PRISMA Systematic Review of Electroencephalographic (EEG) Microstates as Biomarkers: Secondary Findings in Memory Functions
Abstract
1. Introduction
2. Methodology
2.1. Microstate Analysis
2.2. Functional Associations of Microstates
2.3. Review of Research Articles
3. Results
3.1. Microstates A and B
3.2. Microstate C
3.3. Microstate D
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ADHD | Attention Deficit Hyperactivity Disorder |
| ASR | Artifact Subspace Reconstruction |
| BART | Balloon Analogue Risk Task |
| BPRS | Brief Psychiatric Rating Scale |
| CDSS | Calgary Depression Scale for Schizophrenia |
| ESRD | End-Stage Renal Disease |
| FASTER | Fully Automated Statistical Thresholding for eeg artifact Rejection |
| FEP | First Episode Psychosis |
| GFP | Global Field Power |
| GMD | Global Map Dissimilarity |
| HC | Healthy Controls |
| ICA | Independent Component Analysis |
| MCCB | MATRICS Cognitive Consensus Battery |
| MCI | Mild Cognitive Impairment |
| MIS | Malnutrition-Inflammation Score |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| PANSS | Positive and Negative Syndrome Scale |
| PD | Parkinson’s Disease |
| qEEG | Quantitative Electroencephalography |
| RAVLT | Rey Auditory Verbal Learning Test |
| SARA | Statistical Artifact Rejection Algorithm |
| SIPS | Structured Interview for Prodromal Syndromes |
| SNR | Signal-to-noise ratio |
| UHR-NT | Ultra High-Risk Non-Transition |
| UHR-T | Ultra High-Risk Transition |
| UPDRS-III | Uniform Parkinson’s Disease Rating Scale-iii |
References
- Niedermeyer, E.; Da Silva, F.H.L. Electroencephalography; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Ropper, A.H.; Samuels, M.A.; Klein, J.; Prasad, S. Adams and Victor’s Principles of Neurology, 12th ed.; McGraw-Hill Education: Columbus, OH, USA, 2023. [Google Scholar]
- Yamada, T.; Meng, E. Pruebas Neurofisiológicas Clínicas. Electroencefalografía, 2nd ed.; LWW: Philadelphia, PA, USA, 2020. [Google Scholar]
- Kandel, E.R. Principles of Neural Science, 5th ed.; McGraw Hill Professional: Columbus, OH, USA, 2013. [Google Scholar]
- Afifi, A.K.; Bergman, R.A. Neuroanatomía Funcional: Texto y Atlas, 3rd ed.; McGraw-Hill Interamericana: Mexico City, Mexico, 2020. [Google Scholar]
- Gazzaniga, M.; Ivry, R.B.; Mangun, G.R. Cognitive Neuroscience, 5th ed.; W.W. Norton & Company: New York, NY, USA, 2018. [Google Scholar]
- Sporns, O. Brain networks. In Networks of the Brain; The MIT Press: Cambridge, MA, USA, 2010; pp. 31–50. [Google Scholar] [CrossRef]
