Contrasting Effects of an Atherogenic Diet and High-Protein/Unsaturated Fatty Acids Diet on the Accelerated Aging Mouse Model SAMP8 Phenotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mice Treatment
2.3. Behavioral Tests
2.4. Reagents and Antibodies
2.5. Measurement of ROS Content
2.6. Measurement of ATP Content
2.7. Immunoblotting
2.8. Statistical Analysis
3. Results
3.1. The Cocoa Diet Increases the Body Weights of SAMP8 Mice
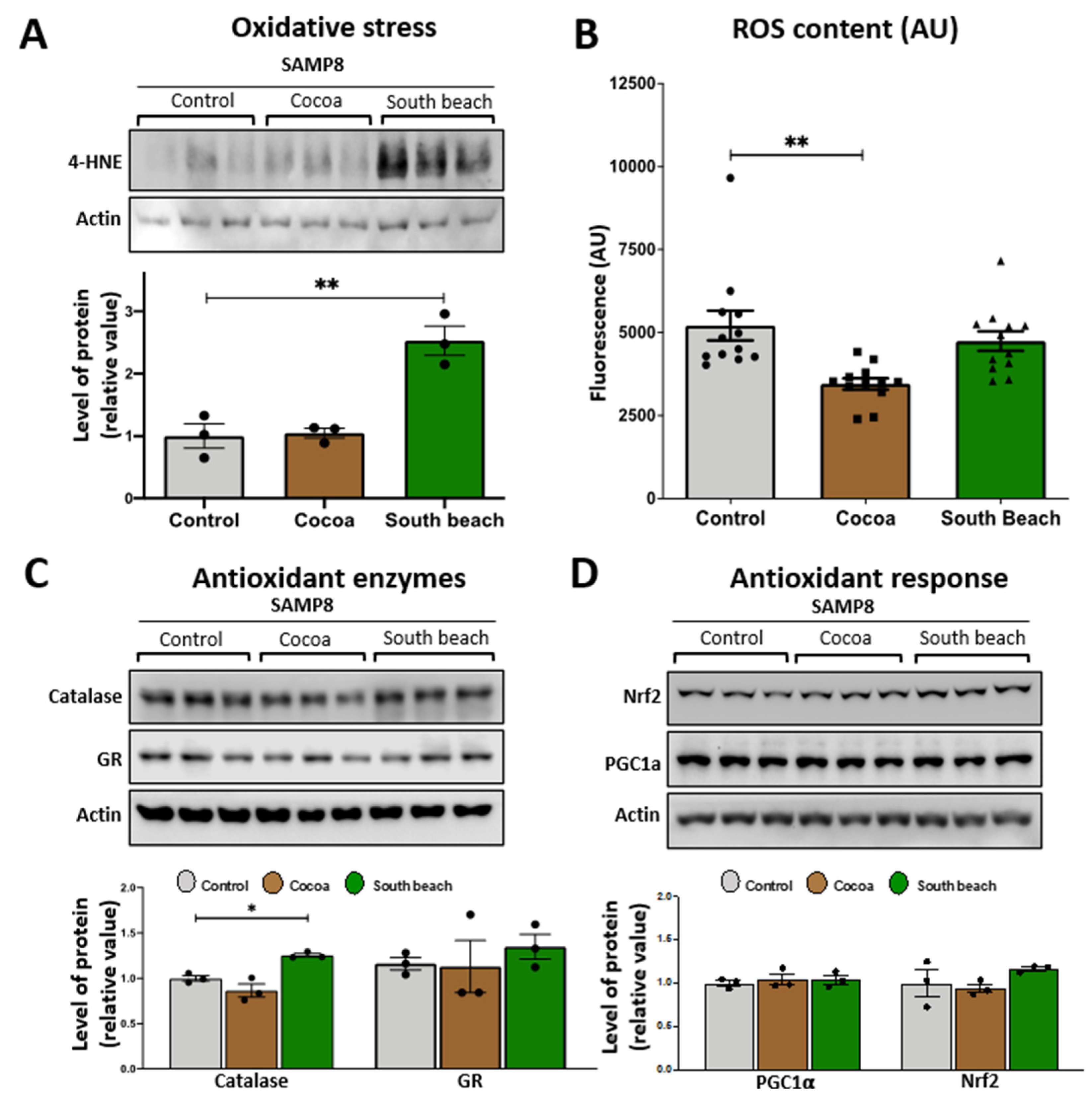
3.2. The South Beach Diet Has an Enhanced Level of Oxidative Damage
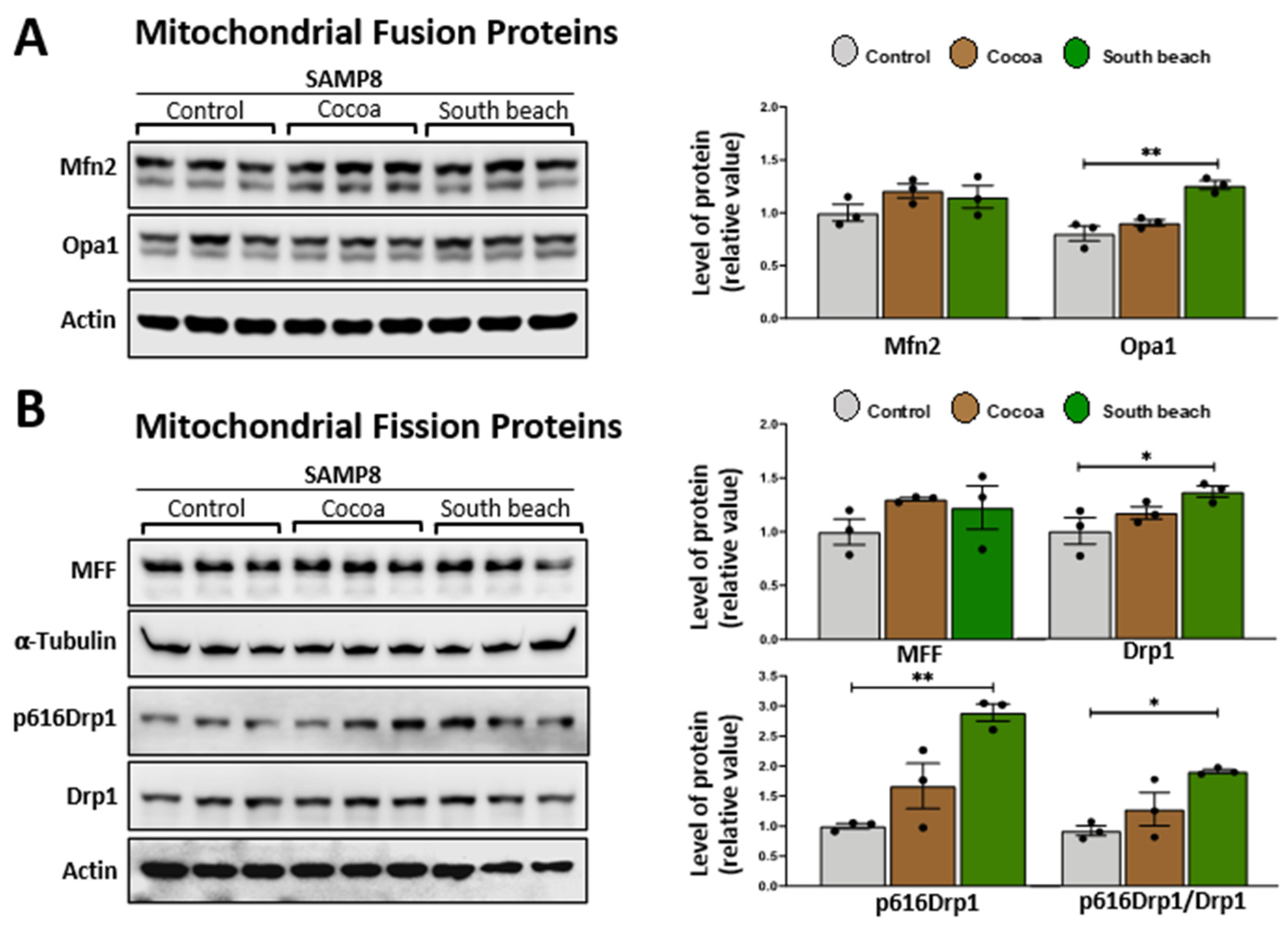
3.3. The South Beach Diet Increases the Levels of Proteins Involved in Mitochondrial Fusion and Fission, Suggesting Increased Mitochondrial Dynamic Processes in the Hippocampus of SAMP8 Mice
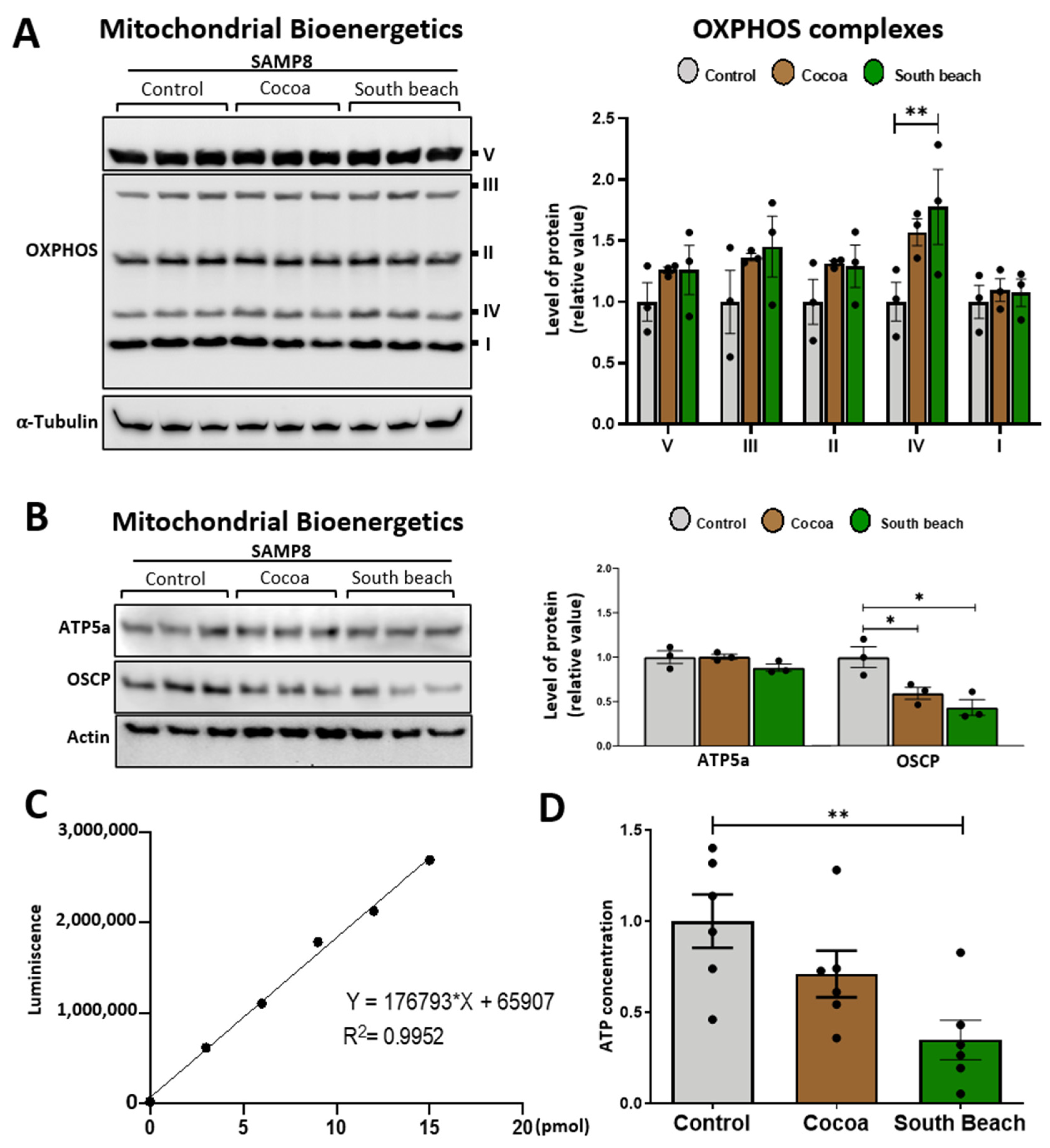
3.4. The Cocoa and South Beach Diets Reduce the Levels of an Essential Protein Implicated in Energy Production, but Only the South Beach Diet Reduced the Energy State in the Hippocampus of SAMP8 Mice
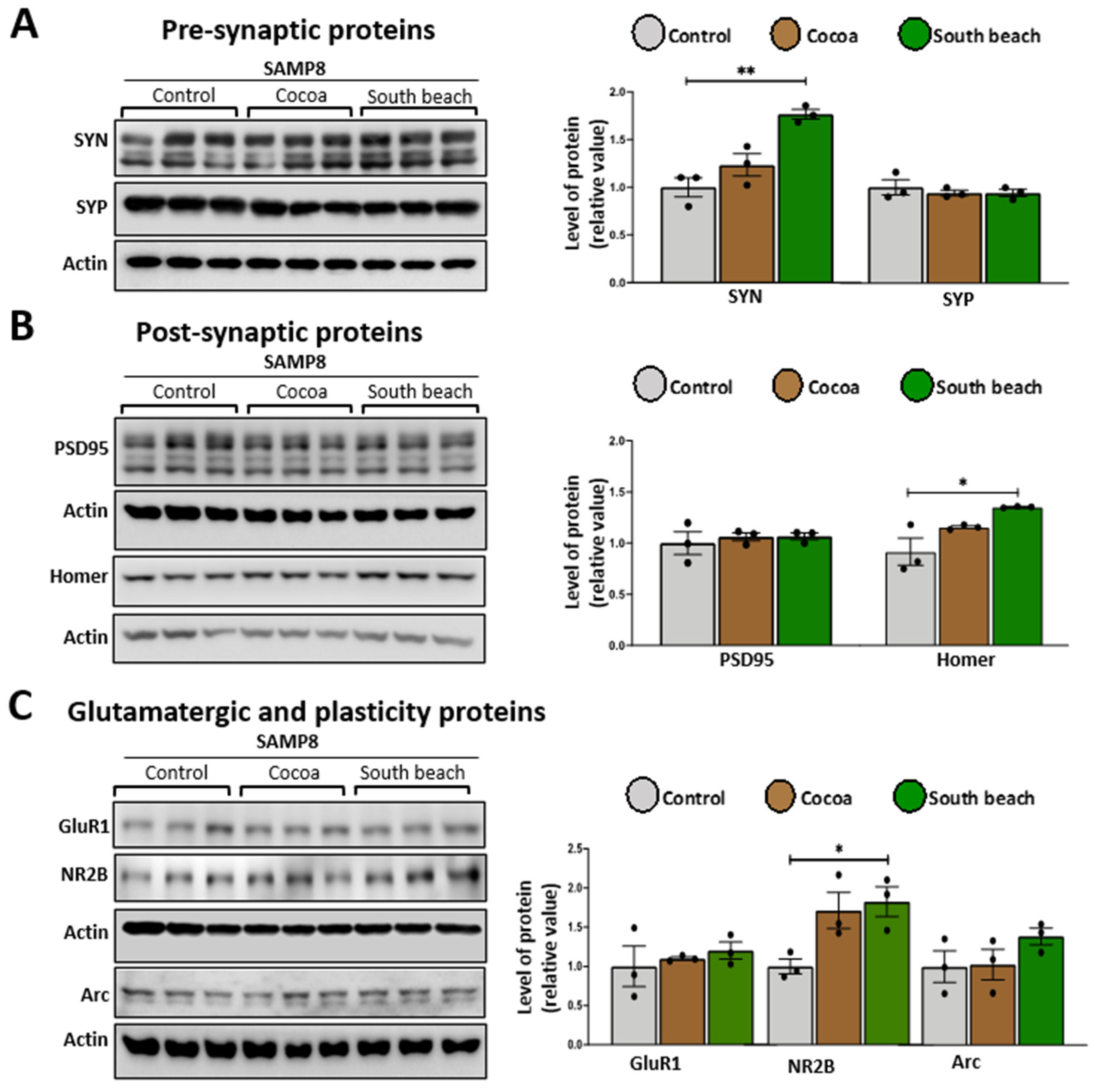
3.5. The South Beach Diet Increases the Levels of Synaptic Proteins, Suggesting Excitotoxicity in the Hippocampus of SAMP8 Mice
3.6. Cocoa and South Beach Diets Show Reduced Hippocampus-Dependent Spatial Acuity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trtica Majnaric, L.; Bosnic, Z.; Kurevija, T.; Wittlinger, T. Cardiovascular risk and aging: The need for a more comprehensive understanding. J. Geriatr. Cardiol. 2021, 18, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J. Appl. Physiol. (1985) 2005, 98, 203–210. [Google Scholar] [CrossRef]
- Granholm, A.C.; Bimonte-Nelson, H.A.; Moore, A.B.; Nelson, M.E.; Freeman, L.R.; Sambamurti, K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008, 14, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Stouffer, E.M.; Warninger, E.E.; Michener, P.N. A high-fat diet impairs learning that is dependent on the dorsal hippocampus but spares other forms of learning. Hippocampus 2015, 25, 1567–1576. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef]
