Pulmonary Embolism After Acute Ischaemic Stroke (PEARL-AIS): Global Prevalence, Risk Factors, Outcomes, and Evidence Grading from a Meta-Analysis
Abstract
1. Background
2. Methodology
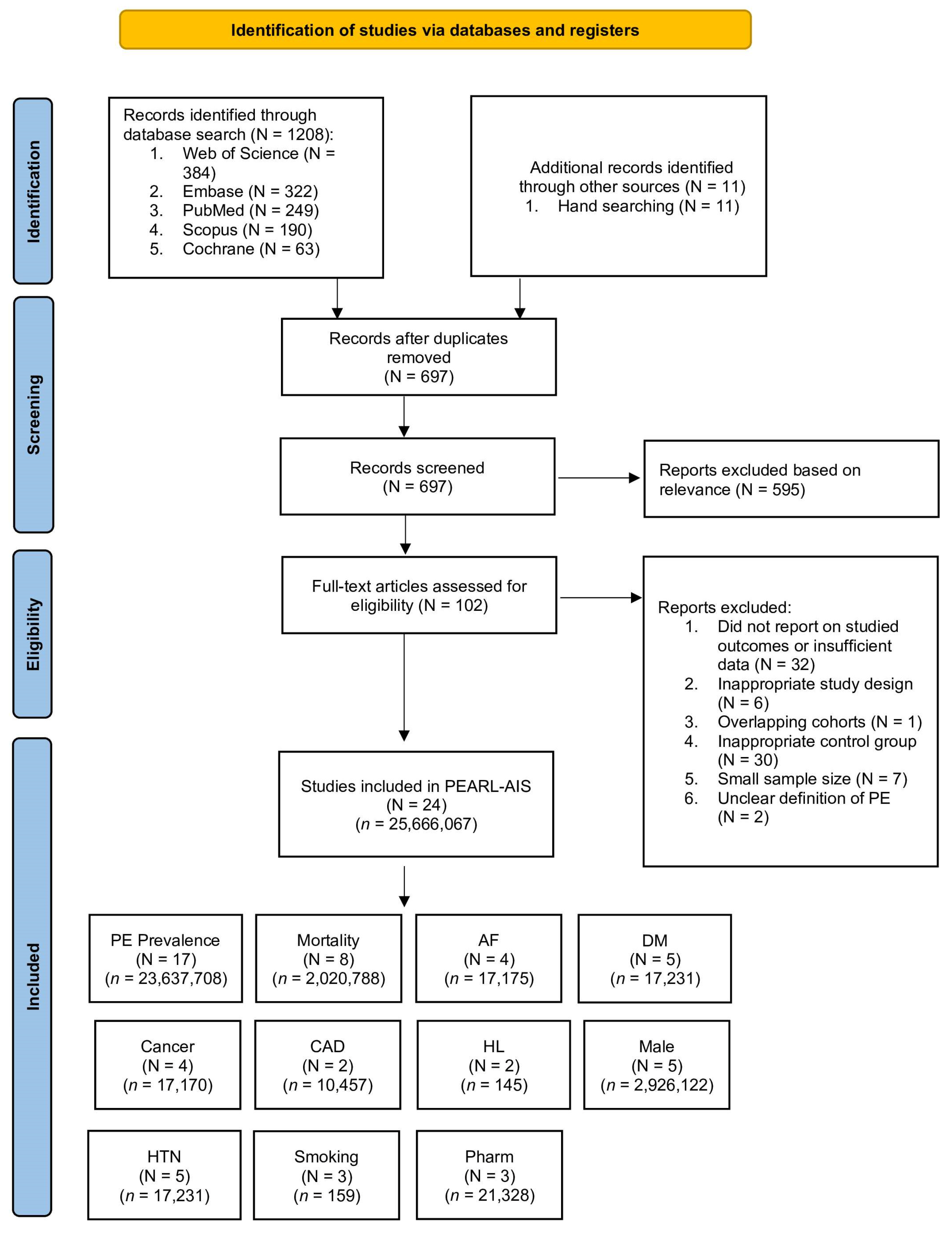
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Ethics Statement
2.5. Methodological Quality Assessment of Included Studies
2.6. Statistical Analysis
| Author (Year) | Country | Study Design | Mean Age (Years, SD) | PE (n) | PE (%) | Male (%) | Mortality (n, %) |
|---|---|---|---|---|---|---|---|
| Abdelsalam et al. (2020) [32] | Egypt | Prospective | – | 9 | 5.69 | – | – |
| Ahmed et al. (2023) [33] | USA | Retrospective | – | 26,758 | 0.50 | – | – |
| Ali et al. (2009) [34] | Multiple | Retrospective | 74.7 (9.7) | 22 | 4.14 | 53 | – |
| Allendorfer et al. (2007) [35] | Germany | Prospective | 54.3 (19.2) | – | – | – | – |
| Amin et al. (2013) [36] | USA | Retrospective | 62.2 (12.2) | 5 | 0.33 | – | – |
| CAST Collaboration Group (1997) [37] | China | Prospective | – | 32 | 0.15 | – | 15 (PE), 726 (no PE) |
| Che et al. (2024) [38] | China | Prospective | – | 4 | 1.31 | – | – |
| Chen et al. (2012) [39] | Taiwan | Retrospective | 70.1 (16.8) | – | – | – | 5 (PE), 9 (no PE) |
| Dennis et al. (2011) [40] | Multiple | Prospective | 75.3 (11.9) | 75 | 1.33 | 49 | – |
| Eswaradass et al. (2018) [41] | Canada | Retrospective | – | 10 | 0.32 | – | – |
| Huang et al. (2021) [42] | China | Retrospective | – | 1743 | 0.21 | – | 55 (PE), 1688 (no PE) |
| IST Collaborative Group (1997) [43] | – | Prospective | – | – | – | – | – |
| Keller et al. (2024) [44] | Germany | Retrospective | – | – | – | 46 | 1938 (PE), 4766 (no PE) |
| Keller et al. (2020) [45] | Germany | Retrospective | 75.3 (11.9) | 10,368 | 0.36 | – | 2943 (PE), 7425 (no PE) |
| Kelly et al. (2004) [46] | USA | Prospective | 70.1 (11.9) | 12 | 11.54 | 46 | – |
| Pongmoragot et al. (2013) [47] | Canada | Retrospective | – | 89 | 0.79 | 52 | 28 (PE), 61 (no PE) |
| Sherman et al. (2007) [48] | Multiple | Prospective | – | 7 | 0.52 | – | – |
| Skaf et al. (2005) [49] | USA | Retrospective | – | 72,000 | 0.51 | – | – |
| Skaf et al. (2006) [50] | USA | Retrospective | – | – | – | – | 11,101 (PE), 1,989,862 (no PE) |
| Sluis et al. (2021) [51] | Multiple | Retrospective/Prospective | 68.7 (13.4) | 8 | 21.05 | 64 | – |
| Sprigg et al. (2005) [52] | UK | Prospective | 73.3 (10.4) | 20 | 1.35 | 54 | – |
| Tanislav et al. (2011) [53] | Germany | Prospective | 55.2 | – | – | 57 | – |
| TOAST Investigators (1998) [54] | USA | Prospective | 65.5 (11.4) | 6 | 0.47 | – | – |
| Turpie et al. (2013) [55] | Multiple | Prospective | – | – | – | 56 | 10 (PE), 156 (no PE) |
2.7. Evidence Grading
3. Results
3.1. Prevalence of Pulmonary Embolism
3.2. Mortality in AIS Patients with Pulmonary Embolism
3.3. Predictive Indicators of Pulmonary Embolism
3.4. Clinical Outcomes Following Pulmonary Embolism
3.5. Prevalence of Risk Factors in AIS Patients with PE
3.6. Pharmacological Prophylaxis
3.7. Evidence Grading Assessment Findings
