quality (PSQI) | 10.50 ± 6.49 | 9.25 ± 5.41 | ↓ | 13.5 | 0.078 | 0.591 | [0.011, 0.873] | Trend |
| Perceived Stress (PSS-10) | 20.17 ± 8.96 | 18.42 ± 6.43 | ↓ | 23 | 0.373 | 0.282 | [−0.337, 0.731] | No |
| Perceived Anxiety (STAI Y 1) | 46.33 ± 10.17 | 42.50 ± NA | ↓ | 28 | 0.424 | 0.303 | [−0.341, 0.753] | No |
| Mindfulness (FFMQ total) | 124.33 ± 19.15 | 117.67 ± 25.21 | ↓ | 18 | 0.11 | 0.397 | [−0.217, 0.786] | No |
| Non Reactivity (FFMQ) | 21.33 ± 5.80 | 19.75 ± 5.97 | ↓ | 23.5 | 0.266 | −0.236 | [−0.735, 0.429] | No |
| Non Judging (FFMQ) | 27.67 ± 6.37 | 29.08 ± 6.91 | ↑ | 21 | 0.506 | 0.115 | [−0.481, 0.639] | No |
| Observing (FFMQ) | 20.42 ± 5.92 | 19.83 ± 5.87 | ↓ | 34.5 | 0.791 | 0.697 | [0.191, 0.910] | No |
| Describing (FFMQ) | 26.92 ± 7.20 | 23.67 ± 7.74 | ↓ | 10 | 0.04 | 0.564 | [−0.002, 0.856] | Yes |
| Acting with Awareness (FFMQ) | 27.75 ± 6.02 | 25.33 ± 6.11 | ↓ | 17 | 0.092 | 0.538 | [−0.039, 0.846] | Trend |
| Interoception (MAIA total) | 2.61 ± 1.94 | 3.14 ± 1.45 | ↑ | 9 | 0.109 | −0.036 | [−0.626, 0.580] | No |
| Noticing (MAIA) | 2.54 ± 1.33 | 2.60 ± 1.17 | ↑ | 26.5 | 0.919 | −0.127 | [−0.679, 0.516] | No |
| Not Distracting (MAIA) | 2.11 ± 1.04 | 2.44 ± 0.97 | ↑ | 24 | 0.719 | −0.038 | [−0.591, 0.539] | No |
| Not Worrying (MAIA) | 2.91 ± 0.94 | 2.92 ± 0.75 | ↑ | 37.5 | 0.91 | −0.273 | [−0.739, 0.370] | No |
| Attention Regulation (MAIA) | 2.10 ± 1.36 | 2.26 ± 1.18 | ↑ | 24 | 0.423 | 0.333 | [−0.311, 0.768] | No |
| Emotional Awareness (MAIA) | 3.80 ± 0.93 | 3.33 ± 1.00 | ↓ | 22 | 0.328 | −0.530 | [−0.851, 0.077] | No |
| Self-Regulation (MAIA) | 2.12 ± 1.33 | 2.88 ± 1.13 | ↑ | 15.5 | 0.119 | −0.167 | [−0.669, 0.440] | No |
| Body Listening (MAIA) | 1.87 ± 1.57 | 2.39 ± 1.33 | ↑ | 32.5 | 0.622 | −0.600 | [−0.891, 0.042] | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciacchini, R.; Conversano, C.; Orrù, G.; Pizzanelli, C.; Scarpitta, C.; Turco, F.; Bonanni, E.; Bressan, A.; Reali, T.; Gemignani, A. Preliminary Findings from a Mindfulness-Based Intervention in Patients with Psychogenic Non-Epileptic Seizures. Neurol. Int. 2025, 17, 171. https://doi.org/10.3390/neurolint17100171
Ciacchini R, Conversano C, Orrù G, Pizzanelli C, Scarpitta C, Turco F, Bonanni E, Bressan A, Reali T, Gemignani A. Preliminary Findings from a Mindfulness-Based Intervention in Patients with Psychogenic Non-Epileptic Seizures. Neurology International. 2025; 17(10):171. https://doi.org/10.3390/neurolint17100171
Chicago/Turabian StyleCiacchini, Rebecca, Ciro Conversano, Graziella Orrù, Chiara Pizzanelli, Claudia Scarpitta, Francesco Turco, Enrica Bonanni, Annachiara Bressan, Thomas Reali, and Angelo Gemignani. 2025. "Preliminary Findings from a Mindfulness-Based Intervention in Patients with Psychogenic Non-Epileptic Seizures" Neurology International 17, no. 10: 171. https://doi.org/10.3390/neurolint17100171
APA StyleCiacchini, R., Conversano, C., Orrù, G., Pizzanelli, C., Scarpitta, C., Turco, F., Bonanni, E., Bressan, A., Reali, T., & Gemignani, A. (2025). Preliminary Findings from a Mindfulness-Based Intervention in Patients with Psychogenic Non-Epileptic Seizures. Neurology International, 17(10), 171. https://doi.org/10.3390/neurolint17100171