- Freeman, L.R.; Haley-Zitlin, V.; Stevens, C.; Granholm, A.C. Diet-induced effects on neuronal and glial elements in the middle-aged rat hippocampus. Nutr. Neurosci. 2011, 14, 32–44. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Laricchiuta, D.; Foti, F.; Ronci, M.; Rossi, C.; Neri, C.; Spalletta, G.; Caltagirone, C.; et al. n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front. Aging Neurosci. 2014, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstadter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Floel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 2014, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef]
- Andreo-Lopez, M.C.; Contreras-Bolivar, V.; Munoz-Torres, M.; Garcia-Fontana, B.; Garcia-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef]
- Mendez, M.A.; Newman, A.B. Can a Mediterranean Diet Pattern Slow Aging? J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 315–317. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; D’Antona, G.; Nisoli, E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging 2011, 3, 464–478. [Google Scholar] [CrossRef]
- Bajracharya, R.; Youngson, N.A.; Ballard, J.W.O. Dietary Macronutrient Management to Treat Mitochondrial Dysfunction in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1850. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.; Takechi, R.; Nesbit, M.; Pallebage-Gamarallage, M.M.; Lam, V.; Mamo, J.C.L. The differential effects of fatty acids on enterocytic abundance of amyloid-beta. Lipids Health Dis. 2019, 18, 209. [Google Scholar] [CrossRef]
- Polley, K.R.; Miller, M.K.; Johnson, M.; Vaughan, R.; Paton, C.M.; Cooper, J.A. Metabolic responses to high-fat diets rich in MUFA v. PUFA. Br. J. Nutr. 2018, 120, 13–22. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.B.; Pulliam, C.F.; Patel, A.; Del Piero, F.; Watanabe, T.T.N.; Wankhade, U.D.; Shankar, K.; Hicks, C.; Ronis, M.J. Liver tumorigenesis is promoted by a high saturated fat diet specifically in male mice and is associated with hepatic expression of the proto-oncogene Agap2 and enrichment of the intestinal microbiome with Coprococcus. Carcinogenesis 2019, 40, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Liisberg, U.; Myrmel, L.S.; Fjaere, E.; Ronnevik, A.K.; Bjelland, S.; Fauske, K.R.; Holm, J.B.; Basse, A.L.; Hansen, J.B.; Liaset, B.; et al. The protein source determines the potential of