Clinical Methods Supporting Initial Recognition of Early Post-Stroke Seizures: A Systematic Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question and Eligibility Criteria
2.2. Searching for Relevant Papers
2.3. Selection of Sources of Evidence and Charting the Data
2.4. Data Charting Process
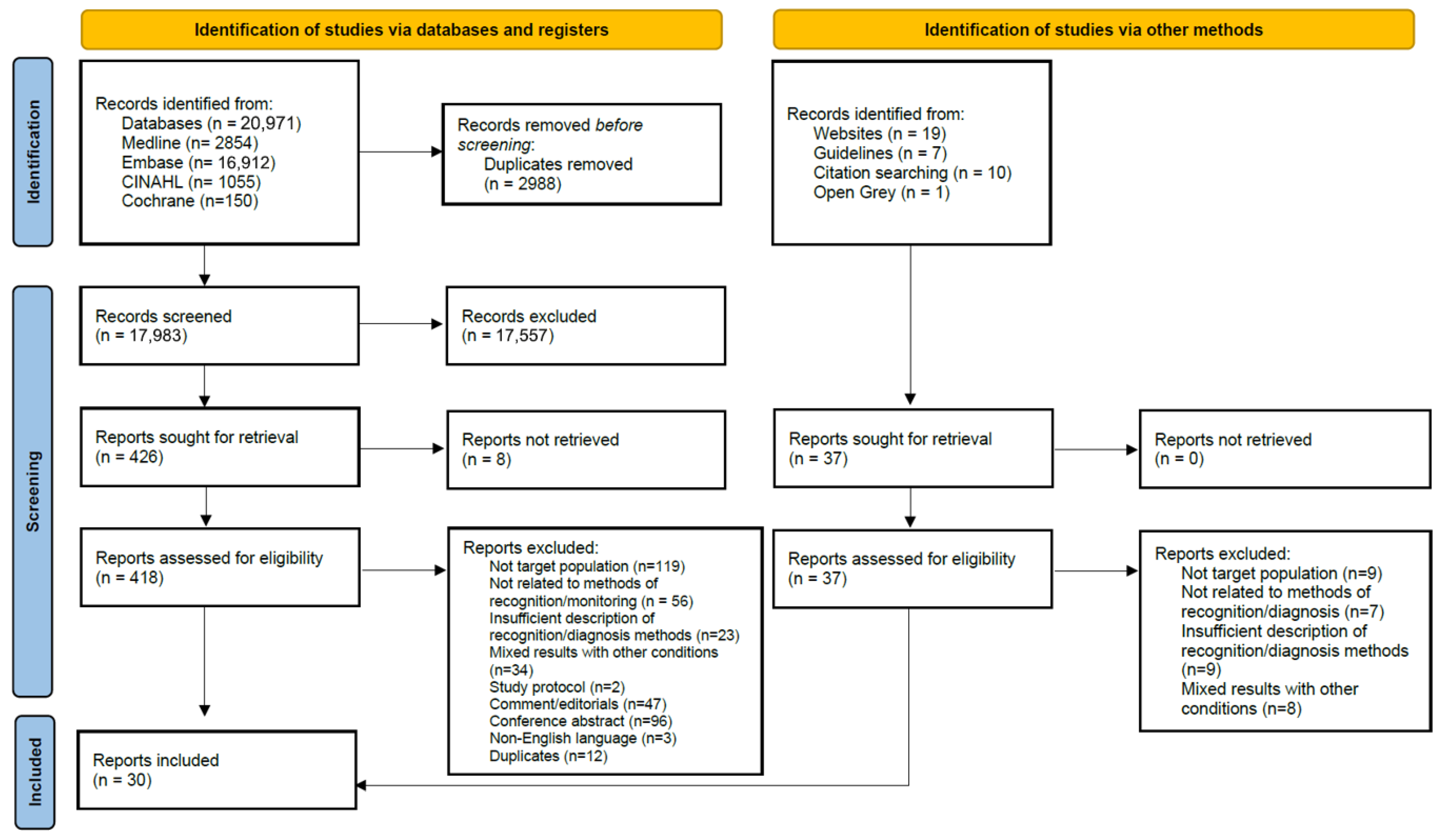
3. Results
3.1. Selection of Sources of Evidence
| First Author, Year | Country | Study Type | Study Aim | Number of Participants | Male (%) | Ischaemic Stroke (%) | Clinical Method to Identify Seizure |
|---|---|---|---|---|---|---|---|
| Ba, 2021 [33] | France | Cohort | To evaluate whether thrombolysis is associated with an increased risk of early epileptic seizures in a cohort of consecutive patients who underwent an angiography in emergency care for cerebral ischaemia due to large-vessel occlusion | 1638 | 783 (48%) | 1638 (100%) | Clinical diagnosis. EEG used to diagnose seizures with atypical manifestation. EEG not used systematically. |
| Beghi, 2011 [42] | Italy | Cohort | To identify incidence and predictors of acute symptomatic seizures in a cohort of patients with first stroke | 714 | 399 (56%) | 609 (85%) | Direct observation by medical staff or reliable witness. Simple loss of consciousness or short episodes of confusion excluded. EEG only when indicated by medical staff. |
| Belcastro, 2014 [43] | Italy | Cohort | To evaluate in a stroke unit the usefulness of a prolonged, at least 6 h, video-EEG recording (VEEG) in identifying episodes of non-convulsive status epilepticus after an acute ischemic stroke | 889 | 566 (64%) | 889 (100%) | Prolonged VEEG routinely within first 7 days of admission or immediately upon suspected seizure activity |
| Bentes, 2017 [44] | Portugal | Cohort | To compare the frequency of seizures and EEG abnormalities between stroke patients treated and not treated with thrombolysis | 151 | 89 (59%) | 151 (100%) | Continuous VEEG in first 72 h, daily for first 7 days + if neurological worsening, at discharge |
| Bentes, 2018 [13] | Portugal | Cohort | To investigate whether early EEG abnormalities are independent predictors of post-stroke epilepsy | 151 | 112 (74%) | 151 (100%) | Continuous VEEG in first 72 h, daily for first 7 days + if neurological worsening, at discharge |
| Carrera, 2006 [45] | Switzerland | Case control | To determine the incidence and risk factors of electrical seizures and other electrical epileptic activity using cEEG in patients with acute stroke | 100 | 58 (58%) | 91 (91%) | cEEG routinely on first full admission day |
| Daniele, 1996 [34] | Italy | Cohort | To evaluate the incidence of seizures and relationship between the various types of seizures and lesion location | 217 | 125 (58%) | 187 (86%) | Observation and description by either experienced departmental staff or by witness relatives of the patient |
| Jung, 2012 [35] | Switzerland | Cohort | To analyse the influence of early and late epileptic seizures on the outcomes of patients with acute ischemic stroke treated with thrombolytic therapy | 805 | 438 (56%) | 805 (100%) | Symptom information from the patient, from a witness, or both |
| Kim, 2016 [36] | South Korea | Cohort | To define clinical predictors of seizure recurrence after first post-stroke seizure in ischaemic stroke | 48 | 29 (60%) | 48 (100%) | Seizure diagnosed clinically. Standard EEG within 24–72 h of seizure onset. |
| Lasek-Bal, 2023 [46] | Poland | Cohort | To determine the prevalence and nature of changes in EEG recordings from the stroke hemisphere and contralateral hemisphere | 131 | 62 (47%) | 131 (100%) | Two EEGs in first 72 h and one before discharge |