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Kelly, J.; Rudd, A.; Lewis, R.; Hunt, B.J. Venous thromboembolism after acute stroke. Stroke 2001, 32, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrom, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef]
- Chen, D.; Bhaskar, S.M.M. Pulmonary Embolism in Acute Ischaemic Stroke: Evolving Evidence, Diagnostic Challenges, and a Novel Thromboinflammatory Axis Hypothesis. Int. J. Mol. Sci. 2025, 26, 6733. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L.; Roth, E.J.; Yarnold, P.R.; Durham, J.R.; Green, D. Deep vein thrombosis in stroke. The use of plasma D-dimer level as a screening test in the rehabilitation setting. Stroke 1996, 27, 1516–1520. [Google Scholar] [CrossRef]
- Dennis, M.; Caso, V.; Kappelle, L.J.; Pavlovic, A.; Sandercock, P. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur. Stroke J. 2016, 1, 6–19. [Google Scholar] [CrossRef]
- Haybar, H.; Bandar, B.; Torfi, E.; Mohebbi, A.; Saki, N. Cytokines and Their Role in Cardiovascular Diseases. Cytokine. 2023, 169, 156261. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Schulze, J.; Vogelgesang, A.; Dressel, A. Catecholamines, steroids and immune alterations in ischemic stroke and other acute diseases. Aging Dis. 2014, 5, 327–339. [Google Scholar] [CrossRef]
- Mallard, C.; Ferriero, D.M.; Vexler, Z.S. Immune-Neurovascular Interactions in Experimental Perinatal and Childhood Arterial Ischemic Stroke. Stroke 2024, 55, 506–518. [Google Scholar] [CrossRef]
- Bhaskar, S.; Stanwell, P.; Cordato, D.; Attia, J.; Levi, C. Reperfusion therapy in acute ischemic stroke: Dawn of a new era? BMC Neurol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Han, L.; Yang, J.M.; Qian, W.Y.; Xu, X.P.; Tung, T.H.; Liu, Y.; Wang, F. Risk factors for lower extremity deep vein thrombosis in acute stroke patients following endovascular thrombectomy: A retrospective cohort study. Front. Neurol. 2023, 14, 1249365. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.D.; Guo, Z.N.; Jin, H.; Sun, T.; Ni, C.P.; Yan, X.L. Incidence and Risk Factors of Lower-Extremity Deep Vein Thrombosis After Thrombolysis Among Patients with Acute Ischemic Stroke. Pharmacogenom. Pers. Med. 2021, 14, 1107–1114. [Google Scholar]
- Bajenaru, O.; Antochi, F.; Balasa, R.; Buraga, I.; Patrichi, S.; Simu, M.; Szabolcs, S.; Tiu, C.; Zaharia, C. Assessment of Venous Thromboembolism Prophylaxis in Neurological Patients with Restricted Mobility—VTE-NEURO Study. Maedica 2014, 9, 6–14. [Google Scholar] [PubMed]
- Kurtoglu, M.; Guloglu, R.; Ertekin, C.; Taviloglu, K.; Alimoglu, O. Intermittent pneumatic compression in the prevention of venous thromboembolism in high-risk trauma and surgical ICU patients. Ulus Travma Acil Cerrahi Derg. 2005, 11, 38–42. [Google Scholar]
- Dennis, M.; Sandercock, P.; Reid, J.; Graham, C.; Forbes, J.; Murray, G. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): A multicentre randomised controlled trial. Lancet 2013, 382, 516–524. [Google Scholar] [CrossRef]
- Nyquist, P.; Bautista, C.; Jichici, D.; Burns, J.; Chhangani, S.; DeFilippis, M.; Goldenberg, F.D.; Kim, K.; Liu-DeRyke, X.; Mack, W.; et al. Prophylaxis of Venous Thrombosis in Neurocritical Care Patients: An Evidence-Based Guideline: A Statement for Healthcare Professionals from the Neurocritical Care Society. Neurocrit. Care 2016, 24, 47–60. [Google Scholar] [CrossRef]
