Mapping Motor Neuroplasticity after Successful Surgical Brachial Plexus Reconstruction Using Navigated Transcranial Magnetic Stimulation (nTMS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
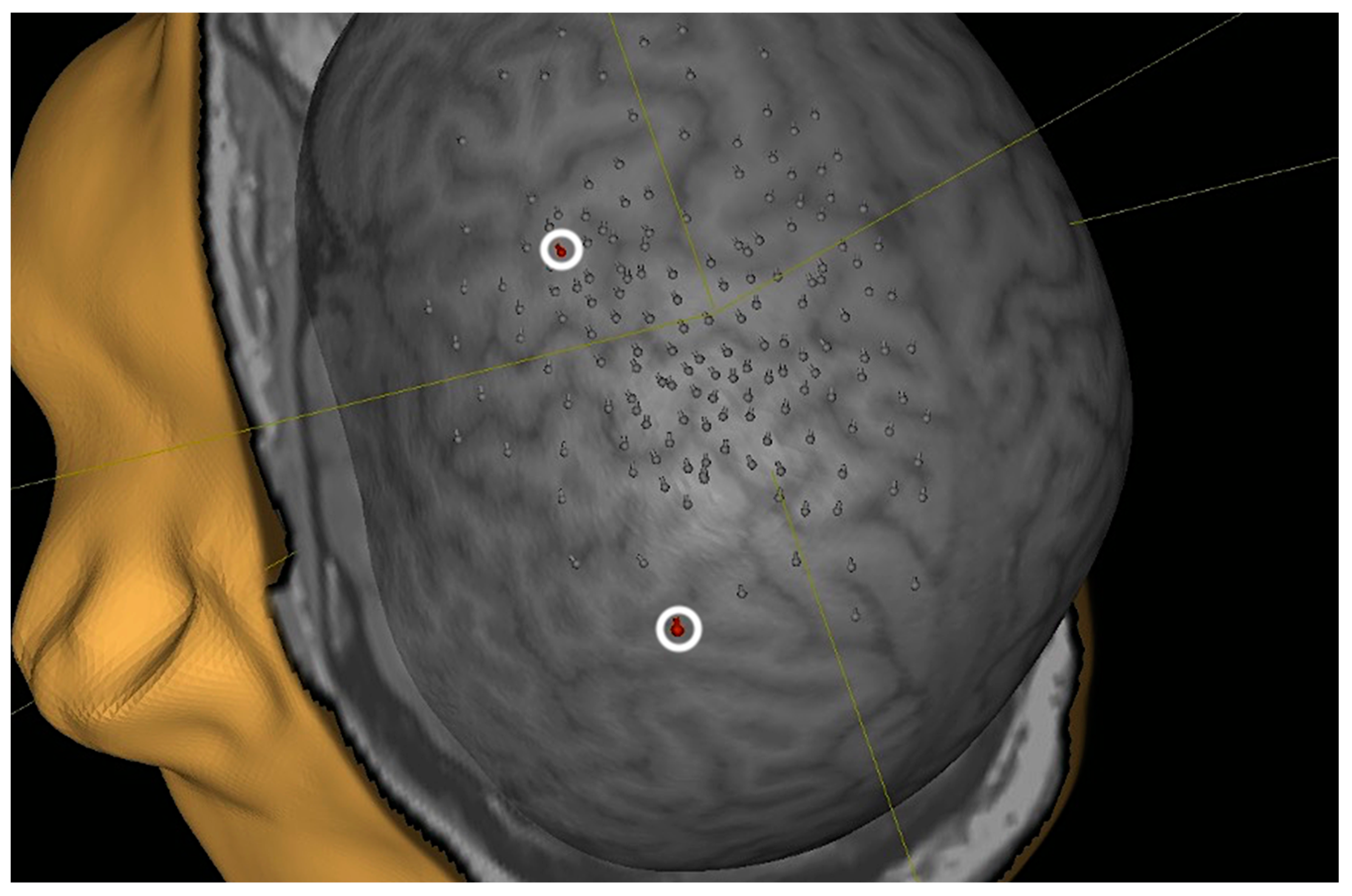
2.2. nTMS Protocol
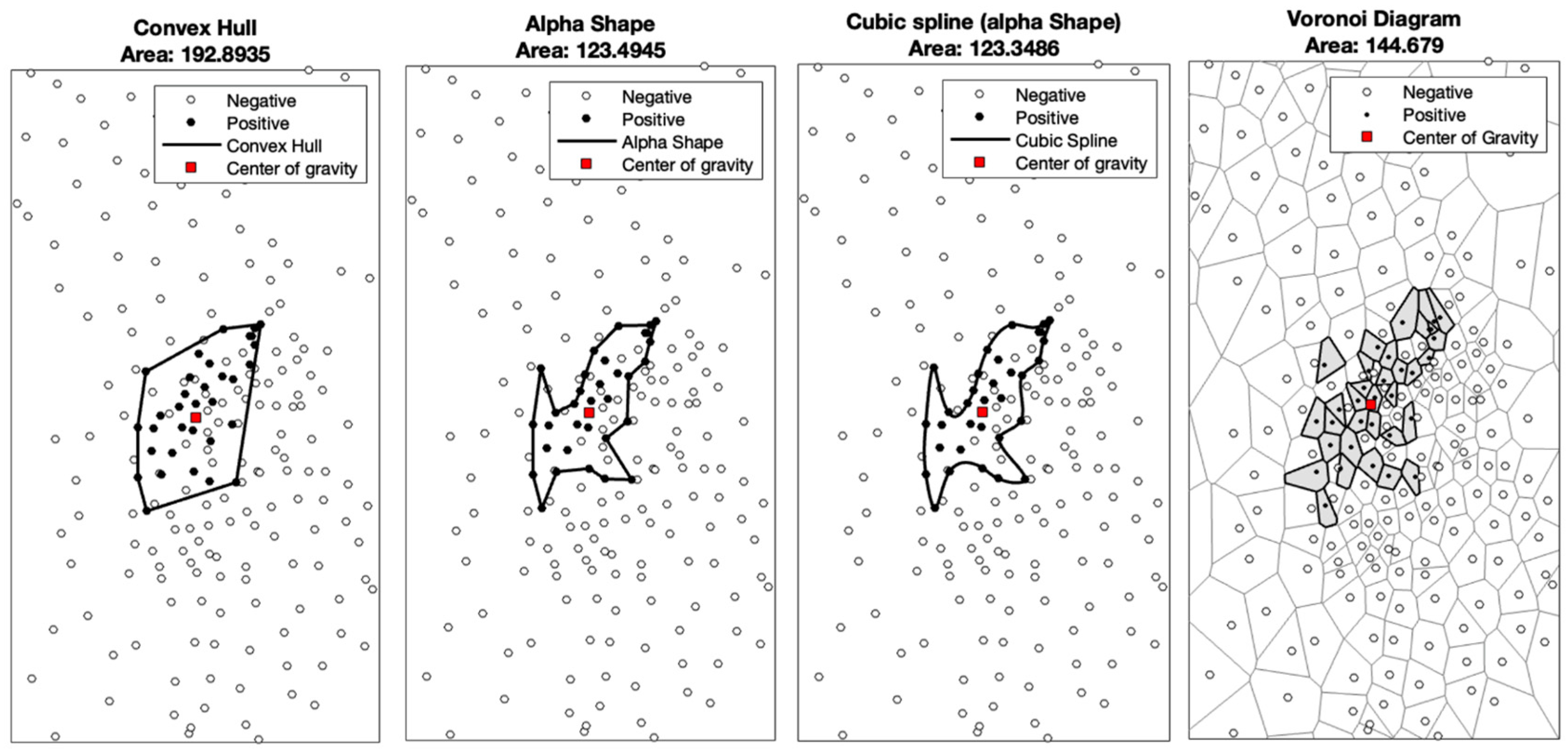
2.3. Computation of the Cortical Representation Area and Statistical Analysis
- The TMS coil stimulates the cortex area closest to the coil.
- The TMS stimulating field is similar in every location of the cortex, and there are no relevant variations in the MEPs induced by TMS.