| Mecarelli, 2011 [47] | Italy | Cohort | To analyse EEG patterns performed within 24 h of stroke onset | 232 | 107 (46%) | 177 (76%) | EEG within 24 h of admission |
| Onder, 2017 [40] | Turkey | Cohort | To identify whether EEG findings could be a marker for post-stroke seizure development and survival in patients with acute ischemic or haemorrhagic stroke, who were followed up in a neurological intensive care unit | 50 | 23 (46%) | 37 (74%) | Continuous EEG in neurological intensive care unit |
| Sarfo, 2020 [41] | Africa | Cohort | To assess the frequency and factors associated with post-stroke seizures by stroke types across 15 hospitals in Nigeria and Ghana | 3344 | 1870 (66%) | 2091 (62%) | Seizure diagnosed clinically. No EEG. |
| Scoppettulo, 2019 [37] | Belgium | Cohort | To assess if epileptic activities were associated with neurological deterioration in acute ischaemic stroke | 81 | 46 (56%) | 81 (100%) | EEG |
| Tako, 2022 [38] | Germany | Cohort | To analyse predictive factors for acute symptomatic seizures in a well-defined patient population who experienced an ischemic stroke due to large vessel occlusion and treated after mechanical recanalisation | 979 | 509 (52%) | 979(100%) | Clinically observed ictal stigmas. EEG only when indicated by medical staff. |
| Vespa, 2003 [48] | USA | Cohort | To determine whether early seizures that occur frequently after intracerebral haemorrhage led to increased brain oedema | 109 | 60 (55%) | 46 (42%) | EEG within 24 h of stroke onset and admission to intensive care |
| Yerram, 2019 [39] | USA | Cohort | To evaluate risk factors from examination, imaging, and cEEG for the development of seizures in critically ill patients with ICH | 57 | 26 (46%) | 0 (0%) | Continuous EEG at the order of the physician |
| First Author, Year | Country | Clinical Setting | Clinical Method to Identify Seizure | Duration of Method | Indications |
|---|---|---|---|---|---|
| Green, 2021 [23] | USA | Acute ischaemic stroke | Standardised approach to recognition, assessment, and documentation of the seizure Neurological examination EEG | Not reported | Monitor with EEG for change in mental status or depressed level of consciousness out of proportion to the stroke |
| Hemphill, 2015 [49] | USA | Acute intracerebral haemorrhage | cEEG | At least 24 h | Depressed mental status out of proportion to the stroke |
| Tatum, 2022 [50] | USA | Inpatient | Continuous VEEG monitoring | Condition-specific | Continuous VEEG should be used to differentiate between epileptic and non-epileptic events |
3.2. Clinical Methods for the Identification and Observation of Seizures
- (i)
- cEEG. Seventeen papers, including eight research studies, referred to cEEG for EPSS detection and monitoring. Technical parameters for EEG were reported in nine papers with varying detail and no standardisation. cEEG was typically initiated at the earliest opportunity after suspected seizure or stroke onset. Duration of monitoring ranged from >6 h to 7 days; one intensive care study monitored for up to 38 days. Three papers described bedside cEEG visible to nursing staff [25,31,45], and two highlighted the need for nursing and physician competency in recognising electrographic seizure patterns [25,31]. Bautista [25] specified essential bedside EEG interpretation skills, including waveform frequency, amplitude, morphology, and symmetry. Two papers reported retrospective cEEG review by either trained physicians or electroencephalographers [31,45]. Several papers highlighted the utility of cEEG in detecting non-convulsive seizures and periodic discharges (associated with increased seizure risk) [2,28,31,32].