high protein diets to attenuate obesity development in C57BL/6J mice. Adipocyte 2016, 5, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.A.; Torres, A.K.; Jara, C.; Murphy, M.P.; Tapia-Rojas, C. Premature synaptic mitochondrial dysfunction in the hippocampus during aging contributes to memory loss. Redox Biol. 2020, 34, 101558. [Google Scholar] [CrossRef]
- Torres, A.K.; Jara, C.; Llanquinao, J.; Lira, M.; Cortes-Diaz, D.; Tapia-Rojas, C. Mitochondrial Bioenergetics, Redox Balance, and Calcium Homeostasis Dysfunction with Defective Ultrastructure and Quality Control in the Hippocampus of Aged Female C57BL/6J Mice. Int. J. Mol. Sci. 2023, 24, 5476. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Shiwaku, K.; Katsube, T.; Kitajima, K.; Anuurad, E.; Yamasaki, M.; Yamane, Y. Mulberry (Morus alba L.) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice. J. Nutr. 2005, 135, 729–734. [Google Scholar] [CrossRef]
- Wierenga, K.A.; Pestka, J.J. Omega-3 Fatty Acids and Inflammation—You Are What You Eat! Front. Young Minds 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Sebastian, D.; Palacin, M.; Zorzano, A. Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol. Med. 2017, 23, 201–215. [Google Scholar] [CrossRef]
- Jara, C.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Tau Deletion Prevents Cognitive Impairment and Mitochondrial Dysfunction Age Associated by a Mechanism Dependent on Cyclophilin-D. Front. Neurosci. 2020, 14, 586710. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Sharma, A.; Smith, H.J.; Yao, P.; Mair, W.B. Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep. 2019, 20, e48395. [Google Scholar] [CrossRef]
- Yang, M.; He, Y.; Deng, S.; Xiao, L.; Tian, M.; Xin, Y.; Lu, C.; Zhao, F.; Gong, Y. Mitochondrial Quality Control: A Pathophysiological Mechanism and Therapeutic Target for Stroke. Front. Mol. Neurosci. 2021, 14, 786099. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.T.; Wang, Z.Z.; Yuan, Y.H.; Wang, X.L.; Sun, H.M.; Chen, N.H.; Zhang, Y. Dynamin-related protein 1: A protein critical for mitochondrial fission, mitophagy, and neuronal death in Parkinson’s disease. Pharmacol. Res. 2020, 151, 104553. [Google Scholar] [CrossRef]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet impact on mitochondrial bioenergetics and dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef]