- Menounos, S.; Shen, H.; Bhaskar, S.M.M. Integrated Management of Stroke Risk in Brain Cancer: Insights from the Tumoral Bleeding Classification System and CanStroke Protocol. J. Stroke Med. 2025, 25166085251335378. [Google Scholar] [CrossRef]
- Esmon, C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003, 1, 1343–1348. [Google Scholar] [CrossRef]
- Stanimirovic, D.; Satoh, K. Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation. Brain Pathol. 2000, 10, 113–126. [Google Scholar] [CrossRef]
- Simats, A.; Liesz, A. Systemic inflammation after stroke: Implications for post-stroke comorbidities. EMBO Mol. Med. 2022, 14, e16269. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Bhaskar, S. Unifying Vascular Injury and Neurodegeneration: A Mechanistic Continuum in Cerebral Small Vessel Disease and Dementia. Eur. J. Neurosci. 2025, 62, e70246. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132, 1–17. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Won, T.; Wood, M.K.; Hughes, D.M.; Talor, M.V.; Ma, Z.; Schneider, J.; Skinner, J.T.; Asady, B.; Goerlich, E.; Halushka, M.K.; et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine 2022, 75, 103812. [Google Scholar] [CrossRef]
- Colling, M.E.; Tourdot, B.E.; Kanthi, Y. Inflammation, Infection and Venous Thromboembolism. Circ. Res. 2021, 128, 2017–2036. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Douglas, D.A.A.; Birks, J.; Borenstein, M.; Campbell, M.; Deeks, J.; Egger, M.; Higgins, J.P.T.; Lau, J.; O’Rourke, K.; Rücker, G. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- Abdelsalam, M.; Abu-Hegazy, M.; El-Hadaad, H.A.; Wahba, H.; Egila, H.; Esmael, A. Pathophysiology, Mechanism, and Outcome of Ischemic Stroke in Cancer Patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 105299. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Mhina, C.; Philip, K.; Patel, S.D.; Aneni, E.; Osondu, C.; Lamikanra, O.; Akano, E.O.; Anikpezie, N.; Albright, K.C.; et al. Age- and Sex-Specific Trends in Medical Complications After Acute Ischemic Stroke in the United States. Neurology 2023, 100, e1282–e1295. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Sacco, R.L.; Lees, K.R.; Bath, P.M.W.; Bluhmki, E.; Claesson, L.; Curram, J.; Davis, S.M.; Diener, H.C.; Donnan, G.A.; et al. Primary end-point times, functional outcome and adverse event profile after acute ischaemic stroke. Int. J. Stroke 2009, 4, 432–442. [Google Scholar] [CrossRef]
- Allendorfer, J.; Tanislav, C.; Puille, M.; Grebe, M.; Stolz, E.; Jauss, M. Risk factors for pulmonary embolism in patients with stroke and patent foramen ovale. Cerebrovasc. Dis. 2007, 24, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.N.; Lin, J.; Thompson, S.; Wiederkehr, D. Rate of deep-vein thrombosis and pulmonary embolism during the care continuum in patients with acute ischemic stroke in the United States. BMC Neurol. 2013, 13, 17. [Google Scholar] [CrossRef]
- Group, C.C. CAST: Randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997, 349, 1641–1649. [Google Scholar]