- (ii)
- Periodic EEG. Eleven papers referred to periodic EEG, reported as 20 to 30 min in duration (Table 6). One paper [28] initiated an emergency EEG performed soon after stroke presentation due to fluctuant confusion, followed by cEEG. Two research papers used periodic EEG systematically on all acute stroke patients [46,47]. They were also the only papers reporting on technical parameters, both using the International 10–20 system with 14 [47] or 21 [46] electrodes. In one study, serial EEGs over several days were indicated if the first EEG showed abnormal epileptiform activity [47].
- (iii)
- VEEG. Concurrent video recording with EEG is considered best practice, supplementing clinical assessments and linking electrographic seizures with clinical changes [2]. Tatum et al. [50] recommend a single camera setup and provide guidance on EEG and video synchronisation and digital memory requirements. Video use was reported in one out 11 papers using periodic EEG and in 10 out of 19 cEEG papers (Table 6). Mader’s case report [30] described a 28 s clonic seizure observed on video but obscured on EEG due to movement artefact. This case drew attention to the narrow time window for observing seizures if relying on human observation as well as the value of concurrent video recording with EEG.
- (iv)
- Clinician observation. Nine papers, six research papers, addressed clinical observation in EPSS. In five papers, seizures were diagnosed clinically without details on observation procedures or staff training [34,35,38,41,42]. Among papers reporting criteria, seizure definitions and thresholds were varied [24,35,36]. The International League Against Epilepsy definitions were used in three papers [36,38,41]. Green et al. [23] recommended a standardised nursing observation approach for post-stroke complications, including seizures, but did not specify a method. Bautista [50] recommended a systematic assessment covering onset and duration, level of consciousness, eye deviation, gaze, pupil size, urinary incontinence, body movements, and motor function, with periodic assessments until baseline recovery [50].
- (v)
- Family witness. Four research papers [34,35,41,42] and case report [30] referred to a family witness description contributing to the diagnosis of seizure at stroke onset or on hospitalisation. No papers referred to supplemental video information recorded on smart phones provided as part of the witness account.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPSS | Early post-stroke seizure |
| EEG | Electroencephalogram |
| cEEG | Continuous electroencephalogram |
| VEEG | Video-electroencephalogram |
References
- Holtkamp, M.; Beghi, E.; Benninger, F.; Kälviäinen, R.; Rocamora, R.; Christensen, H. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur. Stroke J. 2017, 2, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Zelano, J.; Holtkamp, M.; Agarwal, N.; Lattanzi, S.; Trinka, E.; Brigo, F. How to diagnose and treat post-stroke seizures and epilepsy. Epileptic Disord. 2020, 22, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Carpio, A.; Forsgren, L.; Hesdorffer, D.; Malmgren, K.; Sander, J.; Tomson, T.; Hauser, A.W. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010, 51, 671–675. [Google Scholar] [CrossRef]
- Chung, J.M. Seizures in the acute stroke setting. Neurol. Res. 2014, 36, 403–406. [Google Scholar] [CrossRef]
- Blum, D.E.; Eskola, J.; Bortz, J.J.; Fisher, R.S. Patient awareness of seizures. Neurology 1996, 47, 260–264. [Google Scholar] [CrossRef]
- Brigo, F.; Lattanzi, S. Poststroke seizures as stroke mimics: Clinical assessment and management. Epilepsy Behav. 2020, 104, 106297. [Google Scholar] [CrossRef]
- Stefanidou, M.; Das, R.R.; Beiser, A.S.; Sundar, B.; Kelly-Hayes, M.; Kase, C.S.; Devinsky, O.; Seshadri, S.; Friedman, D. Incidence of seizures following initial ischemic stroke in a community-based cohort: The Framingham Heart Study. Seizure 2017, 47, 105–110. [Google Scholar] [CrossRef]
- Xu, Y.; Hackett, M.L.; Chalmers, J.; Lindley, R.I.; Wang, X.; Li, Q.; Robinson, T.; Arima, H.; Lavados, P.M.; Anderson, C.S.; et al. Frequency, determinants, and effects of early seizures after thrombolysis for acute ischemic stroke: The ENCHANTED trial. Neurol 2017, 7, 324–332. [Google Scholar] [CrossRef]
- Nandan, A.; Zhou, Y.M.; Demoe, L.; Waheed, A.; Jain, P.; Widjaja, E. Incidence and risk factors of post-stroke seizures and epilepsy: Systematic review and meta-analysis. J. Int. Med. Res. 2023, 51, 11. [Google Scholar] [CrossRef]
- Procaccianti, G.; Zaniboni, A.; Rondelli, F.; Crisci, M.; Sacquegna, T. Seizures in acute stroke: Incidence, risk factors and prognosis. Neuroepidemiology 2012, 39, 45–50. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, Y.; Zhang, J.G.; Hu, W.; Ge, M.; Zhang, K.; Shao, X. Risk factors for post-stroke seizures: A systematic review and meta-analysis. Epilepsy Res. 2014, 108, 1806–1816. [Google Scholar] [CrossRef]
- Bentes, C.; Martins, H.; Peralta, A.R.; Morgado, C.; Casimiro, C.; Franco, A.C.; Fonseca, A.C.; Geraldes, R.; Canhao, P.; Pinho, E.M.T.; et al. Early EEG predicts poststroke epilepsy. Epilepsia Open 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Ryu, H.U.; Kim, H.J.; Shin, B.S.; Kang, H.G. Clinical approaches for poststroke seizure: A review. Front. Neurol. 2024, 15, 1337960. [Google Scholar] [CrossRef] [PubMed]
- The Association of Neurophysiological Scientists (ANS) and British Society for Clinical Neurophysiology (BSCN). ANS-BSCN Recommendations and Guidelines for the Practice of Clinical Neurophysiology in the United Kingdom. 2024. Version 14. Available online: https://www.bscn.org.uk/data/files/Guidelines/ANS%20BSCN%20%20Neurophys%20Guidelines%20V14%20final.pdf (accessed on 1 July 2025).