- Torres, A.K.; Jara, C.; Olesen, M.A.; Tapia-Rojas, C. Pathologically phosphorylated tau at S396/404 (PHF-1) is accumulated inside of hippocampal synaptic mitochondria of aged Wild-type mice. Sci. Rep. 2021, 11, 4448. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Currais, A.; Prior, M.; Fischer, W.; Chiruta, C.; Ratliff, E.; Daugherty, D.; Dargusch, R.; Finley, K.; Esparza-Molto, P.B.; et al. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell 2018, 17, e12715. [Google Scholar] [CrossRef]
- Beck, S.J.; Guo, L.; Phensy, A.; Tian, J.; Wang, L.; Tandon, N.; Gauba, E.; Lu, L.; Pascual, J.M.; Kroener, S.; et al. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease. Nat. Commun. 2016, 7, 11483. [Google Scholar] [CrossRef]
- Gauba, E.; Sui, S.; Tian, J.; Driskill, C.; Jia, K.; Yu, C.; Rughwani, T.; Wang, Q.; Kroener, S.; Guo, L.; et al. Modulation of OSCP mitigates mitochondrial and synaptic deficits in a mouse model of Alzheimer’s pathology. Neurobiol. Aging 2021, 98, 63–77. [Google Scholar] [CrossRef]
- Duarte, F.V.; Ciampi, D.; Duarte, C.B. Mitochondria as central hubs in synaptic modulation. Cell Mol. Life Sci. 2023, 80, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Chen, C.; Li, S.; Zhang, Z.; Xu, H.; Zhu, F.; Liu, J.; Spencer, P.S.; Dai, Z.; et al. Proteomic Profile of Mouse Brain Aging Contributions to Mitochondrial Dysfunction, DNA Oxidative Damage, Loss of Neurotrophic Factor, and Synaptic and Ribosomal Proteins. Oxid. Med. Cell Longev. 2020, 2020, 5408452. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Sun, X.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.M.; Weistuch, C.; et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl. Acad. Sci. USA 2020, 117, 6170–6177. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Augustine, G.J. Synapsin Isoforms and Synaptic Vesicle Trafficking. Mol. Cells 2015, 38, 936–940. [Google Scholar] [CrossRef]
- Kolos, Y.A.; Grigoriyev, I.P.; Korzhevskyi, D.E. A synaptic marker synaptophysin. Morfologiia 2015, 147, 78–82. [Google Scholar]
