Anti-Collapsin Response Mediator Protein 5(CV2/CRMP5) and Anti-Glutamic Acid Decarboxylase (GAD) Antibodies-Mediated Encephalopathy Mimicking Atypical Parkinsonism
Abstract
1. Introduction
2. Clinical Scenario
2.1. Case Description
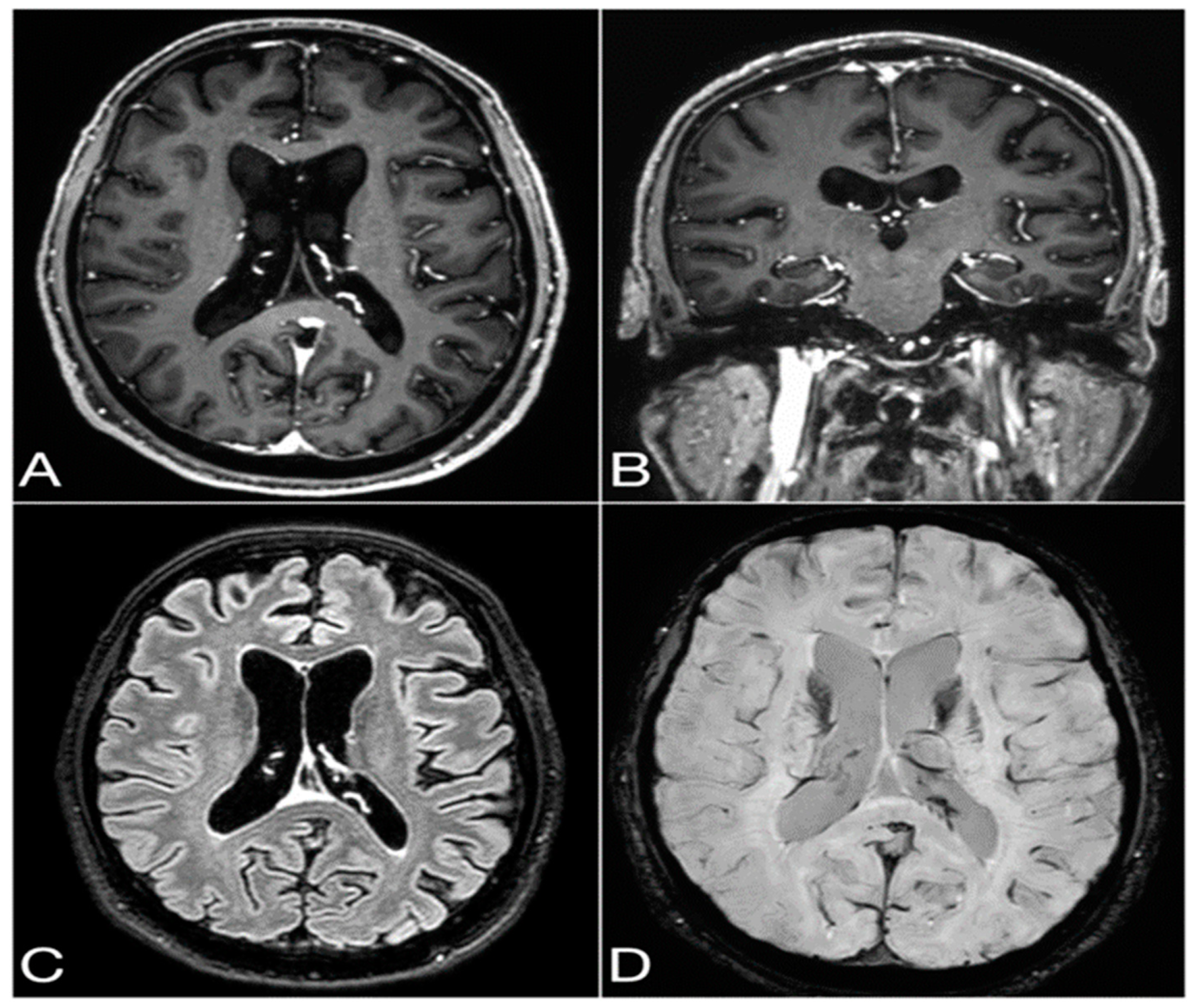
2.2. Diagnostic Findings
2.3. Treatment Performed
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graus, F.; Delattre, J.Y.; Antoine, J.E.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.J.G.M.; et al. Recommended Diagnostic Criteria for Paraneoplastic Neurological Syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Pittock, S.J.; Yoshikawa, H.; Ahlskog, J.E.; Tisch, S.H.; Benarroch, E.E.; Kryzer, T.J.; Lennon, V.A. Glutamic Acid Decarboxylase Autoimmunity with Brainstem, Extrapyramidal, and Spinal Cord Dysfunction. Mayo Clin. Proc. 2006, 81, 1207–1214. [Google Scholar] [CrossRef]
- Dalmau, J.; Graus, F.; Villarejo, A.; Posner, J.B.; Blumenthal, D.; Thiessen, B.; Saiz, A.; Meneses, P.; Rosenfeld, M.R. Clinical Analysis of Anti-Ma2-Associated Encephalitis. Brain A J. Neurol. 2004, 127 Pt 8, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kryzer, T.J.; Griesmann, G.E.; Kim, K.K.; Benarroch, E.E.; Lennon, V.A. CRMP-5 Neuronal Autoantibody: Marker of Lung Cancer and Thymoma-Related Autoimmunity. Ann. Neurol. 2001, 49, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ricken, G.; Schwaiger, C.; De Simoni, D.; Pichler, V.; Lang, J.; Glatter, S.; Macher, S.; Rommer, P.S.; Scholze, P.; Kubista, H.; et al. Detection Methods for Autoantibodies in Suspected Autoimmune Encephalitis. Front. Neurol. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.C.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1014. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Marsili, L.; Truong, D.D. Parkinsonism in Viral, Paraneoplastic, and Autoimmune Diseases. J. Neurol. Sci. 2022, 433, 120014. [Google Scholar] [CrossRef] [PubMed]