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Scoping Reviews. In JBI Manual for Evidence Synthesis; JBI: Singapore, 2020. [Google Scholar] [CrossRef]
- Gordon, C.; Watkins, C.L.; Emsley, H.; Harris, C.; Lightbody, L.; Davidson, C.E. Recognition of Early Post Stroke Seizures and Epilepsy (REPoSS). Available online: https://osf.io/bkejc/ (accessed on 21 April 2023).
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- RAYYAN. rayyan©. Available online: https://www.rayyan.ai/ (accessed on 21 April 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Greenhalgh, T.M.; Dijkstra, P. How to Read a Paper: The Basics of Evidence-Based Healthcare; John Wiley & Sons, Incorporated: Newark, UK, 2024. [Google Scholar]
- Green, T.L.; McNair, N.D.; Hinkle, J.L.; Middleton, S.; Miller, E.T.; Perrin, S.; Power, M.; Southerland, A.M.; Summers, D.V.; American Heart Association Stroke Nursing Committee of the Council on Cardiovascular and Stroke Nursing and the Stroke Council. Care of the Patient with Acute Ischemic Stroke (Posthyperacute and Prehospital Discharge): Update to 2009 Comprehensive Nursing Care Scientific Statement: A Scientific Statement From the American Heart Association. Stroke 2021, 52, e179–e197. [Google Scholar] [CrossRef]
- Algın, D.İ.; Yiğit, E.G.; Yeşilkaya, M.; Erdinç, O.O. Frontal Lacunar Infarct in the Development of Non-Convulsive Status Epilepticus: Case Report. Arch. Epilepsy 2022, 28, 134–137. [Google Scholar] [CrossRef]
- Bautista, C. Monitoring for Poststroke Seizures. Crit. Care Nurs. Clin. N. Am. 2020, 32, 85–95. [Google Scholar] [CrossRef]
- Cyrous, A.; O’Neal, B.; Freeman, W.D. New approaches to bedside monitoring in stroke. Expert Rev. Neurother. 2012, 12, 915–928. [Google Scholar] [CrossRef]
- De Reuck, J. Management of stroke-related seizures. Acta Neurol. Belg. 2009, 109, 271–276. [Google Scholar] [CrossRef]
- Elmali, A.D.; Ekizoglu, E.; Ciftci, I.; Yesilot, N.; Coban, O.; Baykan, B. Periodic electroclinical seizures following an ischemic stroke revealed by continuous-EEG. Epilepsy Behav. Rep. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Kraus, J.J.; Metzler, M.D.; Coplin, W.M. Critical care issues in stroke and subarachnoid hemorrhage. Neurol. Res. 2002, 24, 27–57. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.C., Jr.; Losada, V.; Baity, J.C.; McKinnies, E.M.; Branch, L.A. Stroke-Onset Seizures During Midbrain Infarction in a Patient with Top of the Basilar Syndrome. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620940497. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P. Continuous EEG monitoring for the detection of seizures in traumatic brain injury, infarction, and intracerebral hemorrhage: “to detect and protect”. J. Clin. Neurophysiol. 2005, 22, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chu, C.; Jing, C.; Zheng, X.; Lin, W. Non-Convulsive Status Epileptics Presenting with Periodic Lateralized Epileptiform Discharges and Coma after Cerebral Hemorrhage: A Case Report. Neurol. India 2021, 69, 733–736. [Google Scholar] [CrossRef]
- Ba, K.; Casolla, B.; Caparros, F.; Bricout, N.; Della Schiava, L.; Pasi, M.; Dequatre-Ponchelle, N.; Bodenant, M.; Bordet, R.; Cordonnier, C.; et al. Early epileptic seizures in ischaemic stroke treated by mechanical thrombectomy: Influence of rt-PA. J. Neurol. 2021, 268, 305–311. [Google Scholar] [CrossRef]
- Daniele, O.; Caravaglios, G.; Ferraro, G.; Mattaliano, A.; Tassinari, C.A.; Natalé, E. Stroke-Related Seizures and the Role of Cortical and Subcortical Structures. J. Epilepsy 1996, 9, 184–188. [Google Scholar] [CrossRef]
- Jung, S.; Schindler, K.; Findling, O.; Mono, M.L.; Fischer, U.; Gralla, J.; El-Koussy, M.; Weck, A.; Galimanis, A.; Brekenfeld, C.; et al. Adverse effect of early epileptic seizures in patients receiving endovascular therapy for acute stroke. Stroke 2012, 43, 1584–1590. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.D.; Choi, K.G.; Lee, H.W. Clinical predictors of seizure recurrence after the first post-ischemic stroke seizure. BMC Neurol. 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Scoppettuolo, P.; Gaspard, N.; Depondt, C.; Legros, B.; Ligot, N.; Naeije, G. Epileptic activity in neurological deterioration after ischemic stroke, a continuous EEG study. Clin. Neurophysiol. 2019, 130, 2282–2286. [Google Scholar] [CrossRef] [PubMed]
- Tako, L.M.; Strzelczyk, A.; Rosenow, F.; Pfeilschifter, W.; Steinmetz, H.; Golbach, R.; Schafer, J.H.; Zollner, J.P.; Kohlhase, K. Predictive Factors of Acute Symptomatic Seizures in Patients with Ischemic Stroke Due to Large Vessel Occlusion. Front. Neurol. 2022, 13, 894173. [Google Scholar] [CrossRef] [PubMed]
- Yerram, S.; Katyal, N.; Sarwal, A.; George, P.; Newey, C.R. Lateralized Periodic Discharges are Predictive of Seizures in Patients with Intracerebral Hemorrhage. Ann. Indian Acad. Neurol. 2019, 22, 414–418. [Google Scholar] [CrossRef]
