Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges
Abstract
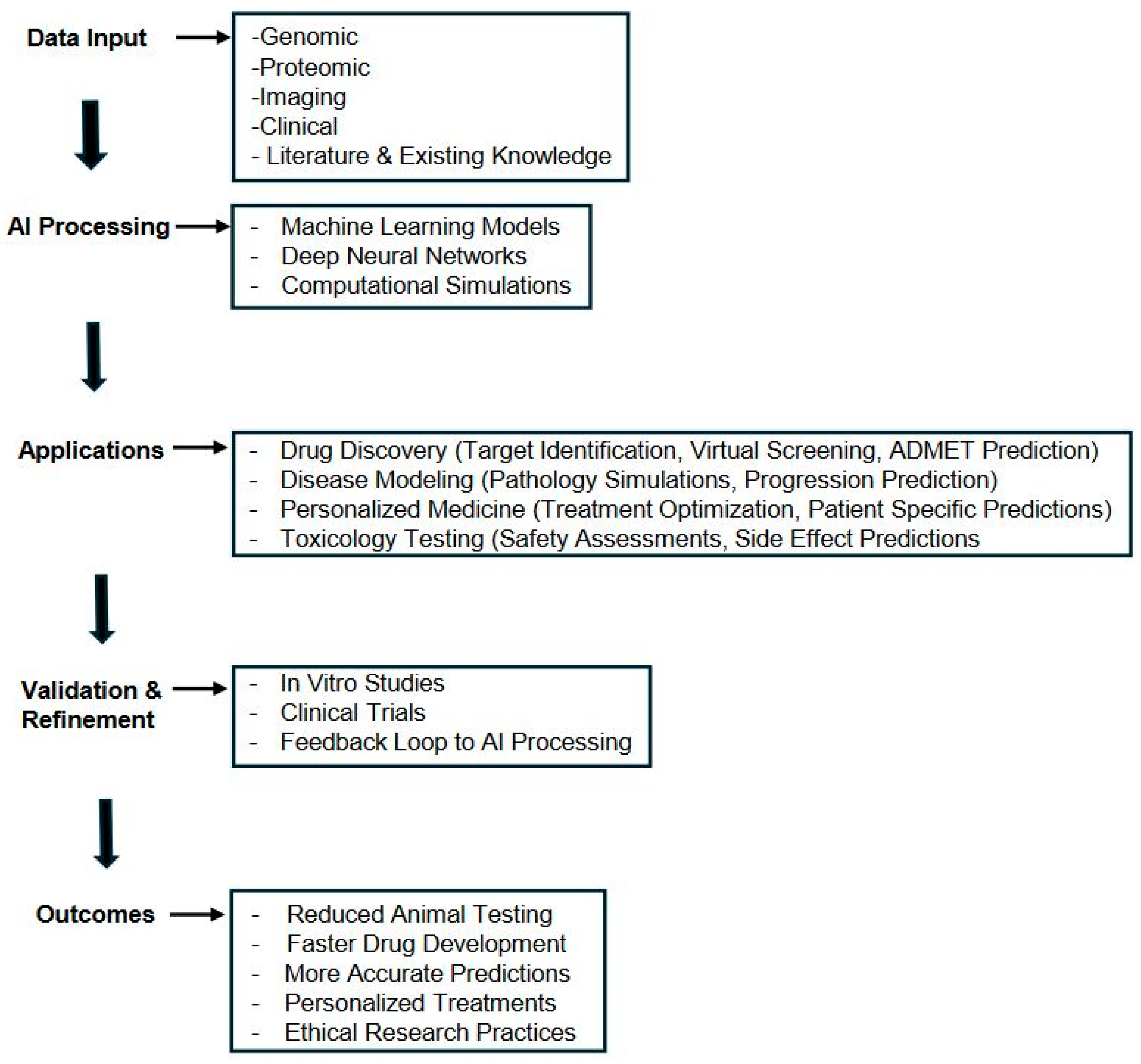
1. Introduction
2. Limitations of Animal Models in Neurology
3. Computer Modeling and Simulation
4. Economic and Practical Benefits of AI in Neurology Research
5. Regulatory and Ethical Considerations for AI in Neurology
6. Challenges and Limitations
7. The Path Forward
8. Summary
Funding
Conflicts of Interest
References
- Roelfsema, P.R.; Treue, S. Basic neuroscience research with nonhuman primates: A small but indispensable component of biomedical research. Neuron 2014, 82, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114. [Google Scholar] [PubMed]
- Bailey, J. Does the stress inherent to laboratory life and experimentation on animals adversely affect research data? Altern. Lab. Anim. 2018, 46, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Bottini, A.A.; Hartung, T. Food for thought... on the economics of animal testing. ALTEX-Altern. Anim. Exp. 2009, 26, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M.; Du, J.L.; Ip, N.Y.; Xiong, Z.Q.; Xu, B.; Tan, T. China brain project: Basic neuroscience, brain diseases, and brain-inspired computing. Neuron 2016, 92, 591–596. [Google Scholar] [CrossRef]
- Erofeeva, M.N.; Cherkasova, I.L. Brain organoids as a new approach to model human brain development and neurodegenerative disorders. Bull. Russ. State Med. Univ. 2018, 5–11. [Google Scholar]
- Basile, A.O.; Yahi, A.; Tatonetti, N.P. Artificial intelligence for drug toxicity and safety. Trends Pharmacol. Sci. 2019, 40, 624–635. [Google Scholar] [CrossRef]
- Ladybug, R.; Jenkins, S.; Papadopoulos, S.; Snutch, T.; Loewen, J.P. LB12 Personalized medicine in epilepsy utilizing integrative AI technology. Clin. Neurophysiol. 2019, 130, e91. [Google Scholar]
- Pound, P.; Nicol, C.J. Retrospective harm benefit analysis of pre-clinical animal research for six treatment interventions. PLoS ONE 2018, 13, e0193758. [Google Scholar] [CrossRef]
- Graham, M.L.; Prescott, M.J. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur. J. Pharmacol. 2015, 759, 19–29. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Ivanenkov, Y.A.; Aliper, A.; Veselov, M.S.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Polykovskiy, D.A.; Kuznetsov, M.D.; Asadulaev, A.; et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol. 2019, 37, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shao, C.; Wang, Y.; Jiang, H.; Huang, J.; Shen, D.; Zhang, Y. A Thalamus-based Deep Learning Model for Predicting Parkinson’s Disease Progression. IEEE J. Biomed. Health Inform. 2020, 25, 2673–2683. [Google Scholar]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Freund, I.; Jelen, B.; Behr, C.; Schenk, B.; Van Ravenzwaay, B. Predictive performance of a sequential toxicity testing strategy using machine learning approaches. Comput. Toxicol. 2021, 18, 100162. [Google Scholar]
- Zhang, Y.; Shao, Y.; Huang, Y.; Zhao, S.; Huang, J.; Zhang, Y. Artificial intelligence-enabled analysis of cerebral organoids reveals key cellular and molecular features of human brain development. Nat. Commun. 2022, 13, 1–14. [Google Scholar]
- Strickland, E. AI-based brain-computer interface rejuvenates paralyzed person’s sense of touch. IEEE Spectr. 2019, 56, 8–9. [Google Scholar]
- Kosoy, E.; Schulz, M.A.; Halchenko, Y.O. Artificial Intelligence in Neuroimaging: A Comprehensive Review of Methods and Applications. Neuroimage 2023, 119372. [Google Scholar]