- Michel, C.M.; Koenig, T. EEG Microstates as a Tool for Studying the Temporal Dynamics of Whole-Brain Neuronal Networks: A Review. Neuroimage 2018, 180, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Strik, W.K.; Henggeler, B.; Koenig, T.; Koukkou, M. Brain Electric Microstates and Momentary Conscious Mind States as Building Blocks of Spontaneous Thinking: I. Visual Imagery and Abstract Thoughts. Int. J. Psychophysiol. 1998, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bréchet, L.; Brunet, D.; Birot, G.; Gruetter, R.; Michel, C.M.; Jorge, J. Capturing the Spatiotemporal Dynamics of Self-Generated, Task-Initiated Thoughts with EEG and FMRI. Neuroimage 2019, 194, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Smailovic, U.; Koenig, T.; Laukka, E.J.; Kalpouzos, G.; Andersson, T.; Winblad, B.; Jelic, V. EEG Time Signature in Alzheimer´s Disease: Functional Brain Networks Falling Apart. Neuroimage Clin. 2019, 24, 102046. [Google Scholar] [CrossRef]
- Tarailis, P.; Koenig, T.; Michel, C.M.; Griškova-Bulanova, I. The Functional Aspects of Resting EEG Microstates: A Systematic Review. Brain Topogr. 2024, 37, 181–217. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in Resting-State EEG: Current Status and Future Direc-tions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Segmentation of Brain Electrical Activity into Microstates: Model Estima-tion and Validation. IEEE Trans. Biomed. Eng. 1995, 42, 658–665. [Google Scholar] [CrossRef]
- Koenig, T.; Prichep, L.; Lehmann, D.; Sosa, P.V.; Braeker, E.; Kleinlogel, H.; Isenhart, R.; John, E.R. Millisecond by Millisecond, Year by Year: Normative EEG Microstates and Developmental Stages. Neuroimage 2002, 16, 41–48. [Google Scholar] [CrossRef]
- Van De Ville, D.; Britz, J.; Michel, C.M. EEG Microstate Sequences in Healthy Humans at Rest Reveal Scale-Free Dynamics. Proc. Natl. Acad. Sci. USA 2010, 107, 18179–18184. [Google Scholar] [CrossRef]
- Milz, P.; Faber, P.L.; Lehmann, D.; Koenig, T.; Kochi, K.; Pascual-Marqui, R.D. The Functional Significance of EEG Mi-crostates—Associations with Modalities of Thinking. Neuroimage 2016, 125, 643–656. [Google Scholar] [CrossRef]
- Murray, M.M.; Brunet, D.; Michel, C.M. Topographic ERP Analyses: A Step-by-Step Tutorial Review. Brain. Topogr. 2008, 20, 249–264. [Google Scholar] [CrossRef]