- Che, F.; Wang, A.; Ju, Y.; Liu, L.; Ma, N.; Cheng, Z.; Duan, H.; Zhao, X.; Geng, X. Prevalence and Impact of Medical Complications on Clinical Outcomes in Acute Ischemic Stroke Patients After Endovascular Therapy—Data from a Comprehensive Stroke Unit in China. World Neurosurg. 2024, 182, e386–e399. [Google Scholar] [CrossRef]
- Chen, C.C.; Lee, T.H.; Chung, C.Y.; Chang, W.H.; Hong, J.P.; Huang, L.T.; Tang, S.F.; Chen, C.K. Symptomatic pulmonary embolism among stroke patients in Taiwan: A retrospective cohort study. Top. Stroke Rehabil. 2012, 19, 361–368. [Google Scholar] [CrossRef]
- Dennis, M.; Mordi, N.; Graham, C.; Sandercock, P. The timing, extent, progression and regression of deep vein thrombosis in immobile stroke patients: Observational data from the CLOTS multicenter randomized trials. J. Thromb. Haemost. 2011, 9, 2193–2200. [Google Scholar] [CrossRef]
- Eswaradass, P.V.; Dey, S.; Singh, D.; Hill, M.D. Pulmonary Embolism in Ischemic Stroke. Can. J. Neurol. Sci. 2018, 45, 343–345. [Google Scholar] [CrossRef]
- Huang, Z.X.; Gu, H.Q.; Yang, X.; Wang, C.J.; Wang, Y.J.; Li, Z.X. Risk factors for in-hospital mortality among acute ischemic stroke patients in China: A nationwide prospective study. Neurol. Res. 2021, 43, 387–395. [Google Scholar] [CrossRef]
- Ceravolo, M.G.; Polonara, S.; Provinciali, L.; Reginelli, R.; International Stroke Trial Collaborative Group. The International Stroke Trial (IST): A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997, 349, 1569–1581. [Google Scholar]
- Keller, K.; Schmitt, V.H.; Hahad, O.; Hobohm, L. Outcome of Pulmonary Embolism with and without Ischemic Stroke. J. Clin. Med. 2024, 13, 2730. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Munzel, T.; Lankeit, M.; Ostad, M.A. Impact of pulmonary embolism on in-hospital mortality of patients with ischemic stroke. J. Neurol. Sci. 2020, 419, 117174. [Google Scholar] [CrossRef]
- Kelly, J.; Rudd, A.; Lewis, R.R.; Coshall, C.; Moody, A.; Hunt, B.J. Venous thromboembolism after acute ischemic stroke: A prospective study using magnetic resonance direct thrombus imaging. Stroke 2004, 35, 2320–2325. [Google Scholar] [CrossRef]
- Pongmoragot, J.; Rabinstein, A.A.; Nilanont, Y.; Swartz, R.H.; Zhou, L.M.; Saposnik, G. Pulmonary Embolism in Ischemic Stroke: Clinical Presentation, Risk Factors, and Outcome. J. Am. Heart Assoc. 2013, 2, e000372. [Google Scholar] [CrossRef]
- Sherman, D.G.; Albers, G.W.; Bladin, C.; Fieschi, C.; Gabbai, A.A.; Kase, C.S.; O’Riordan, W.; Pineo, G.F. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): An open-label randomised comparison. Lancet 2007, 369, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Skaf, E.; Stein, P.D.; Beemath, A.; Sanchez, J.; Bustamante, M.A.; Olson, R.E. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am. J. Cardiol. 2005, 96, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Skaf, E.; Stein, P.D.; Beemath, A.; Sanchez, J.; Olson, R.E. Fatal pulmonary embolism and stroke. Am. J. Cardiol. 2006, 97, 1776–1777. [Google Scholar] [CrossRef]