3. Results
3.1. Patient Characteristics
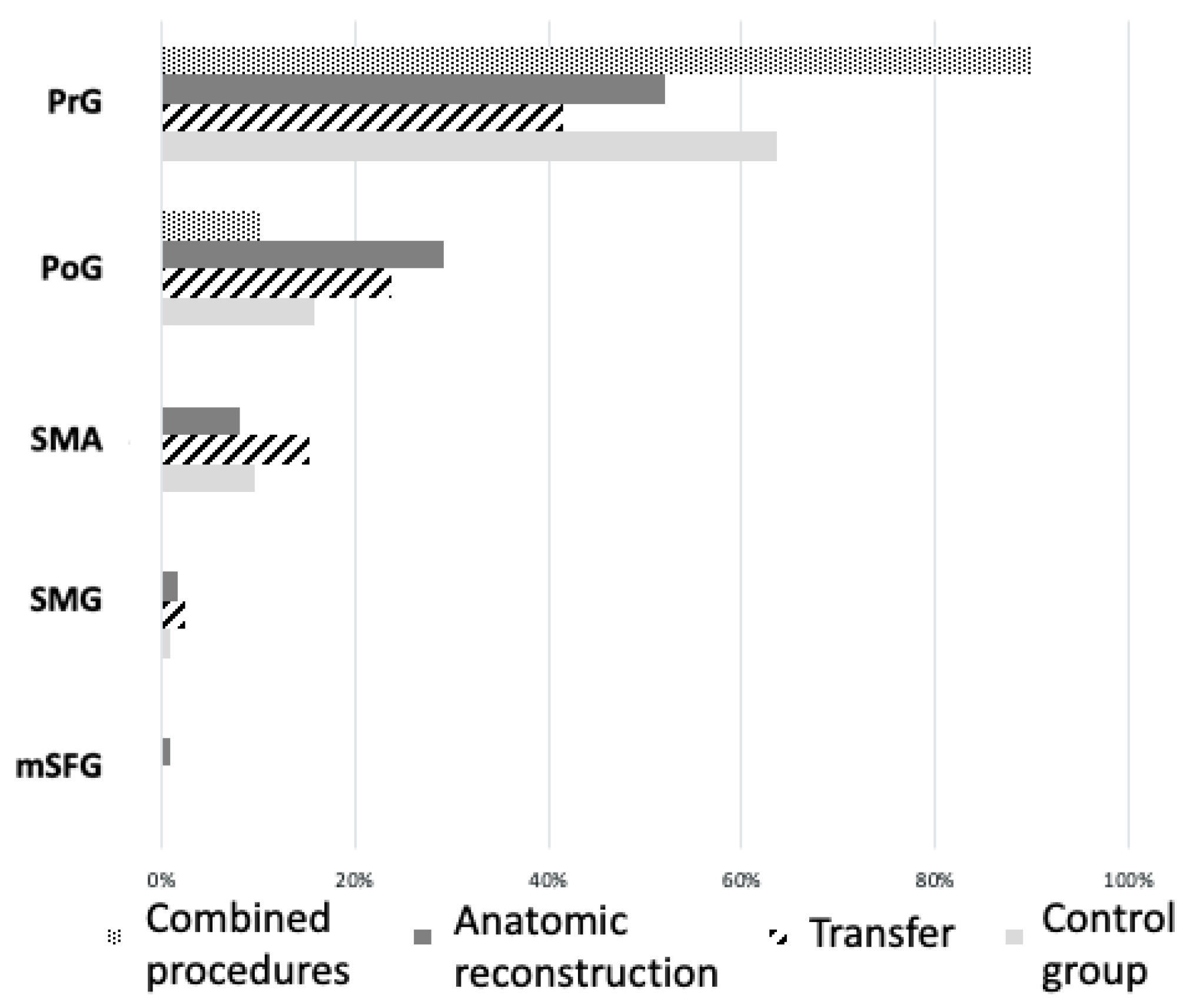
3.2. Cortical Motor Area Location
3.3. Cortical Motor Area Size
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 3D Convex Hull | 2D Convex Hull | 2D Voronoi | 2D Alpha | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | ID | Left | Right | Left | Right | Left | Right | Left | Right |
| volunteer | 1 | 197 | 362 | 192.9 | 339.8 | 144.7 | 350.4 | 123.5 | 279.1 |
| volunteer | 2 | 682.6 | 45 | 644.6 | 36.9 | 510.3 | 26.1 | 571.8 | 27.7 |
| volunteer | 3 | 243 | 132 | 239.1 | 119.5491 | 70.3 | 82.6644 | 170.1 | 88.5177 |
| volunteer | 4 | 227 | 162 | 220.2 | 110.3 | 106.9 | 79.7 | 179.7 | 99.4 |
| volunteer | 5 | 1643 | 361 | 1568.5 | 332.4 | 974 | 193.3 | 1260.5 | 279.4 |
| volunteer | 6 | 299 | 433 | 273.4 | 369.9 | 186.2 | 289.9 | 203.8 | 316 |
| volunteer | 7 | 349 | 378 | 337.2 | 350.4 | 139.3 | 132.8 | 296.6 | 181.9 |
| volunteer | 8 | 380 | 1376 | 370.5 | 1083.5 | 174.1 | 1284.6 | 239.2 | 934.5 |
| volunteer | 9 | 183 | 415 | 181.8 | 402.3 | 97.7 | 308 | 100 | 300.9 |
| volunteer | 10 | 526 | 384 | 502.3 | 358.9 | 425.1 | 225.6 | 380.9 | 283.7 |
| mean | 473.0 | 404.8 | 453.1 | 350.4 | 282.9 | 297.3 | 352.6 | 279.1 | |
| Category | ID | unaffected | affected | unaffected | affected | unaffected | affected | unaffected | affected |
| patient | 1 | 536.9 | 1639 | 525.7 | 1491.7 | 270 | 399.2 | 436.2 | 1105.3 |
| patient | 2 | 1157.3 | 194 | 1132.2 | 190.5 | 278.9 | 86 | 952.8 | 167.5 |
| patient | 3 | 177 | 1013 | 125 | 953.5 | 177.2 | 1252.2 | 103.6 | 686 |
| patient | 5 | 290 | 72 | 286.4 | 63.2 | 174.8 | 65.5 | 260.2 | 45.6 |
| patient | 6 | 344.8 | 449.8 | 236.5 | 435.2 | 119.1 | 394.7 | 195.9 | 381.5 |
| patient | 7 | 359.8 | 735.2 | 340.4 | 620.4 | 156.6 | 459.3 | 315.6 | 510.7 |
| patient | 8 | 143 | 451 | 134.6 | 441.8 | 103.8 | 126.7 | 123.4 | 352 |
| mean | 429.8 | 650.6 | 397.3 | 599.5 | 182.9 | 397.7 | 341.1 | 464.1 | |
| 2D Interpol | SD ABV | SD BLW | SD Tot | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | ID | Left | Right | Left | Right | Left | Right | Left | Right |
| volunteer | 1 | 123.3 | 276.6 | −0.53 | −3.94 | −1.14 | −2.41 | −1.44 | −2.55 |
| volunteer | 2 | 640.6 | 38.1 | −2.12 | −0.70 | −2.26 | −2.89 | −2.24 | −2.91 |
| volunteer | 3 | 200.5 | 94.284 | −0.81 | −5.29 | −2.19 | −2.84 | −2.22 | −2.90 |
| volunteer | 4 | 199.9 | 104 | −1.52 | −1.30 | −2.01 | −2.82 | −2.05 | −2.88 |
| volunteer | 5 | 1276.8 | 283.3 | −1.17 | −3.54 | −1.60 | −2.14 | −1.62 | −2.17 |
| volunteer | 6 | 206.9 | 332.7 | −1.46 | −7.29 | −2.27 | −2.50 | −2.37 | −2.60 |
| volunteer | 7 | 307.5 | 196.6 | −0.65 | −2.08 | −2.11 | −2.75 | −2.16 | −2.79 |