- D’Croz-Baron, D.F.; Baker, M.; Michel, C.M.; Karp, T. EEG Microstates Analysis in Young Adults with Autism Spectrum Dis-order During Resting-State. Front. Hum. Neurosci. 2019, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Seitzman, B.A.; Gratton, C.; Marek, S.; Raut, R.V.; Dosenbach, N.U.F.; Schlaggar, B.L.; Petersen, S.E.; Greene, D.J. A Set of Func-tionally-Defined Brain Regions with Improved Representation of the Subcortex and Cerebellum. Neuroimage 2020, 206, 116290. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.J.; Debener, S.; Eichele, T.; Herrmann, C.S. Methods for Simultaneous EEG-FMRI: An Introductory Review. J. Neuro-Sci. 2012, 32, 6053–6060. [Google Scholar] [CrossRef] [PubMed]
- Tait, L.; Tamagnini, F.; Stothart, G.; Barvas, E.; Monaldini, C.; Frusciante, R.; Volpini, M.; Guttmann, S.; Coulthard, E.; Brown, J.T.; et al. EEG Microstate Complexity for Aiding Early Diagnosis of Alzheimer’s Disease. Sci. Rep. 2020, 10, 17627. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Li, H.-Z.; Wang, W.; Kuang, L. Changes in Oscillatory Patterns of Microstate Sequence in Patients with First-Episode Psychosis. Sci. Data 2024, 11, 38. [Google Scholar] [CrossRef]
- de Bock, R.; Mackintosh, A.J.; Maier, F.; Borgwardt, S.; Riecher-Rössler, A.; Andreou, C. EEG Microstates as Biomarker for Psychosis in Ultra-High-Risk Patients. Transl. Psychiatry 2020, 10, 300. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Khoo, S.Y.; Lai, W.H.; On, S.H.; On, Y.Y.; Adam, B.M.; Law, W.C.; Ng, B.H.S.; Fong, A.Y.Y.; Anselm, S.T. Resting-State Electroen-cephalography (EEG) Microstates of Healthy Individuals Following Mild Sleep Deprivation. Sci. Rep. 2024, 14, 16820. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Farzan, F. Reliability of Resting-State Microstate Features in Electroencephalography. PLoS ONE 2014, 9, e114163. [Google Scholar] [CrossRef]
- Custo, A.; Van De Ville, D.; Wells, W.M.; Tomescu, M.I.; Brunet, D.; Michel, C.M. Electroencephalographic Resting-State Net-works: Source Localization of Microstates. Brain. Connect. 2017, 7, 671–682. [Google Scholar] [CrossRef]
- Rieger, K.; Diaz Hernandez, L.; Baenninger, A.; Koenig, T. 15 Years of Microstate Research in Schizophrenia—Where Are We? A Meta-Anal. Front. Psychiatry 2016, 7, 22. [Google Scholar] [CrossRef]
- Chivu, A.; Pascal, S.A.; Damborská, A.; Tomescu, M.I. EEG Microstates in Mood and Anxiety Disorders: A Meta-Analysis. Brain. Topogr. 2024, 37, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Catrambone, V.; Valenza, G. Nervous-System-Wise Functional Estimation of Directed Brain–Heart Interplay Through Mi-crostate Occurrences. IEEE Trans. Biomed. Eng. 2023, 70, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Iftimovici, A.; Marchi, A.; Férat, V.; Pruvost-Robieux, E.; Guinard, E.; Morin, V.; Elandaloussi, Y.; D’Halluin, A.; Krebs, M.-O.; Chaumette, B.; et al. Electroencephalography Microstates Imbalance across the Spectrum of Early Psychosis, Autism, and Mood Disorders. Eur. Psychiatry 2023, 66, e41. [Google Scholar] [CrossRef]