- Sluis, W.M.; Linschoten, M.; Buijs, J.E.; Biesbroek, J.M.; den Hertog, H.M.; Ribbers, T.; Nieuwkamp, D.J.; van Houwelingen, R.C.; Dias, A.; van Uden, I.W.M.; et al. Risk, Clinical Course, and Outcome of Ischemic Stroke in Patients Hospitalized with COVID-19 A Multicenter Cohort Study. Stroke 2021, 52, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Sprigg, N.; Gray, L.J.; Bath, P.M.W.; Boysen, G.; De Deyn, P.P.; Leys, D.; Lindenstrom, E.; O’Neill, D.; Ringelstein, B.; Van Der Sande, J.J. Compression stockings and the prevention of symptomatic venous thromboembolism: Data from the Tinzaparin in Acute Ischemic Stroke Trial. J. Stroke Cerebrovasc. Dis. 2005, 14, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tanislav, C.; Puille, M.; Pabst, W.; Reichenberger, F.; Grebe, M.; Nedelmann, M.; Kaps, M.; Allendorfer, J. High frequency of silent pulmonary embolism in patients with cryptogenic stroke and patent foramen ovale. Stroke 2011, 42, 822–824. [Google Scholar] [CrossRef]
- Investigators, T. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: A randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA 1998, 279, 1265–1272. [Google Scholar]
- Turpie, A.G.; Hull, R.D.; Schellong, S.M.; Tapson, V.F.; Monreal, M.; Samama, M.M.; Chen, M.; Yusen, R.D. Venous thromboembolism risk in ischemic stroke patients receiving extended-duration enoxaparin prophylaxis: Results from the EXCLAIM study. Stroke 2013, 44, 249–251. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Valerio, L.; Gallo, A.; Turatti, G.; Mahmoudpour, S.H.; Ageno, W.; Castellucci, L.A.; Cesarman-Maus, G.; Ddungu, H.; De Paula, E.V.; et al. Global reporting of pulmonary embolism-related deaths in the World Health Organization mortality database: Vital registration data from 123 countries. Res. Pract. Thromb. Haemost. 2021, 5, e12520. [Google Scholar] [CrossRef] [PubMed]
- Laporte, S.; Mismetti, P.; Decousus, H.; Uresandi, F.; Otero, R.; Lobo, J.L.; Monreal, M. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: Findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation 2008, 117, 1711–1716. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Nie, J.; Zhou, L.; Tian, W.; Liu, X.; Yang, L.; Yang, X.; Zhang, Y.; Wei, S.; Wang, D.W.; Wei, J. Deep insight into cytokine storm: From pathogenesis to treatment. Signal Transduct. Target. Ther. 2025, 10, 112. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer cell-derived tissue factor-positive extracellular vesicles: Biomarkers of thrombosis and survival. Curr. Opin. Hematol. 2019, 26, 349–356. [Google Scholar] [CrossRef]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sinha, A.; Banach, M.; Mittoo, S.; Weissert, R.; Kass, J.S.; Rajagopal, S.; Pai, A.R.; Kutty, S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front. Immunol. 2020, 11, 1648. [Google Scholar] [CrossRef]
- Malik, A.; Ahmed, M.; Hamid, S.; Ahmed, E.; Ifzaal, M. Computed Tomography Pulmonary Angiography (CTPA) Utilization in Suspected Pulmonary Embolism Patients Based on Age-Adjusted D-dimer Thresholds and Pulmonary Embolism Rule-Out Criteria (PERC) Score: A Retrospective Analysis. Cureus 2025, 17, e79743. [Google Scholar] [CrossRef]