- Onder, H.; Arsava, E.M.; Topcuoglu, M.A.; Dericioglu, N. Do Video-EEG Monitoring Findings in ICU Patients with Acute Stroke Predict Development of Seizures and Survival During Follow-up? Clin. EEG Neurosci. Off. J. EEG Clin. Neurosci. Soc. 2017, 48, 417–421. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Akinyemi, J.; Akpalu, A.; Wahab, K.; Yaria, J.; Adebayo, O.; Komolafe, M.; Obiako, R.; Owolabi, L.; Osaigbovo, G.O.; et al. Frequency and factors associated with post-stroke seizures in a large multicenter study in West Africa. J. Neurol. Sci. 2021, 427, 117535. [Google Scholar] [CrossRef]
- Beghi, E.; D’Alessandro, R.; Beretta, S.; Consoli, D.; Crespi, V.; Delaj, L.; Gandolfo, C.; Greco, G.; La Neve, A.; Manfredi, M.; et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology 2011, 77, 1785–1793. [Google Scholar] [CrossRef]
- Belcastro, V.; Vidale, S.; Gorgone, G.; Pisani, L.R.; Sironi, L.; Arnaboldi, M.; Pisani, F. Non-convulsive status epilepticus after ischemic stroke: A hospital-based stroke cohort study. J. Neurol. 2014, 261, 2136–2142. [Google Scholar] [CrossRef]
- Bentes, C.; Martins, H.; Peralta, A.R.; Morgado, C.; Casimiro, C.; Franco, A.C.; Fonseca, A.C.; Geraldes, R.; Canhao, P.; Pinho, E.M.T.; et al. Epileptic manifestations in stroke patients treated with intravenous alteplase. Eur. J. Neurol. 2017, 24, 755–761. [Google Scholar] [CrossRef]
- Carrera, E.; Michel, P.; Despl, P.A.; Maeder-Ingvar, M.; Ruffieux, C.; Debatisse, D.; Ghika, J.; Devuyst, G.; Bogousslavsky, J. Continuous assessment of electrical epileptic activity in acute stroke. Neurology 2006, 67, 99–104. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Dewerenda-Sikora, M.; Binek, L.; Student, S.; Labuz-Roszak, B.; Krzystanek, E.; Kaczmarczyk, A.; Krzan, A.; Zak, A.; Cieslik, A.; et al. Epileptiform activity in the acute phase of stroke predicts the outcomes in patients without seizures. Front. Neurol 2023, 14, 1096876. [Google Scholar] [CrossRef]
- Mecarelli, O.; Pro, S.; Randi, F.; Dispenza, S.; Correnti, A.; Pulitano, P.; Vanacore, N.; Vicenzini, E.; Toni, D. EEG patterns and epileptic seizures in acute phase stroke. Cerebrovasc. Dis. 2011, 31, 191–198. [Google Scholar] [CrossRef]
- Vespa, P.M.; O’Phelan, K.; Shah, M.; Mirabelli, J.; Starkman, S.; Kidwell, C.; Saver, J.; Nuwer, M.R.; Frazee, J.G.; McArthur, D.A.; et al. Acute seizures after intracerebral hemorrhage: A factor in progressive midline shift and outcome. Neurology 2003, 60, 1441–1446. [Google Scholar] [CrossRef]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; MacDonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Tatum, W.O.; Mani, J.; Jin, K.; Halford, J.J.; Gloss, D.; Fahoum, F.; Maillard, L.; Mothersill, I.; Beniczky, S. Minimum standards for inpatient long-term video-EEG monitoring: A clinical practice guideline of the international league against epilepsy and international federation of clinical neurophysiology. Clin. Neurophysiol. 2022, 134, 111–128. [Google Scholar] [CrossRef]
- Sheikh, Z.B.; Stretz, C.; Maciel, C.B.; Dhakar, M.B.; Orgass, H.; Petroff, O.A.; Hirsch, L.J.; Gilmore, E.J. Deep Versus Lobar Intraparenchymal Hemorrhage: Seizures, Hyperexcitable Patterns, and Clinical Outcomes. Crit Care Med. 2020, 48, e505–e513. [Google Scholar] [CrossRef] [PubMed]
| Population | Intervention | Context |
|---|---|---|
|
|
|
| Variables | Count |
|---|---|
| Year of publication (all papers) | |
| 1996–2001 | 1 |
| 2002–2007 | 4 |
| 2008–2013 | 5 |
| 2014–2019 | 7 |
| 2020–2023 | 13 |
| Continent of publication (all papers) | |
| Europe | 18 |
| North America | 9 |
| Asia | 2 |
| Africa | 1 |
| Clinical setting (17 research papers) | |
| Neurology/neuroscience | 3 |
| Critical care/intensive care | 3 |
| Stroke specialist | 8 |
| Setting not reported | 3 |
| Stroke type (17 research papers) | |
| Intracerebral haemorrhage | 1 |
| Ischaemic stroke | 9 |
| Mixed sample | 7 |
| Sample size (17 research papers) | |
| 0–25 | 0 |
| 26–50 | 1 |
| 51–100 | 3 |
| 101–200 | 5 |
| 201–300 | 2 |
| 301–500 | 4 |
| 501–1000 | 0 |
| >1001 | 2 |
| First Author, Year | Paper Type | Country | Clinical Setting | Clinical Method to Identify Seizure | Duration of Method | Indications |
|---|---|---|---|---|---|---|
| Algin, 2022 [24] | Case report | Turkey | Neurology | Clinical observation of focal seizure + periodic EEG | Multiple EEG results. Details on timing and type not reported. | Focal seizure observed + subsequent depression in general conscious level |
| Bautista, 2020 [25] | Expert opinion | USA | Critical care | Nurse seizure assessment Bedside EEG recording | Continuous EEG for first 5 min of the seizure and until returned to baseline | On admission EEG as soon as possible after ictal event |
| Cyrous, 2012 [26] | Expert opinion | Sweden | Acute stroke | EEG + continuous EEG | Not reported | For ICU stroke patients who are comatose or sedated and paralysed |
| De Reuck, 2009 [27] | Expert opinion | Belgium | Stroke | EEG | Not reported | As soon as possible after the ictal event |
| Elmali, 2021 [28] | Case report | Turkey | ED | Emergency EEG + continuous video EEG | Emergency EEG details not reported. Continuous video EEG for 48 h. | Episodes of fluctuating confusion suggestive of seizures |
| Kraus, 2002 [29] | Expert opinion | USA | Critical care | Continuous EEG | Not reported | For ICU stroke patients who are comatose |
| Mader, 2020 [30] | Case report | USA | Not reported | Continuous EEG | 48 h, days 2 and 3 post-stroke | Not reported |