- Purks, J.; Samkoff, L. Rare Development of Anti-CRMP5 Parkinsonism and Worsening Ataxia While on Atezolizumab for SCLC: A Case Report (P4-5.031). Neurology 2023, 100 (Suppl. 2), 4940. [Google Scholar] [CrossRef]
- Tada, S.; Furuta, M.; Fukada, K.; Hirozawa, D.; Matsui, M.; Aoike, F.; Okuno, T.; Sawada, J.I.; Mochizuki, H.; Hazama, T. Severe Parkinsonism Associated with Anti-CRMP5 Antibody-Positive Paraneoplastic Neurological Syndrome and Abnormal Signal Intensity in the Bilateral Basal Ganglia. J. Neurol. Neurosurg. Psychiatry 2016, 87, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.M.; Lynch, T.; MacMahon, P.; Murray, B. Paraneoplastic Atypical Parkinsonism with Anti-CRMP5 Antibodies and Severe Caudate and Putaminal Hypometabolism on 18-Fluorodeoxyglucose Positron Emission Tomography of the Brain. Mov. Disord. Clin. Pract. 2017, 4, 263. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Y.; Lang, Y.; Wang, Y.H.; Shao, J.; Cui, L. Parkinsonism and Dysautonomia with Anti-CV2/CRMP5 Associated Paraneoplastic Neurological Syndromes Mimicking Multiple System Atrophy: A Case Report. BMC Neurol. 2021, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, H.; Xu, G.; Lin, Y. Anti-CV2 Autoimmune Encephalitis with Parkinson-Like Symptoms and Bilateral Leukoencephalopathy—A Case Report. Front. Neurol. 2019, 10, 489240. [Google Scholar] [CrossRef] [PubMed]