- Seeburun, S.; Valladares, C.; Iglesias, J. Strategies in Management of Pulmonary Embolism With Acute Ischemic Stroke: A Systematic Review. J. Clin. Med. Res. 2025, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Wijeratne, T.; Zelenak, K.; Huasen, B.B.; Iacobucci, M.; Killingsworth, M.C.; Beran, R.G.; Gebreyohanns, M.; Sekhar, A.; Khurana, D.; et al. Disparities in Access to Reperfusion Therapy for Acute Ischemic Stroke (DARTS): A Comprehensive Meta-Analysis of Ethnicity, Socioeconomic Status, and Geographical Factors. CNS Drugs 2025, 39, 417–442. [Google Scholar] [CrossRef]
- Menounos, S.; Shen, H.; Tipirneni, S.; Bhaskar, S.M.M. Decoding the Nexus: Cellular and Molecular Mechanisms Linking Stroke and Neurotoxic Microenvironments in Brain Cancer Patients. Biomolecules 2024, 14, 1507. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Bhaskar, S.M.M. Bridging the Gap in Cancer-Related Stroke Management: Update on Therapeutic and Preventive Approaches. Int. J. Mol. Sci. 2023, 24, 7981. [Google Scholar] [CrossRef]
- Davidson, B.L.; Schryver, N. Pulmonary embolism prophylaxis and treatment: What’s right, what’s wrong, and the future. Chin. Med. J. Pulm. Crit. Care Med. 2025, 3, 1–5. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, W.J.; Choi, S.K.; Kwun, B.D. Deep Vein Thrombosis and Pulmonary Embolism following Hemorrhagic Stroke. J. Neurointensive Care 2018, 1, 20–24. [Google Scholar] [CrossRef]
- Bhaskar, S.; Stanwell, P.; Bivard, A.; Spratt, N.; Walker, R.; Kitsos, G.H.; Parsons, M.W.; Evans, M.; Jordan, L.; Nilsson, M.; et al. The influence of initial stroke severity on mortality, overall functional outcome and in-hospital placement at 90 days following acute ischemic stroke: A tertiary hospital stroke register study. Neurol. India 2017, 65, 1252–1259. [Google Scholar] [CrossRef]
- Sharma, D.; Spring, K.J.; Bhaskar, S.M.M. Neutrophil-lymphocyte ratio in acute ischemic stroke: Immunopathology, management, and prognosis. Acta Neurol. Scand. 2021, 144, 486–499. [Google Scholar] [CrossRef] [PubMed]
- McFadden, P.M.; Ochsner, J.L. A history of the diagnosis and treatment of venous thrombosis and pulmonary embolism. Ochsner J. 2002, 4, 9–13. [Google Scholar] [PubMed]



| Subgroup/Risk Factor | Studies (Patients) N (n) | Crude Prevalence (%) | Pooled Prevalence (95% CI) | I2 (%) | z-Score | p-Value |
|---|---|---|---|---|---|---|
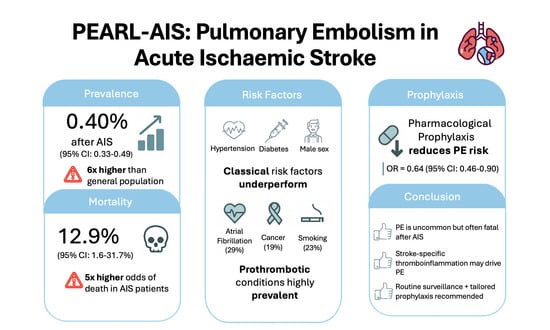
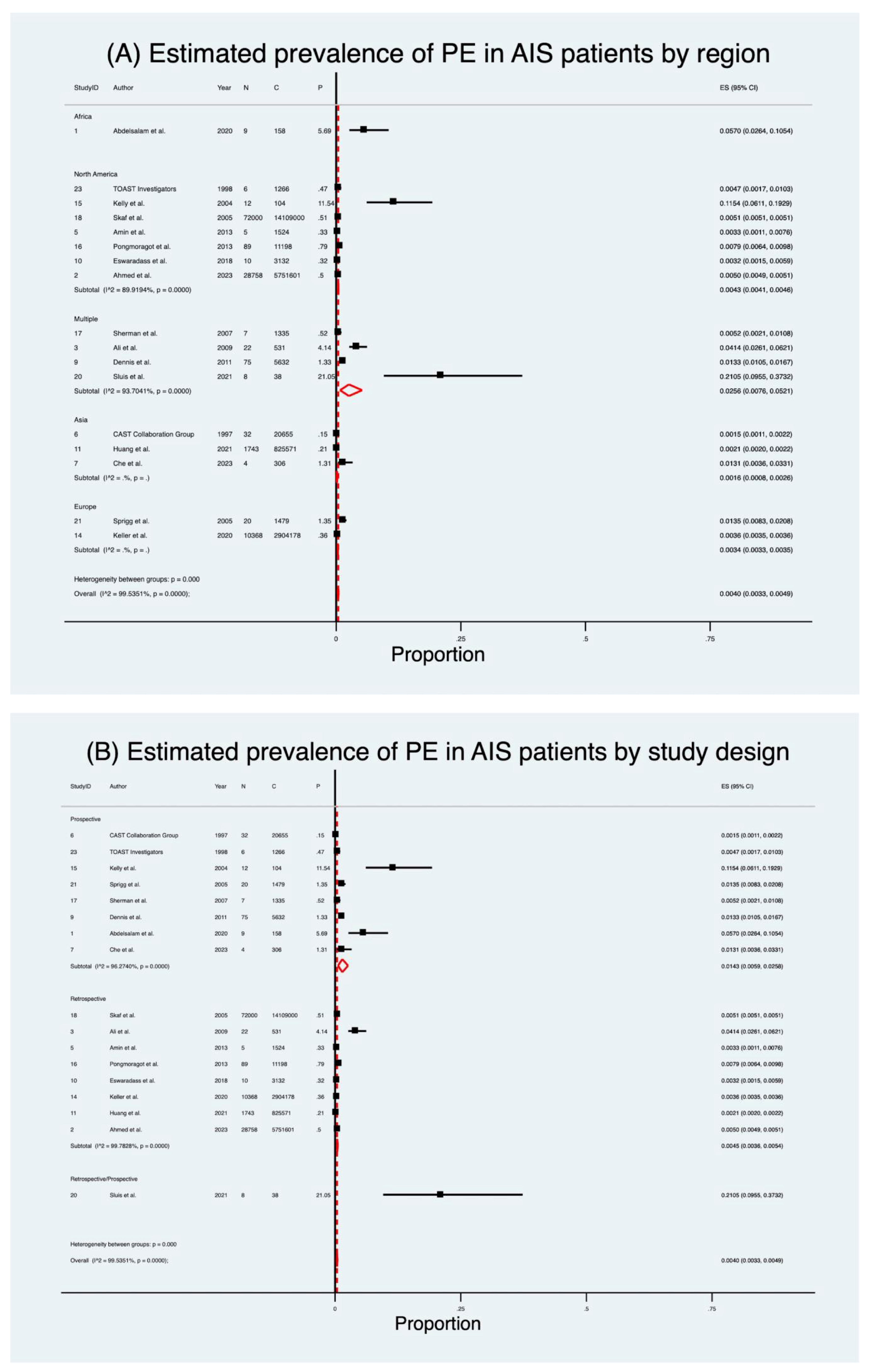
| Overall prevalence of PE | 17 (23,637,708) | 0.48 | 0.40 (0.33–0.49) | 99.5 | 16.34 | <0.001 |
| Retrospective | 8 (23,606,735) | 0.48 | 0.45 (0.36–0.54) | 99.8 | 17.79 | <0.001 |
| Prospective | 8 (30,935) | 0.53 | 1.43 (0.59–2.58) | 96.3 | 4.84 | <0.001 |
| Prospective + retrospective | 1 (38) | 21.00 | – | – | – | – |
| Asia | 3 (846,532) | 0.21 | 0.16 (0.08–0.26) | – | 5.59 | <0.001 |
| Europe | 2 (2,905,657) | 0.36 | 0.34 (0.33–0.35) | – | 172.58 | <0.001 |
| Africa | 1 (158) | 5.69 | – | – | – | – |
| North America | 7 (19,877,825) | 0.51 | 0.43 (0.41–0.46) | 90.0 | 57.92 | <0.001 |
| Multiple countries | 4 (7,536) | 1.49 | 2.56 (0.76–5.21) | 93.7 | 3.79 | <0.001 |
| Mortality in AIS patients with PE | 8 (2,020,788) | 0.80 | 12.9 (1.6–31.7) | 100.0 | 2.77 | 0.006 |