| Vespa, 2005 [31] | Review and expert opinion | USA | Critical care | Continuous EEG on monitor at bedside, nurse continuous review with routine periodic review by physician | 5 days | Lack of clinical seizure activity not an indication to avoid continuous EEG (cEEG) |
| Wang, 2021 [32] | Case report | China | Neurology | Video EEG | Not reported | Persistent coma 4 days post-stroke |
| Zelano, 2020 [2] | Review and expert opinion | Sweden | Not reported | Continuous EEG + concurrent video recording | ≥24 h In patients who are comatose, have periodic discharges, or sedated ≥48 h monitoring advised | As soon as possible after sudden onset of unexplained behavioural changes, or impairment of consciousness (including coma), or clinical paroxysmal events suspected to be seizures |
| First Author, Year | Method Description | Initiation Timing | Duration | Indication |
|---|---|---|---|---|
| Continuous EEG | ||||
| Bautista, 2020 [25] | Observe bedside EEG similar to cardiac monitoring. EEG should be reviewed for frequency, repetition, amplitude, distribution, timing, persistence, morphology, symmetry. | Not reported | Not reported | Not reported |
| Belcastro, 2014 [43] | Prolonged > 6 h VEEG. Cap with 21 fixed gel electrodes, International 10–20 system. | Within the first week: promptly in those with clear or suspected seizure, or routinely at any time | >6 h | Routinely in all patients within the first week of admission. Performed promptly if seizure suspected. |
| Bentes, 2017 [44] | 64-channel VEEG, including an eyes closed wake resting condition and eyes open, hyperventilation, and photic stimulation manoeuvres | First 72 h after stroke | Maximum 60 min | All patients received routine EEG |
| Bentes, 2018 [13] | 64-channel synchronised VEEG | As early as possible in the first 72 h, daily for the first 7 days | Maximum 60 min | All patients received routine EEG for the first 7 days. Additional VEEG if unexplained neurological worsening. |
| Carrera, 2006 [45] | 10 electrodes, International 10–20 system with 8 channel sub-set. Displayed continuously at the bedside. | After 24 h of admission | Typically started in the morning and stopped the following day | All patients admitted for longer than 24 h |
| Cyrous, 2012 [26] | Not reported | Not reported | Not reported | In the intensive care unit |
| Elmali, 2021 [28] | Long-term VEEG | Within first days of admission | 48 h | After seizures observed in the emergency department and emergency EEG performed |
| Hemphill, 2015 [49] | Not reported | Not reported | Not reported | In ICH patients with depressed mental status that is out of proportion to the degree of brain injury |
| Kraus, 2002 [29] | Not reported | Not reported | Not reported | Not reported |
| Mader, 2020 [30] | Not reported | At stroke onset | Not reported | Depressed level of consciousness |
| Onder, 2017 [40] | Continuous VEEG monitoring 10–20 system | Not reported | 1–38 days (mean 7.9) | In a neurological intensive care unit in patients with suspected seizures, unexplained alterations in consciousness or behaviour, or witnessed seizures |
| Scoppettulo, 2019 [37] | cEEG 21 scalp electrodes, International 10–20 system | 0.5 to 4 days | Not reported | Neurological deterioration: worse neurological deficit increase in NIHSS ≥2 points; fluctuating mental state or drop in GCS ≥ 1 point; new clinical symptoms not attributable to the initial stroke lesion |
| Sheikh, 2020 [51] | cEEG, 21 disc electrodes, International 10–20 system | Admission day 1–3 | ≥6 h | Based on clinical indication |
| Tatum, 2022 [50] | Standard configurations apply the International 10–20 system in common bipolar and referential montages for clinical EEG. A minimum of 16 channels for diagnostic long-term VEEG monitoring. Consensus endorsed using more than the 21 electrodes of the International 10–20 system of electrode placement. | Not reported | Duration will vary relative to the indication for performance and number of seizures and events captured | Long-term VEEG monitoring should be used to differentiate between epileptic and non-epileptic events in patients where the diagnosis is in question |
| Vespa, 2003 [48] and 2005 [31] | 14 channel EEG. At bedside with monitor for nurse to observe. Physician trained in EEG interpretation review EEG at least three times per day and when nurse identifies suspicious activity. Seizures were detected in one of three ways: on-line identification of seizures by the neuro-ICU nurse or neuro-intensivist, by the total power trend seizure detection method, or by detection during regularly scheduled EEG segment review. | Earliest opportunity after admission to ICU | 5 to 7 days | If resource are limited, intracerebral haemorrhage should have priority over ischaemic stroke due to higher risk Lack of clinical seizure activity not an indication to avoid EEG |
| Wang, 2021 [32] | VEEG monitoring | Four days post stroke | Not reported | With EEG monitoring. Initiated due to persistent coma after observed seizure and continued until EEG abnormalities had disappeared. |