- Gladstone, D.J.; Black, S.E.; Hakim, A.M. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 2002, 33, 2123–2136. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Passingham, R.E.; Stephan, K.E.; Kötter, R. The anatomical basis of functional localization in the cortex. Nat. Rev. Neurosci. 2002, 3, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Premack, D. Human and animal cognition: Continuity and discontinuity. Proc. Natl. Acad. Sci. USA 2007, 104, 13861–13867. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Hwang, T.J.; Franklin, J.M. Two decades of new drug development for central nervous system disorders. Nat. Rev. Drug Discov. 2015, 14, 815–816. [Google Scholar] [CrossRef]
- Jucker, M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat. Med. 2010, 16, 1210–1214. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.P. The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J. 2014, 55, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Attarwala, H. TGN1412: From discovery to disaster. J. Young Pharm. 2010, 2, 332–336. [Google Scholar] [CrossRef]
- Einevoll, G.T.; Destexhe, A.; Diesmann, M.; Grün, S.; Jirsa, V.; de Kamps, M.; Schürmann, F. The scientific case for brain simulations. Neuron 2019, 102, 735–744. [Google Scholar] [CrossRef]
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.Z. XAI—Explainable artificial intelligence. Sci. Robot. 2019, 4, eaay7120. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 1–19. [Google Scholar] [CrossRef]
- Mak, K.K.; Pichika, M.R.; Desai, M. Artificial intelligence in drug discovery and development: A comprehensive review. Drug Discov. Today 2021, 26, 1695–1709. [Google Scholar]
- Zeng, H.; Gifford, D.K.; Zhang, C.; Feinberg, T.E. Combining deep learning and neuroimaging to map the neural substrates of cognitive function. Nat. Rev. Neurosci. 2022, 23, 547–566. [Google Scholar]
- Ramsundar, B.; Eastman, P.; Walters, P.; Pande, V.; Leswing, K.; Wu, Z. Deep Learning for the Life Sciences; O’Reilly Media, Inc.: Sebastopol., CA, USA, 2019. [Google Scholar]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Krauss, J.K. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Gilron, R.; Little, S.; Perrone, R.; Wilt, R.; de Hemptinne, C.; Yaroshinsky, M.S.; Starr, P.A. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 2021, 39, 1078–1085. [Google Scholar] [CrossRef]
- Anderson, C.J.; Anderson, D.N.; Pulst, S.M.; Butson, C.R. Subthalamic deep brain stimulation reduces pathological information transmission to the thalamus in a computational model. Front. Comput. Neurosci. 2020, 14, 51. [Google Scholar]
- Zhu, F.; Guo, R.; Cheng, Z.; Xue, J.H.; Wang, L.; Lei, B. Personalized Parkinson’s disease treatment via brain modeling and deep brain stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1244–1252. [Google Scholar]
- Nozari, E.; Jafari, A.H.; Zu, Q.; Mailman, R.B.; Yoon, J.H. Computational modeling of basal ganglia circuitry and its application in Parkinson’s disease. J. Neural Eng. 2022, 19, 011001. [Google Scholar]
- Shenoy, K.V.; Carmena, J.M. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron 2014, 84, 665–680. [Google Scholar] [CrossRef]
- Schwemmer, M.A.; Skomrock, N.D.; Sederberg, P.B.; Ting, J.E.; Sharma, G.; Bockbrader, M.A.; Friedenberg, D.A. Meeting brain–computer interface user performance expectations using a deep neural network decoding framework. Nat. Med. 2018, 24, 1669–1676. [Google Scholar]