- Verpelli, C.; Schmeisser, M.J.; Sala, C.; Boeckers, T.M. Scaffold proteins at the postsynaptic density. Adv. Exp. Med. Biol. 2012, 970, 29–61. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Q.; Zhang, K.; Li, Y.J.; Wu, Y.M.; Liu, S.B.; Zheng, L.H.; Zhao, M.G. Neuroprotective effects of daphnetin against NMDA receptor-mediated excitotoxicity. Molecules 2014, 19, 14542–14555. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, Y.; Yamazaki, N.; Nagaoka, A.; Matsuo, T.; Nagawa, Y.; Takeda, T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 1986, 38, 399–406. [Google Scholar] [CrossRef]
- Orejana, L.; Barros-Minones, L.; Jordan, J.; Puerta, E.; Aguirre, N. Sildenafil ameliorates cognitive deficits and tau pathology in a senescence-accelerated mouse model. Neurobiol. Aging 2012, 33, 625.e11–625.e20. [Google Scholar] [CrossRef]
- Oike, H.; Ogawa, Y.; Azami, K. Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice. Nutrients 2020, 12, 1416. [Google Scholar] [CrossRef]
- McLean, F.H.; Campbell, F.M.; Langston, R.F.; Sergi, D.; Resch, C.; Grant, C.; Morris, A.C.; Mayer, C.D.; Williams, L.M. A high-fat diet induces rapid changes in the mouse hypothalamic proteome. Nutr. Metab. 2019, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Shafie, A.; Rahimi, A.M.; Ahmadi, I.; Nabavizadeh, F.; Ranjbaran, M.; Ashabi, G. High-protein and low-calorie diets improved the anti-aging Klotho protein in the rats’ brain: The toxic role of high-fat diet. Nutr. Metab. 2020, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Jiang, Z.; Lu, H.; Lu, N.; Zhu, R.; Zhu, C.; Zhou, P.; Tang, X. A Study on the Amelioration of Circadian Rhythm Disorders in Fat Mice Using High-Protein Diets. Nutrients 2023, 15, 3459. [Google Scholar] [CrossRef]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; LeGrand, P.; et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.H.; Song, M.K.; Kim, Y.J. NXP032 Ameliorates Aging-Induced Oxidative Stress and Cognitive Impairment in Mice through Activation of Nrf2 Signaling. Antioxidants 2022, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Venegas, B.; Munoz-Arenas, G.; Moran, C.; Vazquez-Roque, R.A.; Flores, G.; Trevino, S.; Diaz, A.; Guevara, J. High-carbohydrate and fat diet consumption causes metabolic deterioration, neuronal damage, and loss of recognition memory in rats. J. Chem. Neuroanat. 2023, 129, 102237. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.; Aranguiz, A.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018, 18, 279–294. [Google Scholar] [CrossRef]