| volunteer | 8 | 252.4 | 933.4 | −0.20 | −1.66 | −1.47 | 1.17 | −1.51 | −2.10 |
| volunteer | 9 | 98.7 | 312 | −0.21 | −1.51 | −1.71 | −2.89 | −1.71 | −3.03 |
| volunteer | 10 | 372.3 | 279.4 | −1.34 | −0.69 | −2.61 | −2.93 | −2.89 | −3.11 |
| mean | 367.9 | 285.0 | −1.00 | −2.80 | −1.94 | −2.30 | −2.02 | −2.70 | |
| Category | ID | unaffected | affected | unaffected | affected | unaffected | affected | unaffected | affected |
| patient | 1 | 470.7 | 1274.2 | −0.37 | −1.38 | −1.14 | −3.06 | −1.67 | −3.07 |
| patient | 2 | 1109.9 | 194 | −0.39 | −0.48 | −1.28 | −2.28 | −1.28 | −2.35 |
| patient | 3 | 102.7 | 695 | −2.17 | −1.66 | −3.09 | −1.40 | −3.13 | −1.44 |
| patient | 5 | 277.8 | 50.8 | −0.29 | −1.38 | −2.38 | −2.31 | −2.46 | −2.36 |
| patient | 6 | 232.3 | 385 | −0.49 | −0.98 | −1.60 | −1.50 | −1.62 | −1.58 |
| patient | 7 | 324.6 | 527.1 | −1.32 | −1.19 | −1.08 | −1.73 | −1.09 | −1.79 |
| patient | 8 | 117.7 | 363.1 | −1.54 | −0.49 | −3.13 | −2.78 | −3.18 | −2.80 |
| mean | 376.5 | 498.5 | −0.94 | −1.08 | −1.96 | −2.15 | −2.06 | −2.20 | |
References
- Wassermann, E.M.; Pascual-Leone, A.; Hallett, M. Cortical motor representation of the ipsilateral hand and arm. Exp. Brain Res. 1994, 100, 121–132. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Grafman, J.; Hallett, M. Modulation of Cortical Motor Output Maps During Development of Implicit and Explicit Knowledge. Science 1994, 263, 1287–1289. [Google Scholar] [CrossRef]
- Wilson, S.A.; Thickbroom, G.W.; Mastaglia, F.L. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects The representation of two intrinsic hand muscles. J. Neurol. Sci. 1993, 118, 134–144. [Google Scholar] [CrossRef]
- Kew, J.J.; Ridding, M.C.; Rothwell, J.C.; Passingham, R.E.; Leigh, P.N.; Sooriakumaran, S.; Frackowiak, R.S.; Brooks, D.J. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. J. Neurophysiol. 1994, 72, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Torres, F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain 1993, 116, 39–52. [Google Scholar] [CrossRef]
- Ziemann, U.; Corwell, B.; Cohen, L.G. Modulation of Plasticity in Human Motor Cortex after Forearm Ischemic Nerve Block. J. Neurosci. 1998, 18, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Miltner, W.H.R.; Bauder, H.; Sommer, M.; Dettmers, C.; Taub, E.; Weiller, C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci. Lett. 1998, 250, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Altamura, C.; Ferreri, F.; Melgari, J.-M. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eur. Medicophysica 2007, 43, 241–254. [Google Scholar]