- Skomrock, N.D.; Schwemmer, M.A.; Ting, J.E.; Trivedi, H.R.; Sharma, G.; Bockbrader, M.A.; Friedenberg, D.A. A reinforcement learning approach for optimizing the user experience of brain-computer interfaces. Nat. Biomed. Eng. 2021, 5, 740–753. [Google Scholar]
- Rastogi, A.; Vargas-Irwin, C.E.; Willett, F.R.; Abreu, J.; Crowder, D.C.; Murphy, B.A.; Henderson, J.M. Neural representation of observed, imagined, and attempted grasping force in motor cortex of individuals with chronic tetraplegia. Sci. Rep. 2022, 12, 1429. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ritskes-Hoitinga, M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 2018, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed]
- Deloitte. The Future of Biopharma: Reimagining Drug Development through AI; Deloitte Insights: Beijing, China, 2022. [Google Scholar]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [PubMed]
- Mak, K.K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.H.; Bunnage, M.E.; Martino Cortez, A. Artificial intelligence in drug discovery: A practical perspective. Med. Chem. Res. 2021, 30, 3–12. [Google Scholar]
- Denayer, T.; Stöhr, T.; Van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Smith, S.K.; Petrella, J.R.; Doraiswamy, P.M. The role of artificial intelligence in diagnosing and treating Alzheimer’s disease. Expert Rev. Neurother. 2019, 19, 435–445. [Google Scholar]
- McCall, B. COVID-19 and artificial intelligence: Protecting health-care workers and curbing the spread. Lancet Digit. Health 2020, 2, e166–e167. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Ebell, C.; Muller, J.; Telefont, M.; Knoll, A.; Lippert, T. The Human Brain Project: Creating a European research infrastructure to decode the human brain. Neuron 2016, 92, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Gordon, N.; Langley, G.; Higgins, W. Estimates for worldwide laboratory animal use in 2005. Altern. Lab. Anim. 2008, 36, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, B.; Altman, N.S.; Forsman, A.; Forstmeier, W.; Gurevitch, J.; Jaric, I.; Würbel, H. Reproducibility of animal research in light of biological variation. Nat. Rev. Neurosci. 2020, 21, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Ehrenstein, V.; Vandenbroucke, J.P. Confounding in observational studies based on large health care databases: Problems and potential solutions—A primer for the clinician. Clin. Epidemiol. 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Nonclinical testing of drugs and biological products. In Guidance for Industry; US Food and Drug Administration: Fishers Lane Rockville, MD, USA, 2021. [Google Scholar]
- European Medicines Agency. Guideline on the Principles of Regulatory Acceptance of 3Rs (Replacement, Reduction, Refinement) Testing Approaches. Available online: https://norecopa.no/3r-guide/guideline-on-the-principles-of-regulatory-acceptance-of-3rs-replacement-reduction-refinement-testing-approaches/ (accessed on 5 June 2024).
- US Food and Drug Administration. Alternative Methods Working Group—Implementing the FDA’s Roadmap to Reduce Animal Testing by 2025. Available online: https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda (accessed on 5 June 2024).
- van Norman, G.A. Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach? JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Jobin, A.; Ienca, M.; Vayena, E. The global landscape of AI ethics guidelines. Nat. Mach. Intell. 2019, 1, 389–399. [Google Scholar] [CrossRef]
- Mehrabi, N.; Morstatter, F.; Saxena, N.; Lerman, K.; Galstyan, A. A survey on bias and fairness in machine learning. ACM Comput. Surv. 2021, 54, 1–35. [Google Scholar] [CrossRef]