- Yuan, H.; Zotev, V.; Phillips, R.; Drevets, W.C.; Bodurka, J. Spatiotemporal Dynamics of the Brain at Rest—Exploring EEG Mi-crostates as Electrophysiological Signatures of BOLD Resting State Networks. Neuroimage 2012, 60, 2062–2072. [Google Scholar] [CrossRef]
- Zanesco, A.P.; Denkova, E.; Jha, A.P. Associations between Self-Reported Spontaneous Thought and Temporal Sequences of EEG Microstates. Brain Cogn. 2021, 150, 105696. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5, (updated August 2024); Cochrane: London, UK, 2024. [Google Scholar]
- Shor, O.; Yaniv-Rosenfeld, A.; Valevski, A.; Weizman, A.; Khrennikov, A.; Benninger, F. EEG-Based Spatio-Temporal Relation Signatures for the Diagnosis of Depression and Schizophrenia. Sci. Rep. 2023, 13, 776. [Google Scholar] [CrossRef]
- Chen, Y.; Fazli, S.; Wallraven, C. An EEG Dataset of Neural Signatures in a Competitive Two-Player Game Encouraging De-ceptive Behavior. Sci. Data 2024, 11, 389. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Z.; Wang, J.; Li, Z.; Shen, X.; Han, X.; Bai, L.; Liu, C.; Zhu, X. Temporal and Spatial Variability of Dynamic Mi-crostate Brain Network in Early Parkinson’s Disease. NPJ Park. Dis. 2023, 9, 57. [Google Scholar] [CrossRef]
- Jatupornpoonsub, T.; Thimachai, P.; Supasyndh, O.; Wongsawat, Y. EEG Delta/Theta Ratio and Microstate Analysis Origi-nating Novel Biomarkers for Malnutrition-Inflammation Complex Syndrome in ESRD Patients. Front. Hum. Neurosci. 2022, 15, 795237. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, Q.; Wang, C.; Zhang, K.; Wang, C.; Zhang, J. Biomarkers for Prediction of Schizophrenia: Insights from Rest-ing-State EEG Microstates. IEEE Access 2020, 8, 213078–213093. [Google Scholar] [CrossRef]
- Soni, S.; Muthukrishnan, S.P.; Samanchi, R.; Sood, M.; Kaur, S.; Sharma, R. Pre-Trial and Pre-Response EEG Microstates in Schizophrenia: An Endophenotypic Marker. Behav. Brain Res. 2019, 371, 111964. [Google Scholar] [CrossRef] [PubMed]
- Bagdasarov, A.; Roberts, K.; Bréchet, L.; Brunet, D.; Michel, C.M.; Gaffrey, M.S. Spatiotemporal Dynamics of EEG Microstates in Four- to Eight-Year-Old Children: Age- and Sex-Related Effects. Dev. Cogn. Neurosci. 2022, 57, 101134. [Google Scholar] [CrossRef]