- Fridman, E.A.; Hanakawa, T.; Chung, M.; Hummel, F.; Leiguarda, R.C.; Cohen, L.G. Reorganization of the human ipsilesional premotor cortex after stroke. Brain 2004, 127, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Conway, N.; Wildschuetz, N.; Moser, T.; Bulubas, L.; Sollmann, N.; Tanigawa, N.; Meyer, B.; Krieg, S.M. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J. Neurosurg. 2017, 127, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Barz, A.; Noack, A.; Baumgarten, P.; Seifert, V.; Forster, M.T. Motor Cortex Reorganization in Patients with Glioma Assessed by Repeated Navigated Transcranial Magnetic Stimulation—A Longitudinal Study. World Neurosurg. 2018, 112, e442–e453. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Caulo, M.; Pieri, V.; Falini, A.; Castellano, A. Role of Functional Imaging Techniques to Assess Motor and Language Cortical Plasticity in Glioma Patients: A Systematic Review. Neural Plast. 2019, 2019, 4056436. [Google Scholar] [CrossRef] [PubMed]
- Pejkova, S.; Filipce, V.; Peev, I.; Nikolovska, B.; Jovanoski, T.; Georgieva, G.; Srbov, B. Brachial Plexus Injuries—Review of the Anatomy and the Treatment Options. PRILOZI 2021, 42, 91–103. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Kim, D.D.; Burkett, C.; Kline, D.G. Nerve Transfer Surgery for Adult Brachial Plexus Injury: A 10-Year Experience at Louisiana State University. Neurosurgery 2009, 65 (Suppl. S4), A55–A62. [Google Scholar] [CrossRef]
- Thomeer, R.T.W.M.; Malessy, M.J.A. Surgical repair of brachial plexus injury. Clin. Neurol. Neurosurg. 1993, 95, 65–72. [Google Scholar] [CrossRef]
- Krieg, S.M.; Lioumis, P.; Mäkelä, J.P.; Wilenius, J.; Karhu, J.; Hannula, H.; Savolainen, P.; Lucas, C.W.; Seidel, K.; Laakso, A.; et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. 2017, 159 (Suppl. S4), 1187–1195. [Google Scholar] [CrossRef]
- Corina, D.P.; Loudermilk, B.C.; Detwiler, L.; Martin, R.F.; Brinkley, J.F.; Ojemann, G. Analysis of naming errors during cortical stimulation mapping: Implications for models of language representation. Brain Lang. 2010, 115, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, P. Methods for estimating cortical motor representation size and location in navigated transcranial magnetic stimulation. J. Neurosci. Methods 2014, 232, 125–133. [Google Scholar] [CrossRef]
- Borghetti, D.; Sartucci, F.; Petacchi, E.; Guzzetta, A.; Piras, M.F.; Murri, L.; Cioni, G. Transcranial magnetic stimulation mapping: A model based on spline interpolation. Brain Res. Bull. 2008, 77, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Durner, G.; Gerst, A.; Ulrich, I.; Mayer, B.; Wirtz, C.R.; König, R.; Antoniadis, G.; Pedro, M.; Pala, A. Restoring musculocutaneous nerve function in 146 brachial plexus operations—A retrospective analysis. Clin. Neurol. Neurosur. 2023, 228, 107677. [Google Scholar] [CrossRef] [PubMed]