- Floridi, L.; Cowls, J.; Beltrametti, M.; Chatila, R.; Chazerand, P.; Dignum, V.; Vayena, E. AI4People—An ethical framework for a good AI society: Opportunities, risks, principles, and recommendations. Minds Mach. 2018, 28, 689–707. [Google Scholar] [CrossRef]
- Favaretto, M.; De Clercq, E.; Elger, B.S. Big Data and discrimination: Perils, promises and solutions. A systematic review. J. Big Data 2019, 6, 1–27. [Google Scholar] [CrossRef]
- Cirillo, D.; Catuara-Solarz, S.; Morey, C.; Guney, E.; Subirats, L.; Mellino, S.; Gigante, A.; Valencia, A.; Rementeria, M.J.; Chadha, A.S.; et al. Sex and gender differences and biases in artificial intelligence for biomedicine and healthcare. NPJ Digit. Med. 2020, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.K.; Wong, Y.H.; Pichika, M.R. Artificial Intelligence in Drug Discovery and Development. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Hock, F.J., Pugsley, M.K., Eds.; Springer: Cham, Switerland, 2023. [Google Scholar]
- Prosperi, M.; Guo, Y.; Sperrin, M.; Koopman, J.S.; Min, J.S.; He, X.; Rich, S.; Wang, M.; Buchan, I.E.; Bian, J. Causal inference and counterfactual prediction in machine learning for actionable healthcare. Nat. Mach. Intell. 2020, 2, 369–375. [Google Scholar] [CrossRef]
- Vo, A.H.; Van Vleet, T.R.; Gupta, R.R.; Liguori, M.J.; Rao, M.S. An Overview of Machine Learning and Big Data for Drug Toxicity Evaluation. Chem. Res. Toxicol. 2020, 33, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Smalley, E. AI-powered drug discovery captures pharma interest. Nat. Biotechnol. 2019, 37, 604–605. [Google Scholar] [CrossRef]
- Steinbeck, J.A. Towards better treatments in neurology and psychiatry: The case for brain-computer interfaces. Lancet Neurol. 2021, 20, 599–600. [Google Scholar]
- Jorgenson, L.A.; Newsome, W.T.; Anderson, D.J.; Bargmann, C.I.; Brown, E.N.; Deisseroth, K.; Wingfield, J.C. The BRAIN Initiative: Developing technology to catalyse neuroscience discovery. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140164. [Google Scholar] [CrossRef]
- Benjaminy, S.; Macdonald, I. AI in neuroscience research: A review of current applications and ethical implications. Neuron 2020, 108, 872–885. [Google Scholar]
- Rommelfanger, K.S.; Jeong, S.J.; Montojo, J.; Zirlinger, M.; Arias-Carrión, O.; Illes, J.; Noll-Hussong, M. Neuroethics: Think global. Neuron 2018, 100, 19–22. [Google Scholar] [CrossRef]
- Greenberg, D.S.; Houweling, A.R. Studying neurological and psychiatric disorders using brain organoids. Nat. Neurosci. 2022, 25, 149–159. [Google Scholar]
- Ioannou, A.; Neff, R.; Larson, M.G. Personalized medicine in neurology: From genetic risk predictions to deep brain stimulation. Curr. Opin. Neurol. 2021, 34, 245–251. [Google Scholar]
- Marblestone, A.H.; Wayne, G.; Kording, K.P. Toward an integration of deep learning and neuroscience. Front. Comput. Neurosci. 2016, 10, 94. [Google Scholar] [CrossRef] [PubMed]
| Author(s) and Year | Study Title | AI Method | Application in Neurology | Key Findings | Implications for Replacing Animal Models |
|---|---|---|---|---|---|
| Zhavoronkov et al. [11] | Deep learning enables rapid identification of potent DDR1 kinase inhibitors | Deep learning | Drug discovery for fibrosis (applicable to neurological disorders) | AI identified novel drug candidate in 21 days | Dramatically accelerated early drug discovery, reducing need for initial animal screening |