- Nabil, A.; Houyam, T.; Adil, B.; Jawad, O.; Ahmed, B. Severe Paraneoplastic Parkinsonism: A Rare Cause Revealing Breast Cancer. J. Clin. Neurol. 2017, 13, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.A.; Joyce, J.; Witek, N.; Afshari, M. GAD65 Antibody-Associated Neurologic Disease Presenting with Hemiparkinsonism at Onset. Neurol. Clin. Pract. 2021, 11, E904–E905. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, H.; Vernino, S. Progress in the management of paraneoplastic neurological disorders. Ther. Adv. Neurol. Disord. 2010, 3, 43–52. [Google Scholar] [CrossRef]
- Chiu, D.; Rhee, J.; Gonzalez Castro, L.N. Diagnosis and Treatment of Paraneoplastic Neurologic Syndromes. Antibodies 2023, 12, 50. [Google Scholar] [CrossRef] [PubMed]
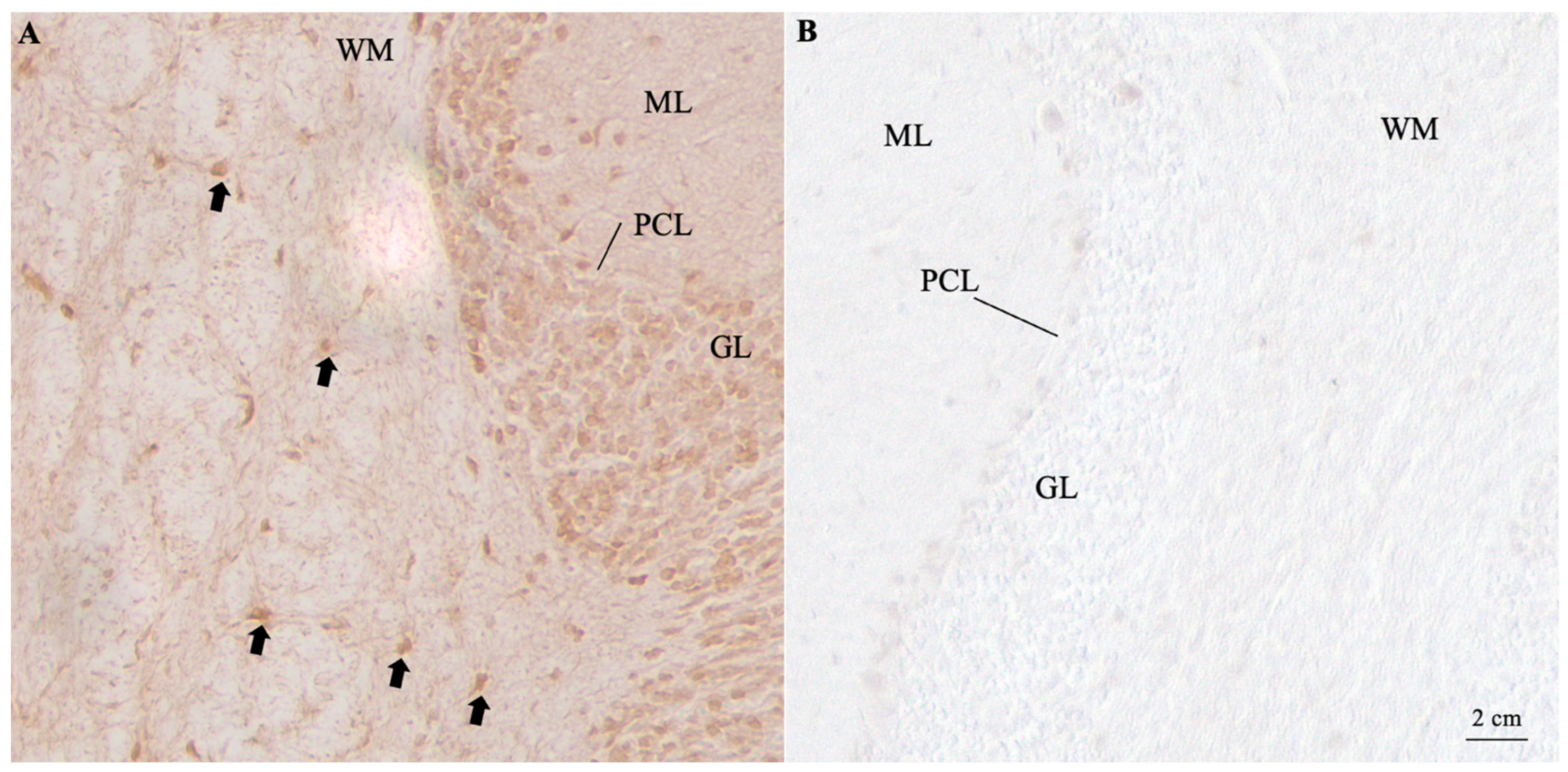
| N | Age | Sex | Antibody | Symptoms Associated with Parkinsonism | Tumor Found | References |
|---|---|---|---|---|---|---|
| 1 | 46 | F | CRMP5 | Ataxia | SCLC | [8] |
| 1 | 72 | M | CRMP5 | Ataxia, dysarthria, and constipation | SCLC | [8] |
| 1 | 72 | M | CRMP5 | Urinary urgency and hoarseness of voice | SCLC | [9] |
| 1 | 70 | M | CRMP5 | Ataxia, erectile dysfunction, severe dizziness while standing, hypotension, constipation, and urinary incontinence | No tumor found | [10] |
| 1 | 52 | F | CRMP5 | Dizziness, speech disfluency, hypophonia, loss of taste, and occasional urinary incontinence | Breast cancer | [11] |
| 3 | NA | M2, F1 | CRMP5 | NA | NA | [12] |
| 1 | 63 | F | CRMP5 | Gait and balance disturbances, pyramidal signs, dysarthria and inability to direct the gaze of the eyes downwards, slow saccades, and broken pursuits | Breast cancer | [4] |
| 1 | 60 | M | GAD65 | Ataxic dysarthria, dysmetria of the left arm and leg, truncal stiffness and instability, and a broad-based ataxic gait | No tumor found | [13] |
| 1 | 58 | M | GAD65 | Mild deficits in visuo-constructive and executive functions | No tumor found | [14] |