- Lewandowska, M.; Tołpa, K.; Rogala, J.; Piotrowski, T.; Dreszer, J. Multivariate Multiscale Entropy (MMSE) as a Tool for Understanding the Resting-State EEG Signal Dynamics: The Spatial Distribution and Sex/Gender-Related Differences. Behav. Brain Funct. 2023, 19, 18. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Xu, W.; Jiang, C. Multi-Scale Convolutional Attention and Riemannian Geometry Network for EEG-Based Motor Imagery Classification. IEEE Access 2024, 12, 79731–79740. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, Z.; Li, L. Deep Learning-Based Multi-Feature Auxiliary Diagnosis Method for Early Detection of Ischemic Stroke. Trait. Du Signal 2023, 40, 433–443. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, C.; Zhong, J.; Xiong, J.; Quan, Y.; Duan, J.; Zhang, Y.; Zhou, Z. SaE-GBLS: An Effective Self-Adaptive Evolution-ary Optimized Graph-Broad Model for EEG-Based Automatic Epileptic Seizure Detection. Front. Comput. Neurosci. 2024, 18, 1379368. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Arechavala, R.J.; Butler, R.; Nolty, A.; Kang, J.J.; Shimojo, S.; Wu, D.-A.; Fonteh, A.N.; Kleinman, M.T.; Kloner, R.A.; et al. Disrupted Brain Functional Connectivity as Early Signature in Cognitively Healthy Individuals with Pathological CSF Amyloid/Tau. Commun. Biol. 2024, 7, 1037. [Google Scholar] [CrossRef]
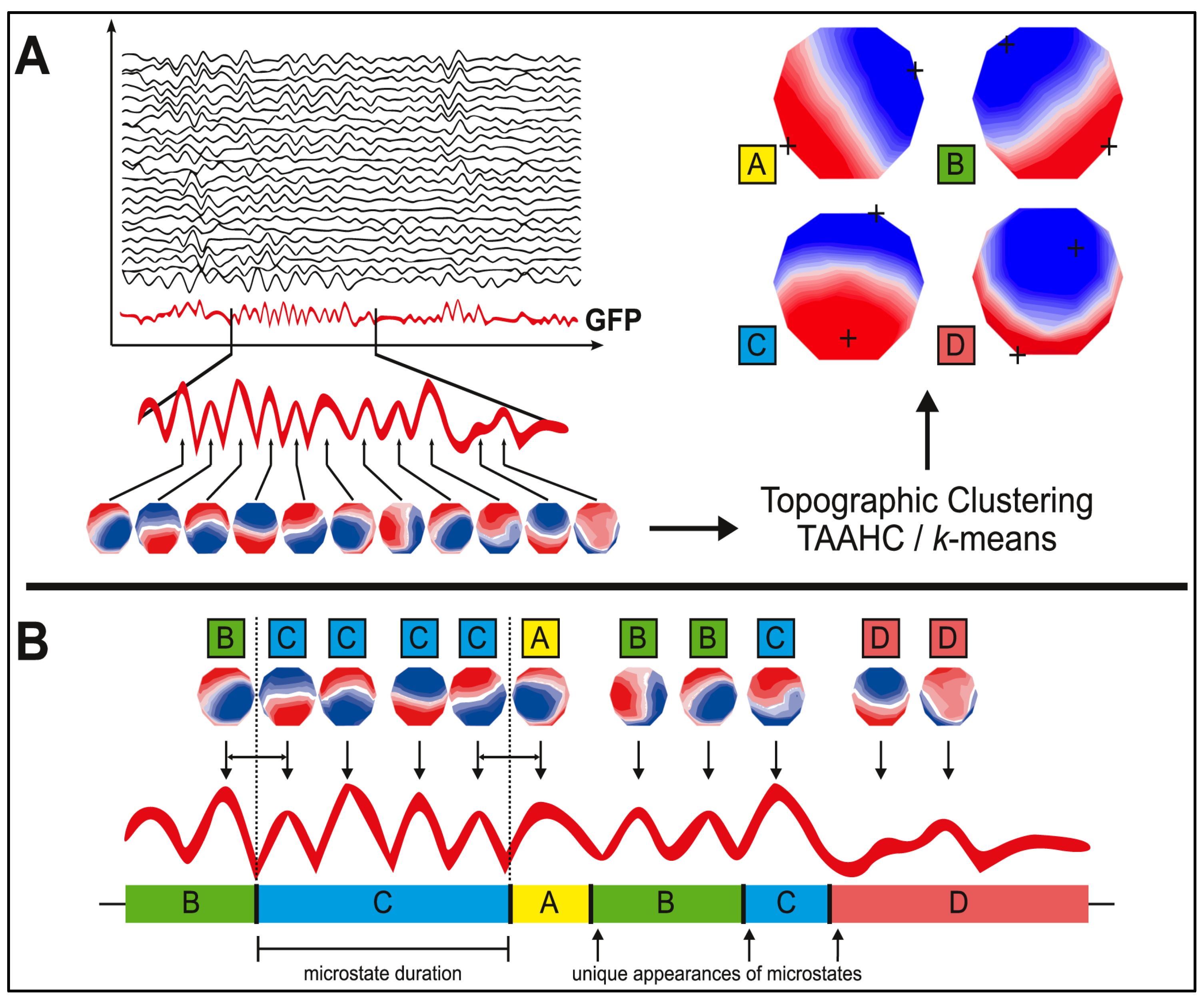
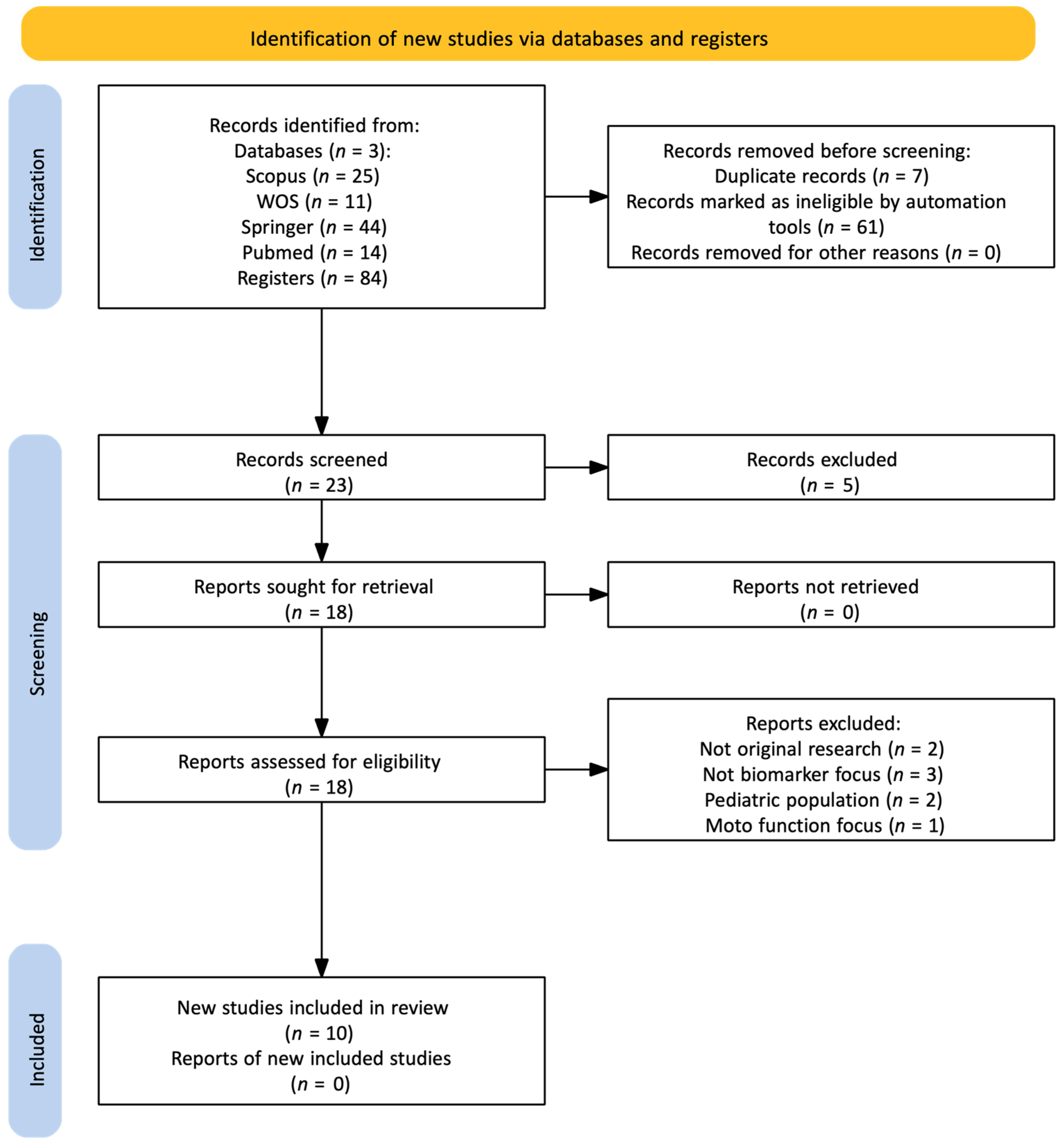
| Title | Age | Gender | Sample Size |
|---|---|---|---|
| Resting-state electroencephalography (EEG) microstates of healthy individuals following mild sleep deprivation [27] | 21–40 years | 66.7% women, 33.3% men | 24 participants |
| EEG-based spatio-temporal relation signatures for the diagnosis of depression and schizophrenia [38] | 18–91 years (media: 52.4 ± 18.7) | 59.4% women | 166 participants (96 controls, 28 with depression, 42 with schizophrenia) |
| EEG microstate complexity for aiding early diagnosis of Alzheimer’s disease [23] | 18–91 years | 52% men, 48% women | 79 participants (21 AD, 25 MCI, 26 controls, 7 MCI) |
| An EEG dataset of neural signatures in a competitive two-player game encouraging deceptive behavior [39] | 19–34 years (media: 25 ± 4.34) | 50% men, 50% women | 24 participants |
| Changes in oscillatory patterns of microstate sequence in patients with first-episode psychosis [24] | 18–34 years (media: 22.8 ± 4.7) | 70% men | 142 participants (81 FEP, 61 controls) |