| Huang et al. [12] | A Thalamus-based Deep Learning Model for Predicting Parkinson’s Disease Progression | Convolutional neural network | Parkinson’s disease progression prediction | AI model predicted disease progression with high accuracy using brain MRI | Could reduce reliance on longitudinal animal studies for understanding disease progression |
| Vamathevan et al. [13] | Applications of machine learning in drug discovery and development | Various machine learning methods | Drug discovery and development for multiple diseases, including neurological | AI can improve efficiency in target validation, biomarker discovery, and toxicology | Potential to reduce animal use across multiple stages of drug development |
| Topol [14] | High-performance medicine: the convergence of human and artificial intelligence | Deep learning, neural networks | Diagnosis and treatment of neurological disorders | AI can match or exceed human performance in diagnosing certain conditions | Could reduce need for animal models in diagnostic method development |
| Freund et al. [15] | Predictive performance of a sequential toxicity testing strategy using machine learning approaches | Machine learning | Neurotoxicity prediction | AI accurately predicted compound toxicity using in vitro data | Could significantly reduce animal use in neurotoxicity testing |
| Zhang et al. [16] | Artificial intelligence-enabled analysis of cerebral organoids reveals key cellular and molecular features of human brain development | Deep learning image analysis | Human brain development study | AI analyzed cerebral organoid development, revealing key insights | Demonstrates potential of AI + organoids to replace some developmental neurobiology animal studies |
| Strickland [17] | AI-based brain–computer interface rejuvenates paralyzed person’s sense of touch | Deep learning | Brain–computer interfaces for paralysis | AI decoded neural signals to restore sense of touch | Reduced need for invasive animal studies in BCI development |
| Kosoy et al. [18] | Artificial Intelligence in Neuroimaging: A Comprehensive Review of Methods and Applications | Various AI methods | Neuroimaging analysis | AI improves efficiency and accuracy in neuroimaging analysis across multiple disorders | Could reduce need for animal imaging studies in method development and validation |
| Research Stage | Traditional Animal Model Approach | AI-Based Approach |
|---|---|---|
| Target identification | - Genetic knockout studies in animals - Observational studies of disease models | - AI analysis of genomic and proteomic databases - Machine learning on large-scale human data sets |
| Drug screening | - High-throughput screening in cell cultures - Initial toxicity testing in animals | - AI-powered virtual screening of compound libraries - In silico prediction of drug–target interactions |
| Lead optimization | - Medicinal chemistry guided by animal study results - Iterative testing in animal models | - AI-driven prediction of ADMET properties - Machine learning models for structure–activity associations |
| Efficacy testing | - Dosing studies in animal disease models - Behavioral and physiological assessments | - AI simulations of drug effects in virtual patient cohorts - Machine learning analysis of human clinical data |
| Safety assessment | - Toxicity studies in multiple animal species - Long-term exposure studies in animals | - AI prediction of toxicity based on chemical structure - Machine learning models trained on human toxicity data |