- Picht, T.; Strack, V.; Schulz, J.; Zdunczyk, A.; Frey, D.; Schmidt, S.; Vajkoczy, P. Assessing the functional status of the motor system in brain tumor patients using transcranial magnetic stimulation. Acta Neurochir. 2012, 154, 2075–2081. [Google Scholar] [CrossRef]
- Julkunen, P.; Könönen, M.; Määttä, S.; Tarkka, I.M.; Hiekkala, S.H.; Säisänen, L.; Vanninen, R.; Karhu, J.; Jäkälä, P. Longitudinal study on modulated corticospinal excitability throughout recovery in supratentorial stroke. Neurosci. Lett. 2016, 617, 88–93. [Google Scholar] [CrossRef]
- Bernabeu, M.; Demirtas-Tatlidede, A.; Opisso, E.; Lopez, R.; Tormos, J.M.; Pascual-Leone, A. Abnormal Corticospinal Excitability in Traumatic Diffuse Axonal Brain Injury. J. Neurotraum 2009, 26, 2185–2193. [Google Scholar] [CrossRef]
- Jussen, D.; Zdunczyk, A.; Schmidt, S.; Rösler, J.; Buchert, R.; Julkunen, P.; Karhu, J.; Brandt, S.; Picht, T.; Vajkoczy, P. Motor plasticity after extra–intracranial bypass surgery in occlusive cerebrovascular disease. Neurology 2016, 87, 27–35. [Google Scholar] [CrossRef]
- Acker, G.; Giampiccolo, D.; Rubarth, K.; Mertens, R.; Zdunczyk, A.; Hardt, J.; Jussen, D.; Schneider, H.; Rosenstock, T.; Mueller, V.; et al. Motor excitability in bilateral moyamoya vasculopathy and the impact of revascularization. Neurosurg. Focus 2021, 51, E7. [Google Scholar] [CrossRef]
- Leao, M.T.D.; Wiesinger, L.; Ziemann, U.; Tatagiba, M.; Naros, G. Rapid motor cortical reorganization following subacute spinal cord dysfunction. Brain Stimul. 2020, 13, 783–785. [Google Scholar] [CrossRef]
- Zdunczyk, A.; Schwarzer, V.; Mikhailov, M.; Bagley, B.; Rosenstock, T.; Picht, T.; Vajkoczy, P. The Corticospinal Reserve Capacity: Reorganization of Motor Area and Excitability As a Novel Pathophysiological Concept in Cervical Myelopathy. Neurosurgery 2018, 83, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Lioumis, P.; Mustanoja, S.; Bikmullina, R.; Vitikainen, A.M.; Kičić, D.; Salonen, O.; Tatlisumak, T.; Kaste, M.; Forss, N.; Mäkelä, J.P. Probing Modifications of Cortical Excitability During Stroke Recovery With Navigated Transcranial Magnetic Stimulation. Top. Stroke Rehabil. 2012, 19, 182–192. [Google Scholar] [CrossRef]
- Bulubas, L.; Sollmann, N.; Tanigawa, N.; Zimmer, C.; Meyer, B.; Krieg, S.M. Reorganization of Motor Representations in Patients with Brain Lesions: A Navigated Transcranial Magnetic Stimulation Study. Brain Topogr. 2018, 31, 288–299. [Google Scholar] [CrossRef]