| 1 | 70 | M | CRMP5 and GAD65 | Ataxia, hypophonia, and cognitive decline | SCLC | Our case report |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirò, G.; Gastaldi, M.; Iacono, S.; Scaranzin, S.; Picciolo, V.; Arnao, V.; Ferrari, A.; Gagliardo, C.; D’Amelio, M. Anti-Collapsin Response Mediator Protein 5(CV2/CRMP5) and Anti-Glutamic Acid Decarboxylase (GAD) Antibodies-Mediated Encephalopathy Mimicking Atypical Parkinsonism. Neurol. Int. 2024, 16, 1849-1855. https://doi.org/10.3390/neurolint16060132
Schirò G, Gastaldi M, Iacono S, Scaranzin S, Picciolo V, Arnao V, Ferrari A, Gagliardo C, D’Amelio M. Anti-Collapsin Response Mediator Protein 5(CV2/CRMP5) and Anti-Glutamic Acid Decarboxylase (GAD) Antibodies-Mediated Encephalopathy Mimicking Atypical Parkinsonism. Neurology International. 2024; 16(6):1849-1855. https://doi.org/10.3390/neurolint16060132
Chicago/Turabian StyleSchirò, Giuseppe, Matteo Gastaldi, Salvatore Iacono, Silvia Scaranzin, Valentina Picciolo, Valentina Arnao, Anita Ferrari, Cesare Gagliardo, and Marco D’Amelio. 2024. "Anti-Collapsin Response Mediator Protein 5(CV2/CRMP5) and Anti-Glutamic Acid Decarboxylase (GAD) Antibodies-Mediated Encephalopathy Mimicking Atypical Parkinsonism" Neurology International 16, no. 6: 1849-1855. https://doi.org/10.3390/neurolint16060132
APA StyleSchirò, G., Gastaldi, M., Iacono, S., Scaranzin, S., Picciolo, V., Arnao, V., Ferrari, A., Gagliardo, C., & D’Amelio, M. (2024). Anti-Collapsin Response Mediator Protein 5(CV2/CRMP5) and Anti-Glutamic Acid Decarboxylase (GAD) Antibodies-Mediated Encephalopathy Mimicking Atypical Parkinsonism. Neurology International, 16(6), 1849-1855. https://doi.org/10.3390/neurolint16060132