- Rindler, P.M.; Plafker, S.M.; Szweda, L.I.; Kinter, M. High dietary fat selectively increases catalase expression within cardiac mitochondria. J. Biol. Chem. 2013, 288, 1979–1990. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef]
- Ramirez, S.; Gomez-Valades, A.G.; Schneeberger, M.; Varela, L.; Haddad-Tovolli, R.; Altirriba, J.; Noguera, E.; Drougard, A.; Flores-Martinez, A.; Imbernon, M.; et al. Mitochondrial Dynamics Mediated by Mitofusin 1 Is Required for POMC Neuron Glucose-Sensing and Insulin Release Control. Cell Metab. 2017, 25, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.W.; Canto, C.; Houtkooper, R.H. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol. Med. 2014, 6, 580–589. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef]
- Lian, W.W.; Zhou, W.; Zhang, B.Y.; Jia, H.; Xu, L.J.; Liu, A.L.; Du, G.H. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef]
- Varanita, T.; Soriano, M.E.; Romanello, V.; Zaglia, T.; Quintana-Cabrera, R.; Semenzato, M.; Menabo, R.; Costa, V.; Civiletto, G.; Pesce, P.; et al. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015, 21, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xia, X.; Gu, Y.; Hu, L.; Li, C.; Ma, X.; Yin, J. Opposite effects of low-carbohydrate high-fat diet on metabolism in humans and mice. Lipids Health Dis. 2023, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, L.; Boukatina, A.; Myers, C.; Oyarzo, J.; Lustgarten, J.; Shi, Y.; Jahangir, A. High calories but not fat content of lard-based diet contribute to impaired mitochondrial oxidative phosphorylation in C57BL/6J mice heart. PLoS ONE 2019, 14, e0217045. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; Fogolari, F.; Lippe, G.; Bernardi, P. OSCP subunit of mitochondrial ATP synthase: Role in regulation of enzyme function and of its transition to a pore. Br. J. Pharmacol. 2019, 176, 4247–4257. [Google Scholar] [CrossRef]
- Canas, P.M.; Duarte, J.M.; Rodrigues, R.J.; Kofalvi, A.; Cunha, R.A. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging 2009, 30, 1877–1884. [Google Scholar] [CrossRef]