- Forster, M.T.; Senft, C.; Hattingen, E.; Lorei, M.; Seifert, V.; Szelényi, A. Motor cortex evaluation by nTMS after surgery of central region tumors: A feasibility study. Acta Neurochir. 2012, 154, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nakamuro, T.; Tamural, R.; Takayanagi, T.; Kawanishi, K.; Tamai, S.; Mayer, R.F. Central motor reorganization after anastomosis of the musculocutaneous and intercostal nerves following cervical root avulsion. Ann. Neurol. 1995, 38, 15–20. [Google Scholar] [CrossRef]
- Chen, R.; Anastakis, D.J.; Haywood, C.T.; Mikulis, D.J.; Manktelow, R.T. Plasticity of the human motor system following muscle reconstruction: A magnetic stimulation and functional magnetic resonance imaging study. Clin. Neurophysiol. 2003, 114, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Rebello, A.; Balaini, N.; Gaba, S.; Mahesh, K.V. Biceps Activity Synchronous with Inspiration After Phrenic Nerve Transfer. Ann. Neurol. 2021, 89, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Socolovsky, M.; Malessy, M.; Bonilla, G.; Masi, G.D.; Conti, M.E.; Lovaglio, A. Phrenic to musculocutaneous nerve transfer for traumatic brachial plexus injuries: Analyzing respiratory effects on elbow flexion control. J. Neurosurg. 2019, 131, 165–174. [Google Scholar] [CrossRef]




| Volunteer | Sex | Age | Handedness | RMT, Left | Total Number of Stimulations, Left | Positive Motor Responses, Left | RMT, Right | Total Number of Stimulations, Right | Positive Motor Responses, Right |
|---|---|---|---|---|---|---|---|---|---|
| volunteer 1 | f | 29 | ambidextrous | 33% | 273 | 33 | 37% | 226 | 33 |
| volunteer 2 | f | 41 | right | 42% | 173 | 32 | 32% | 240 | 9 |
| volunteer 3 | m | 31 | right | 36% | 373 | 14 | 35% | 398 | 19 |
| volunteer 4 | f | 23 | right | 31% | 326 | 23 | 34% | 327 | 15 |
| volunteer 5 | m | 25 | right | 68% | 295 | 73 | 51% | 339 | 34 |
| volunteer 6 | f | 24 | left | 29% | 297 | 31 | 32% | 258 | 30 |
| volunteer 7 | f | 33 | right | 34% | 368 | 23 | 32% | 331 | 22 |
| volunteer 8 | m | 24 | right | 38% | 258 | 27 | 60% | 175 | 54 |
| volunteer 9 | f | 25 | right | 38% | 199 | 14 | 45% | 205 | 38 |
| volunteer 10 | f | 24 | right | 49% | 256 | 70 | 48% | 261 | 44 |
| sum | 2818 | 340 | 2760 | 298 | |||||
| mean | 27.9 | 40% | 41% | ||||||
| median | 37% | 36% |
| Patient | Sex | Age | Age at Accident | Handedness | Affected Hemisphere | Associated TBI | Detailed Procedure | Type of Reconstruction | RMT of Affected Hemisphere | Total Number of Stimuli on Affected Hemisphere | Positive Motor Responses on Affected Hemisphere | RMT of Unaffected Hemisphere | Total Number of Stimuli on Unaffected Hemisphere | Positive Motor Responses on Unaffected Hemisphere |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patient 1 | m | 52 | 45 | right | right | yes | Oberlin transfer | transfer | 37% | 184 | 20 | 59% | 172 | 25 |