| Yerram, 2019 [39] | Continuous VEEG, 21 electrodes, International 10–20 system | Not reported | Not reported | Based on indication by the physician |
| Zelano, 2020 [2] | cEEG monitoring | As soon as possible when non-convulsive seizures suspected | At least 24 h recommended. ≥48 h if comatose, has periodic discharges or sedated | Persistently abnormal mental status following clinically diagnosed seizures or generalised convulsive status epilepticus. Unexplained or fluctuant altered mental status. Clinical paroxysmal events suspected to be seizures. Periodic discharges on routine or emergent EEG. |
| Periodic EEG | ||||
| Algin, 2022 [24] | Portable bedside EEG | In the early period | Not reported | Presence of focal clonic seizures and following day increased drowsiness, meaningless gaze, and reduced speech |
| Ba, 2021 [33] | Not reported | Not reported | Not reported | EEG used in case of atypical manifestation, no systematic EEG |
| Beghi, 2011 [42] | Not reported | At hospitalisation | Within first 7 days | When indicated by the caring physician, according to local practice |
| De Reuck, 2009 [27] | Not reported | Not reported | Not reported | EEG performed as soon as possible after ictal event |
| Elmali, 2021 [28] | Emergency EEG | Not reported | Not reported | Episodes of fluctuating confusion suggestive of seizures |
| Green, 2021 [23] | Not reported | Not reported | Not reported | EEG for change in mental status or depressed level of consciousness out of proportion to the stroke |
| Kim, 2016 [36] | Standard EEG | Within 7 days of stroke onset | 20–30 min | Performed within 24–72 h of PSSi onset |
| Lasek-Bal, 2023 [46] | Standard protocol including hyperventilation and photo stimulation. Conducted at rest and supine. Galileo EEG–EP device with 21 electrodes, International 10–20 system. | Within first 72 h of admission | 20 min | Routinely performed on all eligible patients |
| Mecarelli, 2011 [47] | Micromed digital device, 14 disc electrodes, International 10–20 system | Within 24 h of admission | 30 min minimum | If status epilepticus detected, EEG continued to monitor pharmacological treatment. If first EEG showed abnormal epileptiform activity, series of EEGs were performed over the following days. EEG available weekdays only. |
| Tako, 2022 [38] | Not reported | Not reported | Not reported | Performed according to the indication of the attending physician |
| Clinician Observation | ||||
| Algin, 2022 [24] | Seizure suspected after increased drowsiness, meaningless gaze, and reduced speech. No seizure observed. | Not reported | Not reported | Not reported |
| Bautista, 2020 [25] | Monitor airway, level of consciousness, eye deviation, gaze, pupil size, urinary incontinence, body movements, and motor function. Responsiveness, awareness, motor function, and language should be assessed in ictal and postictal phase. Record onset and duration of seizure. | Not reported | During first 5 min observe continuously. Observe in ictal and post ictal phases until patient back at their baseline | Not reported |
| Beghi, 2011 [42] | Direct observation by medical staff at time of admission or from reliable witness history (e.g., ambulance personnel) | At hospitalisation | Within the first 7 days | Not reported |
| Daniele, 1996 [34] | Diagnosis of seizure based on observation and description by experienced staff from our department. | Not reported | Not reported | Not applicable |
| Green, 2021 [23] | Nurses should have a standardised approach for recognition of seizures. Assessment and documentation of the seizure | Not reported | Not reported | Not reported |
| Jung, 2012 [35] | Patient or witness reporting, or both. Seizure type and time of occurrence. | At stroke onset | Within first 24 h | Seizures in basilar artery occlusion were only assumed if further signs like unequivocal clonic movements, tongue bite, or incontinence were observed |
| Kim, 2016 [36] | Seizure was distinguished as being partial or generalized, according to the 2010 ILAE criteria | Within first 7 days | Within first 7 days | Simple loss of consciousness or short-lasting episodes of mental confusion were not considered for epileptic seizure diagnosis |
| Sarfo, 2021 [41] | Seizures diagnosed clinically and recorded in medical notes or witness reporting | At hospitalisation | Within first 7 days of stroke onset | Classified as acute symptomatic seizures according to ILAE criteria, and focal or generalised |
| Tako, 2022 [38] | Acute symptomatic seizures diagnosed by clinically observed ictal stigmas | Not reported | Within first 7 days of stroke onset | Classified as acute symptomatic seizures according to ILAE criteria, further seizure classification dependent on EEG findings |
| VEEG | ||||
| Belcastro, 2014 [43] | Video recording alongside continuous EEG | Within the first week: promptly in those with clear or suspected seizure, or routinely at any time | >6 h | Routinely in all patients within the first week of admission |
| Bentes, 2017 [44] | Video recording alongside continuous EEG | First 72 h after stroke | Maximum 60 min | All patients received routine EEG |