- Sonnewald, U.; Schousboe, A. Introduction to the Glutamate-Glutamine Cycle. Adv. Neurobiol. 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Carroll, R.C.; Zukin, R.S. NMDA-receptor trafficking and targeting: Implications for synaptic transmission and plasticity. Trends Neurosci. 2002, 25, 571–577. [Google Scholar] [CrossRef]
- Foa, L.; Gasperini, R. Developmental roles for Homer: More than just a pretty scaffold. J. Neurochem. 2009, 108, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Okuma, Y. Age-related defects in lifespan and learning ability in SAMP8 mice. Neurobiol. Aging 1999, 20, 111–115. [Google Scholar] [CrossRef]
- Ueda, Y.; Wang, M.F.; Irei, A.V.; Sarukura, N.; Sakai, T.; Hsu, T.F. Effect of dietary lipids on longevity and memory in the SAMP8 mice. J. Nutr. Sci. Vitaminol. 2011, 57, 36–41. [Google Scholar] [CrossRef]
- Fado, R.; Molins, A.; Rojas, R.; Casals, N. Feeding the Brain: Effect of Nutrients on Cognition, Synaptic Function, and AMPA Receptors. Nutrients 2022, 14, 4137. [Google Scholar] [CrossRef] [PubMed]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Blei, T.; Jaster, R.; Vollmar, B. Aging is associated with a shift of fatty metabolism toward lipogenesis. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Pierrot, N.; Ris, L.; Stancu, I.C.; Doshina, A.; Ribeiro, F.; Tyteca, D.; Bauge, E.; Lalloyer, F.; Malong, L.; Schakman, O.; et al. Sex-regulated gene dosage effect of PPARalpha on synaptic plasticity. Life Sci. Alliance 2019, 2, e201800262. [Google Scholar] [CrossRef]
- Furukawa, M.; Tada, H.; Raju, R.; Wang, J.; Yokoi, H.; Yamada, M.; Shikama, Y.; Matsushita, K. Long-Term Soft-Food Rearing in Young Mice Alters Brain Function and Mood-Related Behavior. Nutrients 2023, 15, 2397. [Google Scholar] [CrossRef]
- Kordestani-Moghadam, P.; Assari, S.; Nouriyengejeh, S.; Mohammadipour, F.; Pourabbasi, A. Cognitive Impairments and Associated Structural Brain Changes in Metabolic Syndrome and Implications of Neurocognitive Intervention. J. Obes. Metab. Syndr. 2020, 29, 174–179. [Google Scholar] [CrossRef]
- Zia, A.; Pourbagher-Shahri, A.M.; Farkhondeh, T.; Samarghandian, S. Molecular and cellular pathways contributing to brain aging. Behav. Brain Funct. 2021, 17, 6. [Google Scholar] [CrossRef]