| patient 2 | m | 29 | 25 | right | right | yes | phrenic nerve on biceps branch of MC + C5 on brachial branch of MC | combination | 36% | 184 | 19 | 49% | 256 | 18 |
| patient 3 | f | 41 | 32 | right | left | yes | phrenic nerve on MC | transfer | 84% | 77 | 32 | 99% | 74 | 8 |
| patient 4 | m | 27 | 17 | right | left | yes | neurolysis | neurolysis | ||||||
| patient 5 | f | 38 | 31 | right | left | no | C5 on MC + pectoral nerve on MC | combination | 44% | 229 | 10 | 48% | 212 | 31 |
| patient 6 | m | 33 | 25 | right | left | no | lateral fascicle on MC | anatomical reconstruction | 37% | 269 | 42 | 35% | 225 | 12 |
| patient 7 | m | 25 | 18 | right | left | no | C6 on MC | anatomical reconstruction | 32% | 313 | 41 | 39% | 285 | 23 |
| patient 8 | m | 36 | 26 | right | left | yes | lateral fascicle on MC | anatomical reconstruction | 60% | 306 | 21 | 51% | 354 | 29 |
| sum | 1562 | 185 | 1578 | 146 | ||||||||||
| mean | 35.1 | 47% | 54% | |||||||||||
| median | 41% | 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durner, G.; Ulrich, I.; Gerst, A.; Becker, R.; Wirtz, C.R.; Antoniadis, G.; Pedro, M.T.; Pala, A. Mapping Motor Neuroplasticity after Successful Surgical Brachial Plexus Reconstruction Using Navigated Transcranial Magnetic Stimulation (nTMS). Neurol. Int. 2024, 16, 239-252. https://doi.org/10.3390/neurolint16010016
Durner G, Ulrich I, Gerst A, Becker R, Wirtz CR, Antoniadis G, Pedro MT, Pala A. Mapping Motor Neuroplasticity after Successful Surgical Brachial Plexus Reconstruction Using Navigated Transcranial Magnetic Stimulation (nTMS). Neurology International. 2024; 16(1):239-252. https://doi.org/10.3390/neurolint16010016
Chicago/Turabian StyleDurner, Gregor, Ina Ulrich, Alexandra Gerst, Ralf Becker, Christian Rainer Wirtz, Gregor Antoniadis, Maria Teresa Pedro, and Andrej Pala. 2024. "Mapping Motor Neuroplasticity after Successful Surgical Brachial Plexus Reconstruction Using Navigated Transcranial Magnetic Stimulation (nTMS)" Neurology International 16, no. 1: 239-252. https://doi.org/10.3390/neurolint16010016
APA StyleDurner, G., Ulrich, I., Gerst, A., Becker, R., Wirtz, C. R., Antoniadis, G., Pedro, M. T., & Pala, A. (2024). Mapping Motor Neuroplasticity after Successful Surgical Brachial Plexus Reconstruction Using Navigated Transcranial Magnetic Stimulation (nTMS). Neurology International, 16(1), 239-252. https://doi.org/10.3390/neurolint16010016