| Temporal and spatial variability of dynamic microstate brain network in early Parkinson’s disease [40] | 62.4 ± 6.3 años (PD) 63.8 ± 5.5 años (HC) | 31% hombres, 69% mujeres (PD) 50% hombres, 50% mujeres (HC) | 51 participantes (29 PD, 22 HC) |
| EEG microstates as biomarker for psychosis in ultra-high-risk patients [25] | 22.39 ± 5.24 years (HC), 25.32 ± 8.14 years (UHR-NT), 25.80 ± 7.20 years (UHR-T), 28.68 ± 7.64 years (FEP) 4o | 12:13 (HC) 26:8 (UHR-NT) 11:9 (UHR-T) 19:10 (FEP) | 108 participants (29 FEP, 20 UHR-T, 34 UHR-NT, 25 HC) |
| EEG Delta/Theta Ratio and microstate analysis originating novel biomarkers for malnutrition-inflammation complex syndrome in ESRD patients [41] | 57.57 ± 14.88 years (mis ≤ 5), 59.13 ± 11.77 years (mis > 5) | 69.6% women (mis ≤ 5), 65.2% women (mis > 5) | 46 participants (23 mis ≤ 5, 23 mis > 5) |
| Biomarkers for prediction of schizophrenia: insights from resting-state EEG microstates [42] | 13–40 years | Not especified | 65 participants (20 FESZ, 19 UHR, 12 h, 14 HC) |
| Pre-trial and pre-response EEG microstates in schizophrenia: an endophenotypic marker [43] | 21–40 years | 66.7% women, 33.3% men | 24 participants |
| Conventions Age: HC: Healthy Controls UHR-NT: Ultra High-Risk Non-Transition UHR-T: Ultra High-Risk Transition. FEP: First Episode Psychosis. Gender: % women, % men: percentage of female and male participants in the study. | Medical Story AD: Alzheimer’s Disease. MCI: Mild Cognitive Impairment. ESRD: End-Stage Renal Disease. PD: Parkinson’s Disease. Source: own elaboration | ||
| Title | Preprocessing Steps | EEG Microstate Evaluation Methods | Memory Evaluation Methods | Summary of Results and Conclusions |
|---|---|---|---|---|
| Resting-state EEG microstates of healthy individuals following mild sleep deprivation [27] | Artifact removal (SARA), band-pass 2–17 Hz, average reference | Microstate analysis, 19 channels, 6 min resting EEG (10–20 system) | Karolinska Sleepiness Scale (Malay) | Mild sleep deprivation (>18 h) increased duration, coverage, and occurrence of microstate C and occurrence of D. C associated with DMN (precuneus, posterior cingulate) and D with attentional networks. Potential early markers of sleep deprivation effects. |
| EEG-based spatio-temporal relation signatures for the diagnosis of depression and schizophrenia [38] | Artifact removal (FASTER), high-pass 1 Hz, 50 Hz notch, interpolation of noisy channels, ICA | Dendrogram analysis, 19 channels, 500 s resting EEG (10–20 system) | Not specified | Did not use classical microstates; proposed dendrogram signature algorithm (PUDHS) differentiating depression, schizophrenia, and controls with high accuracy (AUC > 0.99). Objective tool for differential diagnosis. |