- Giannakou, K.; Golenia, A.; Liabeuf, S.; Malyszko, J.; Mattace-Raso, F.; Farinha, A.; Spasovski, G.; Hafez, G.; Wiecek, A.; Capolongo, G.; et al. Methodological challenges and biases in the field of cognitive function among patients with chronic kidney disease. Front. Med. 2023, 10, 1215583. [Google Scholar] [CrossRef]
- Hanell, A.; Marklund, N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Aranguiz, F.; Varela-Nallar, L.; Inestrosa, N.C. Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer’s Disease. Brain Pathol. 2016, 26, 62–74. [Google Scholar] [CrossRef]
- Lobo, F.; Haase, J.; Brandhorst, S. The Effects of Dietary Interventions on Brain Aging and Neurological Diseases. Nutrients 2022, 14, 5086. [Google Scholar] [CrossRef] [PubMed]
- Akiguchi, I.; Pallas, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H.; et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanquinao, J.; Jara, C.; Cortés-Díaz, D.; Kerr, B.; Tapia-Rojas, C. Contrasting Effects of an Atherogenic Diet and High-Protein/Unsaturated Fatty Acids Diet on the Accelerated Aging Mouse Model SAMP8 Phenotype. Neurol. Int. 2024, 16, 1066-1085. https://doi.org/10.3390/neurolint16050080
Llanquinao J, Jara C, Cortés-Díaz D, Kerr B, Tapia-Rojas C. Contrasting Effects of an Atherogenic Diet and High-Protein/Unsaturated Fatty Acids Diet on the Accelerated Aging Mouse Model SAMP8 Phenotype. Neurology International. 2024; 16(5):1066-1085. https://doi.org/10.3390/neurolint16050080
Chicago/Turabian StyleLlanquinao, Jesús, Claudia Jara, Daniela Cortés-Díaz, Bredford Kerr, and Cheril Tapia-Rojas. 2024. "Contrasting Effects of an Atherogenic Diet and High-Protein/Unsaturated Fatty Acids Diet on the Accelerated Aging Mouse Model SAMP8 Phenotype" Neurology International 16, no. 5: 1066-1085. https://doi.org/10.3390/neurolint16050080
APA StyleLlanquinao, J., Jara, C., Cortés-Díaz, D., Kerr, B., & Tapia-Rojas, C. (2024). Contrasting Effects of an Atherogenic Diet and High-Protein/Unsaturated Fatty Acids Diet on the Accelerated Aging Mouse Model SAMP8 Phenotype. Neurology International, 16(5), 1066-1085. https://doi.org/10.3390/neurolint16050080