| Pharmacokinetics | - ADME studies in animal models - Tissue distribution studies in animals | - AI prediction of ADME properties - Physiologically based pharmacokinetic (PBPK) modeling |
| Biomarker discovery | - Analysis of animal model tissues and fluids - Longitudinal studies in animal models | - AI analysis of multi-omics data from human samples - Machine learning on large-scale clinical datasets |
| Disease modeling | - Genetic or induced disease models in animals - Longitudinal studies of disease progression | - AI-powered simulations of disease mechanisms - Deep learning on patient data for disease trajectory prediction |
| Area of Development | Current Status | Future Direction | Potential Impact |
|---|---|---|---|
| AI model complexity | Models focus on specific aspects of brain function or disease | Development of comprehensive, multi-scale brain models | More accurate prediction of drug effects and disease progression |
| Data integration | Limited integration of diverse data types | Seamless integration of genomic, proteomic, imaging, and clinical data | Holistic understanding of neurological disorders and personalized treatment strategies |
| In silico clinical trials | Early stage simulations for simple scenarios | Full-scale virtual clinical trials for complex neurological disorders | Faster, cheaper, and more ethical drug development process |
| Regulatory acceptance | Limited acceptance of AI-based evidence | Established frameworks for validating and approving AI models in drug development | Accelerated transition from animal models to AI-based approaches |
| AI explainability | Many AI models are “black boxes” | Development of interpretable AI models for neurology | Increased trust and adoption of AI predictions in clinical decision making |
| AI–human collaboration | AI as a tool used by human researchers | AI as an active partner in hypothesis generation and experimental design | More efficient and innovative research processes |
| Neuromorphic computing | Experimental stage | Widespread use of brain-inspired computing architectures | More efficient and biologically relevant AI models of brain function |
| Digital brain twins | Conceptual stage | Personalized brain simulations for individual patients | Highly tailored treatment strategies and improved patient outcomes |
| AI in neurodegenerative diseases | Focus on diagnosis and progression prediction | AI-driven discovery of disease-modifying treatments | Breakthrough therapies for conditions like Alzheimer’s and Parkinson’s |
| Ethical AI in neurology | Emerging discussions on ethical implications | Established ethical frameworks for AI use in neurology research | Responsible and beneficial application of AI technologies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudroff, T. Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges. Neurol. Int. 2024, 16, 805-820. https://doi.org/10.3390/neurolint16040060
Rudroff T. Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges. Neurology International. 2024; 16(4):805-820. https://doi.org/10.3390/neurolint16040060
Chicago/Turabian StyleRudroff, Thorsten. 2024. "Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges" Neurology International 16, no. 4: 805-820. https://doi.org/10.3390/neurolint16040060
APA StyleRudroff, T. (2024). Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges. Neurology International, 16(4), 805-820. https://doi.org/10.3390/neurolint16040060