| Bentes, 2018 [13] | Video recording alongside continuous EEG | As early as possible in the first 72 h, daily for the first 7 days | Maximum 60 min | All patients received video EEG for the first 7 days. Additional video EEG if unexplained neurological worsening. |
| Elmali, 2021 [28] | Long-term video EEG | Within first days of admission | 48 h | After seizures observed in the emergency department and emergency EEG performed |
| Mader, 2020 [30] | Video recording alongside EEG | At stroke onset, duration not reported | Not reported | Continuous with EEG |
| Onder, 2017 [40] | Video recording alongside cEEG | Not reported | 1–38 days (mean 7.9) | With cEEG |
| Tatum, 2022 [50] | One camera is usual practice. Use more than 21 electrodes of the International 10–20 system of electrode placement. Standard digital audio–video data is acquired, provided by standard industry codecs. Specification of time synchronisation between video and EEG has been standardised in the DICOM format and MED format. 24 h VEEG requires up to 30 GB memory. | Not reported | Duration will vary relative to the indication for performance and number of seizures and events captured | Long-term VEEG monitoring should be used to differentiate between epileptic and non-epileptic events in patients where the diagnosis is in question |
| Wang, 2021 [32] | VEEG monitoring | Four days post stroke | Not reported | With EEG monitoring. Initiated due to persistent coma after observed seizure. |
| Yerram, 2019 [39] | Video recording alongside cEEG | Not reported | Not reported | With cEEG |
| Zelano, 2020 [2] | Concurrent video recording is strongly recommended as supplementary to neurologic examination to evaluate clinical behaviour and to assess whether electrographic seizures are associated with clinical changes | In the early phase | Not reported | With cEEG |
| Family Witness | ||||
| Beghi, 2011 [42] | As well as other detection methods, diagnosis of seizure was based on history according to reliable witness description | At hospitalisation | Within the first 7 days | Not appliable |
| Daniele, 1996 [34] | The diagnosis of epileptic seizure was performed by observation, including description by relatives of the patients who witnessed it | Not reported | Not reported | Not applicable |
| Jung, 2012 [35] | Information obtained from the patient or persons who observed the seizure, or both | From stroke onset | Until 3 month post-stroke follow-up | Not applicable |
| Mader, 2020 [30] | Relative noted 30 s episode of bilateral leg jerking 30 min after drop in level of consciousness | 30 min after change in neurology indicating acute stroke | Not applicable | Not applicable |
| Sarfo, 2020 [41] | As well as clinical observation, often a family member witnessed symptoms at time of presentation | On hospitalisation | Within the first 7 days | Not applicable |
| Indications for EEG (References) * | Indication Frequency Based on Number of Papers and Type of Publication |
|---|---|
| Routine at prescribed timepoints post-stroke [13,30,31,43,44,45,46,47,48] | 9 (7 research, 1 case report, 1 opinion paper) |
| Depression in conscious level or coma [2,23,24,26,29,32,40] | 7 (3 opinion papers, 1 case report, 1 guideline paper, 1 research) |
| After direct observation of seizure [24,25,27,33,36,42] | 6 (3 research, 2 opinion papers, 1 case report) |
| Fluctuating confusion or unexplained behavioural changes [2,23,28,49] | 4 (1 case report, 1 opinion paper, 2 guideline papers) |
| Indicated by medical staff [38,39,42] | 3 (3 research papers) |
| Atypical seizure manifestation [33] | 1 (1 research paper) |
| Paroxysmal events suspected to be seizures [2] | 1 (1 opinion paper) |
| Neurological deterioration [37] | 1 (1 research paper) |
| Condition specific [50] | 1 (1 guideline paper) |
| * Studies excluded as EEG not referred to in paper [34,35,41] | 3 (3 research papers) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, C.; Emsley, H.C.A.; Lightbody, C.E.; Clegg, A.; Harris, C.; Harrison, J.; Wall, J.; Davidson, C.E.; Watkins, C.L. Clinical Methods Supporting Initial Recognition of Early Post-Stroke Seizures: A Systematic Scoping Review. Neurol. Int. 2025, 17, 159. https://doi.org/10.3390/neurolint17100159
Gordon C, Emsley HCA, Lightbody CE, Clegg A, Harris C, Harrison J, Wall J, Davidson CE, Watkins CL. Clinical Methods Supporting Initial Recognition of Early Post-Stroke Seizures: A Systematic Scoping Review. Neurology International. 2025; 17(10):159. https://doi.org/10.3390/neurolint17100159
Chicago/Turabian StyleGordon, Clare, Hedley C. A. Emsley, Catherine Elizabeth Lightbody, Andrew Clegg, Catherine Harris, Joanna Harrison, Jasmine Wall, Catherine E. Davidson, and Caroline L. Watkins. 2025. "Clinical Methods Supporting Initial Recognition of Early Post-Stroke Seizures: A Systematic Scoping Review" Neurology International 17, no. 10: 159. https://doi.org/10.3390/neurolint17100159
APA StyleGordon, C., Emsley, H. C. A., Lightbody, C. E., Clegg, A., Harris, C., Harrison, J., Wall, J., Davidson, C. E., & Watkins, C. L. (2025). Clinical Methods Supporting Initial Recognition of Early Post-Stroke Seizures: A Systematic Scoping Review. Neurology International, 17(10), 159. https://doi.org/10.3390/neurolint17100159