| EEG microstate complexity for aiding early diagnosis of Alzheimer’s disease [23] | Artifact removal, band-pass 1–40 Hz, interpolation of noisy channels, ICA | Microstate analysis, 64/19 channels, 20 s resting EEG (10–20 system) | MMSE, RAVLT | Microstate D altered in AD (reduced parietal activation). Lower Lempel-Ziv complexity and longer mean duration of microstates. EEG classifier achieved >80% sensitivity/specificity and predicted MCI conversion to AD. |
| An EEG dataset of neural signatures in a competitive two-player game encouraging deceptive behavior [39] | Downsampling 100 Hz, filters 1/49 Hz, ASR, interpolation, average reference, ICA | Microstate analysis, 31 channels, ERP-based (3500 ms player, 1200 ms observer) | Balloon Analogue Risk Task (BART) | Microstates applied to ERPs: differentiated instructed vs. spontaneous deception and player-observer outcomes. Increased GFP linked to P300. Useful for studying decision-making and deception. |
| Changes in oscillatory patterns of microstate sequence in first-episode psychosis [24] | Artifact removal (ICA), band-pass 1–80 Hz, 60 Hz notch, downsampling 100 Hz, average reference | Microstate analysis, 49 channels, 5 min resting EEG (10–20 system) | BPRS | Separate templates showed shorter A and D, and more frequent B and C in FEP. Introduced Chaos Game Representation (CGR), improving classification (AUC 0.61 vs. 0.46). |
| Temporal and spatial variability of dynamic microstate brain network in early Parkinson’s disease [40] | Artifact removal (ICA), band-pass 2–20 Hz, interpolation of noisy channels, ICA | Microstate analysis, 19 channels, 15–20 min resting EEG (10–20 system) | UPDRS-III, MoCA | Higher temporal variability of B and lower of C in early PD. Frontal variability (C) negatively correlated with MoCA. Spatial variability (D) linked to cognitive and motor symptoms. MCN-SVM classifier reached AUC 0.99. |
| EEG microstates as biomarker for psychosis in ultra-high-risk patients [25] | Artifact removal (ICA), band-pass 0.5–70 Hz, 50 Hz notch, interpolation, ICA | Microstate analysis, 19 channels, 8 min resting EEG (10–20 system) | BPRS | Microstate A ↑ in patients vs. controls; microstate B ↓ in FEP vs. UHR; microstate D ↓ in UHR-T vs. UHR-NT. A and B as state markers, D as trait marker predictive of psychosis transition. |