| Retrospective | 6 (2,019,881) | 0.80 | 17.16 (2.08–41.5) | 99.7 | 2.74 | 0.006 |
| Prospective | 2 (907) | 2.76 | 2.52 (1.57–3.68) | – | 8.11 | <0.001 |
| Asia | 3 (2,498) | 3.00 | 2.94 (0.54–6.61) | 80.1 | 3.08 | 0.002 |
| Europe | 2 (17,072) | 28.59 | 28.59 (27.91–29.27) | 0.0 | 145.99 | <0.001 |
| North America | 2 (2,001,052) | 0.56 | 0.31 (0.30–0.32) | 0.0 | 105.16 | <0.001 |
| Multiple countries | 1 (166) | 6.02 | – | – | – | – |
| Prevalence of risk factors in AIS patients with PE | ||||||
| Atrial fibrillation | 4 (17,175) | 33.62 | 29.0 (25–35) | 68.4 | 18.36 | <0.001 |
| Diabetes mellitus | 5 (17,231) | 26.03 | 23.0 (20–26) | 55.7 | 23.87 | <0.001 |
| Hypertension | 5 (17,231) | 56.91 | 54.0 (49–60) | 70.3 | 28.12 | <0.001 |
| Smoking | 3 (159) | 21.38 | 23.0 (12–37) | 61.9 | 5.59 | <0.001 |
| Cancer | 4 (17,170) | 13.87 | 19.0 (13–25) | 66.2 | 10.51 | <0.001 |
| Coronary artery disease | 2 (10,457) | 15.65 | 15.0 (15–16) | 0.0 | 75.56 | <0.001 |
| Hyperlipidaemia | 2 (145) | 20.69 | 20.0 (14–27) | 0.0 | 9.96 | <0.001 |
| Author (Year) | HTN (PE/No PE) | Cancer (PE/No PE) | AF (PE/No PE) | DM (PE/No PE) | HL (PE/No PE) | CAD (PE/No PE) | Smoking (PE/No PE) |
|---|---|---|---|---|---|---|---|
| Abdelsalam et al. (2020) [32] | – | 8/1 | – | – | – | – | – |
| Chen et al. (2012) [39] | 8/6 | – | 6/8 | 1/14 | – | – | 5/9 |
| Keller et al. (2024) [44] | 3627/3077 | 1113/5591 | 2067/4637 | 1710/4994 | – | – | – |
| Keller et al. (2020) [45] | 6099/4269 | 1234/9134 | 3691/6677 | 2753/7615 | – | 1616/8752 | – |
| Pongmoragot et al. (2013) [47] | 61/28 | 26/63 | 11/78 | 19/70 | 23/66 | 21/67 | 13/76 |
| Tanislav et al. (2011) [53] | 12/44 | – | – | 2/54 | 7/49 | – | 16/40 |
| Outcome | N (Studies) | n (Patients) | Effect Measure | Summary Effect (OR, 95%) | Test of Overall Effect (z, p) | Cochran’s Q | H | I2 * % (95% CI) ¶ | Q p-Value | τ2 Φ |
|---|---|---|---|---|---|---|---|---|---|---|
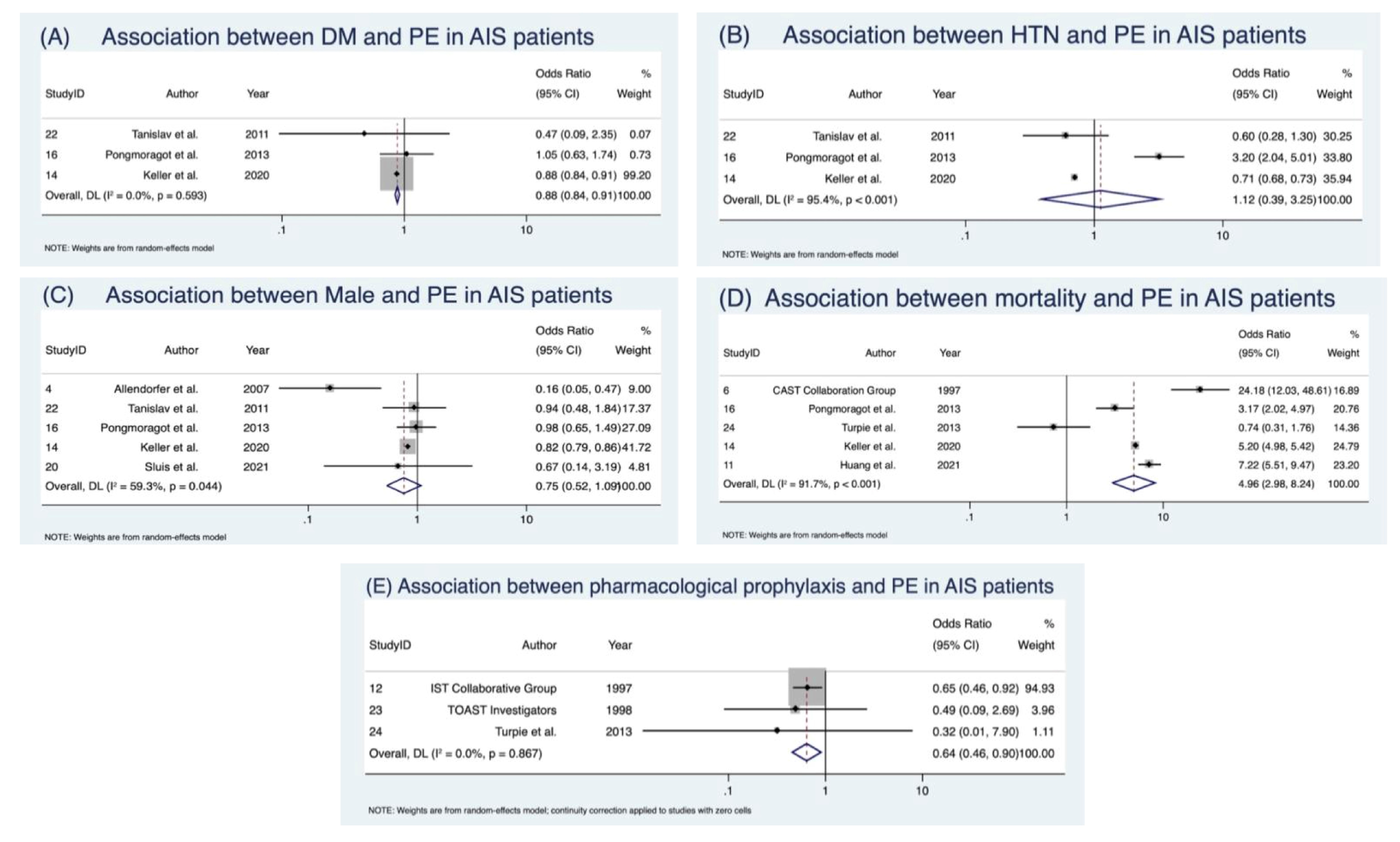
| Male sex | 5 | 2,926,122 | OR | 0.75 (0.52–1.09) | z = –1.52, p = 0.129 | 9.82 | 1.57 | 59.3 (0.0–87.5) | 0.044 | 0.083 |
| Pharmacological prophylaxis | 3 | 21,090 | OR | 0.64 (0.46–0.90) | z = 2.58, p = 0.010 | 0.29 | 0.378 | 0.0 (0.0–37.4) | 0.867 | 0.000 |
| Hypertension | 3 | 2,933,559 | OR | 1.12 (0.39–3.25) | z = 0.209, p = 0.835 | 43.58 | 4.67 | 95.4 (0.0–98.9) | <0.001 | 0.819 |
| Diabetes mellitus | 3 | 2,928,800 | OR | 0.88 (0.84–0.92) | z = –5.97, p < 0.001 | 1.05 | 0.72 | 0.0 (0.0–58.2) | 0.593 | 0.000 |
| Mortality | 5 | 3,774,118 | OR | 4.96 (2.98–8.24) | z = 6.18, p < 0.001 | 48.28 | 3.47 | 91.7 (0.0–97.8) | <0.001 | 0.270 |
| Outcome | No. of Studies (Patients) | Effect Estimate (95% CI) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence (GRADE) | Reasons for Rating |
|---|---|---|---|---|---|---|---|---|---|
| Prevalence of PE after AIS | 17 (23,637,708) | 0.40% (95% CI 0.33–0.49) | Serious (retrospective datasets; coding bias) | Very serious (considerable heterogeneity, I2 > 90%) | Not serious | Serious (wide 95% CI) | Possible (funnel asymmetry) | ⬤⬤◯◯ Low | Considerable heterogeneity (Cochrane classification); reliance on retrospective datasets; imprecision |