| EEG Delta/Theta ratio and microstate analysis in ESRD patients [41] | Artifact removal (ICA), band-pass 1–40 Hz, downsampling 128 Hz, Picard ICA | Microstate analysis, 19 channels, 6 min resting EEG (10–20 system) | MIS | ESRD patients with high MICS risk showed positive correlations with A/B, negative with C. Proposed MIC index combining A–C parameters, with 100% accuracy in discriminating high vs. low risk. |
| Biomarkers for prediction of schizophrenia: insights from resting-state EEG microstates [42] | Artifact removal (ICA), band-pass 1–80 Hz, 50 Hz notch, interpolation | Microstate analysis, 128 channels, 5 min resting EEG (10–20 system) | PANSS, CDSS, SIPS, MCCB | Six microstates (A–F) better explained data. Microstate D progressively decreased with schizophrenia severity. Random forest classifier with EEG + clinical tests reached 92% accuracy. |
| Pre-trial and pre-response EEG microstates in schizophrenia [43] | Band-pass 1–100 Hz, 50 Hz notch, artifact removal (ICA), average reference, downsampling 250 Hz | Microstate analysis, 128 channels, 50 ms pre-trial EEG, GFP map | Visuospatial working memory task | Map 1 (A-like) differentiated patients and controls (state marker); Map 4 (B-like) differentiated controls and relatives (trait marker). Generator localized in rIFG, key for inhibitory control and working memory. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas Osorio, F.A.; Ramirez Lopez, L.J.; Renza Torres, D. PRISMA Systematic Review of Electroencephalographic (EEG) Microstates as Biomarkers: Secondary Findings in Memory Functions. Neurol. Int. 2025, 17, 160. https://doi.org/10.3390/neurolint17100160
Casas Osorio FA, Ramirez Lopez LJ, Renza Torres D. PRISMA Systematic Review of Electroencephalographic (EEG) Microstates as Biomarkers: Secondary Findings in Memory Functions. Neurology International. 2025; 17(10):160. https://doi.org/10.3390/neurolint17100160
Chicago/Turabian StyleCasas Osorio, Fernan Alexis, Leonardo Juan Ramirez Lopez, and Diego Renza Torres. 2025. "PRISMA Systematic Review of Electroencephalographic (EEG) Microstates as Biomarkers: Secondary Findings in Memory Functions" Neurology International 17, no. 10: 160. https://doi.org/10.3390/neurolint17100160
APA StyleCasas Osorio, F. A., Ramirez Lopez, L. J., & Renza Torres, D. (2025). PRISMA Systematic Review of Electroencephalographic (EEG) Microstates as Biomarkers: Secondary Findings in Memory Functions. Neurology International, 17(10), 160. https://doi.org/10.3390/neurolint17100160