| Mortality in AIS patients with PE | 8 (2,020,788) | 12.9% (95% CI 1.6–31.7) | Serious (mixed designs; coding reliance) | Serious (considerable heterogeneity, I2 > 95%) | Not serious | Serious (wide 95% CI) | Undetected (few studies) | ⬤⬤⬤◯ Moderate | Consistent direction but heterogeneity; limited prospective data |
| Mortality risk (OR) | 5 (3,774,118) | OR 4.96 (95% CI 2.98–8.24) | Serious | Serious (considerable heterogeneity, I2 > 90%) | Not serious | Not serious (consistent large effect) | Undetected | ⬤⬤⬤◯ Moderate | Large, consistent effect; down-graded for heterogeneity |
| Atrial fibrillation prevalence in AIS + PE | 4 (17,175) | 29% (95% CI 25–35) | Moderate | Serious (high heterogeneity) | Not serious | Serious (imprecise estimates) | Undetected | ⬤⬤◯◯ Low | Few studies; heterogeneity across datasets |
| Cancer prevalence in AIS + PE | 4 (17,170) | 19% (95% CI 13–25) | Moderate | Serious (reporting variation) | Not serious | Serious (wide 95% CI) | Undetected | ⬤⬤◯◯ Low | Limited data; possible under-reporting |
| Smoking prevalence in AIS + PE | 3 (159) | 23% (95% CI 12–37) | Moderate | Serious (high heterogeneity) | Not serious | Very serious (small sample; wide 95% CI) | Undetected | ⬤◯◯◯ Very low | Small cohorts; wide confidence interval |
| Diabetes as predictor of PE | 3 (2,928,800) | OR 0.88 (95% CI 0.84–0.92) | Low | Not serious (I2 = 0%) | Not serious | Not serious (narrow 95% CI) | Unlikely | ⬤⬤⬤◯ Moderate | Consistent effect, no heterogeneity; inverse association |
| Hypertension as predictor of PE | 3 (2,933,559) | OR 1.12 (95% CI 0.39–3.25) | Moderate | Very serious (considerable heterogeneity, I2 > 95%) | Not serious | Serious (wide 95% CI) | Possible | ⬤◯◯◯ Very low | Extreme heterogeneity; imprecision of effect |
| Pharmacological prophylaxis | 3 (21,090) | OR 0.64 (95% CI 0.46–0.90) | Low | Not serious (I2 = 0%) | Not serious | Serious (few studies) | Undetected | ⬤⬤⬤◯ Moderate | Consistent protective effect; no heterogeneity; limited sample size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yang, Y.; Bhaskar, S.M.M. Pulmonary Embolism After Acute Ischaemic Stroke (PEARL-AIS): Global Prevalence, Risk Factors, Outcomes, and Evidence Grading from a Meta-Analysis. Neurol. Int. 2025, 17, 168. https://doi.org/10.3390/neurolint17100168
Chen D, Yang Y, Bhaskar SMM. Pulmonary Embolism After Acute Ischaemic Stroke (PEARL-AIS): Global Prevalence, Risk Factors, Outcomes, and Evidence Grading from a Meta-Analysis. Neurology International. 2025; 17(10):168. https://doi.org/10.3390/neurolint17100168
Chicago/Turabian StyleChen, Darryl, Yuxiang Yang, and Sonu M. M. Bhaskar. 2025. "Pulmonary Embolism After Acute Ischaemic Stroke (PEARL-AIS): Global Prevalence, Risk Factors, Outcomes, and Evidence Grading from a Meta-Analysis" Neurology International 17, no. 10: 168. https://doi.org/10.3390/neurolint17100168
APA StyleChen, D., Yang, Y., & Bhaskar, S. M. M. (2025). Pulmonary Embolism After Acute Ischaemic Stroke (PEARL-AIS): Global Prevalence, Risk Factors, Outcomes, and Evidence Grading from a Meta-Analysis. Neurology International, 17(10), 168. https://doi.org/10.3390/neurolint17100168