Treatment of Acute Ischaemic Stroke and Concomitant Multiple Arterial Splanchnic Thromboses in a Patient with Immune Thrombocytopenia on Thrombopoietin Agonist: A Case Report
Abstract
1. Introduction
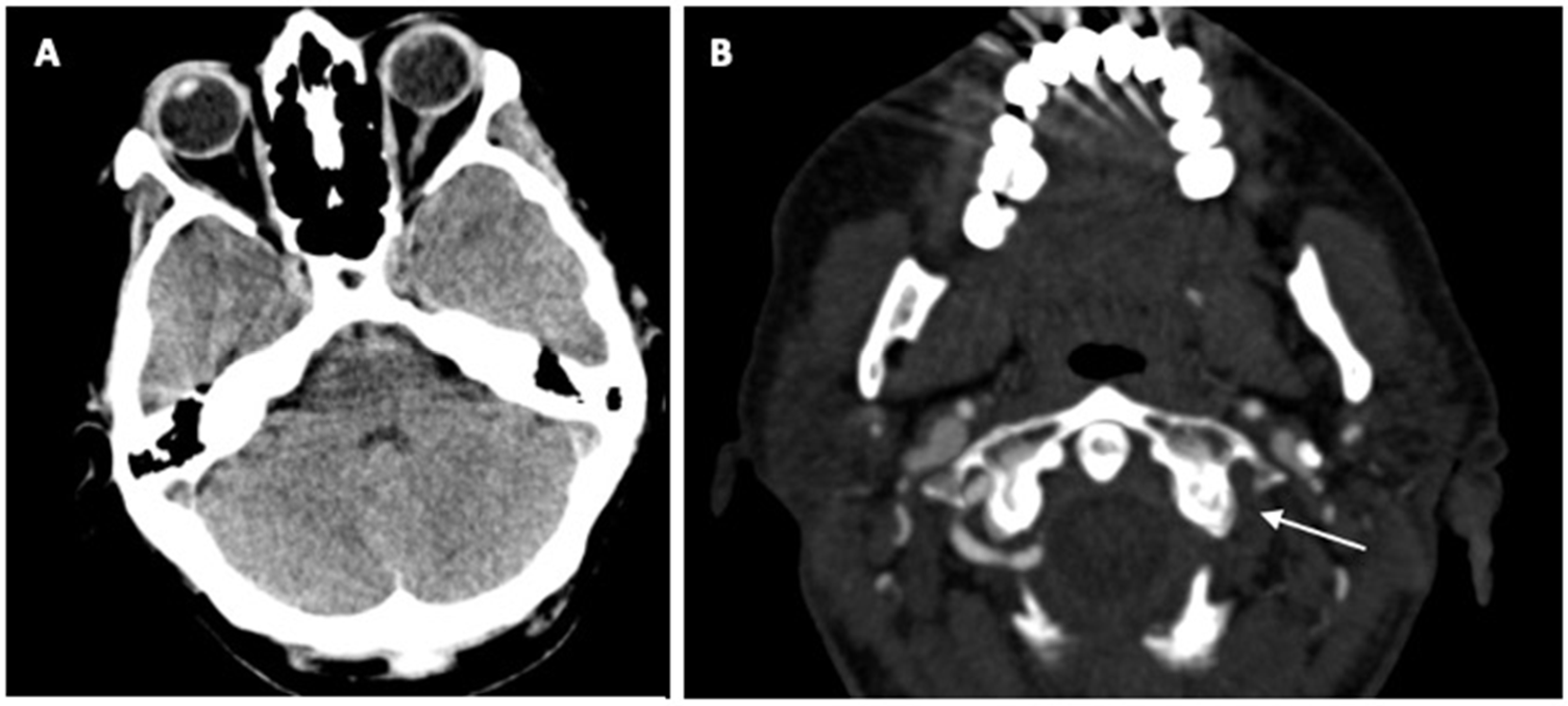
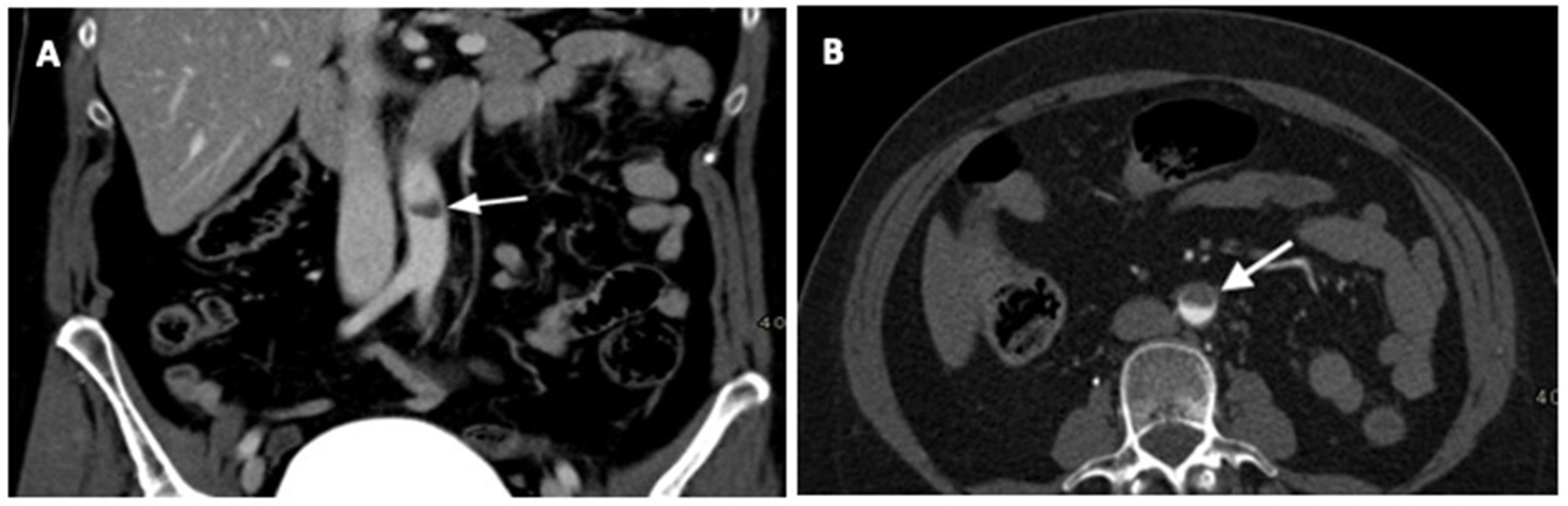
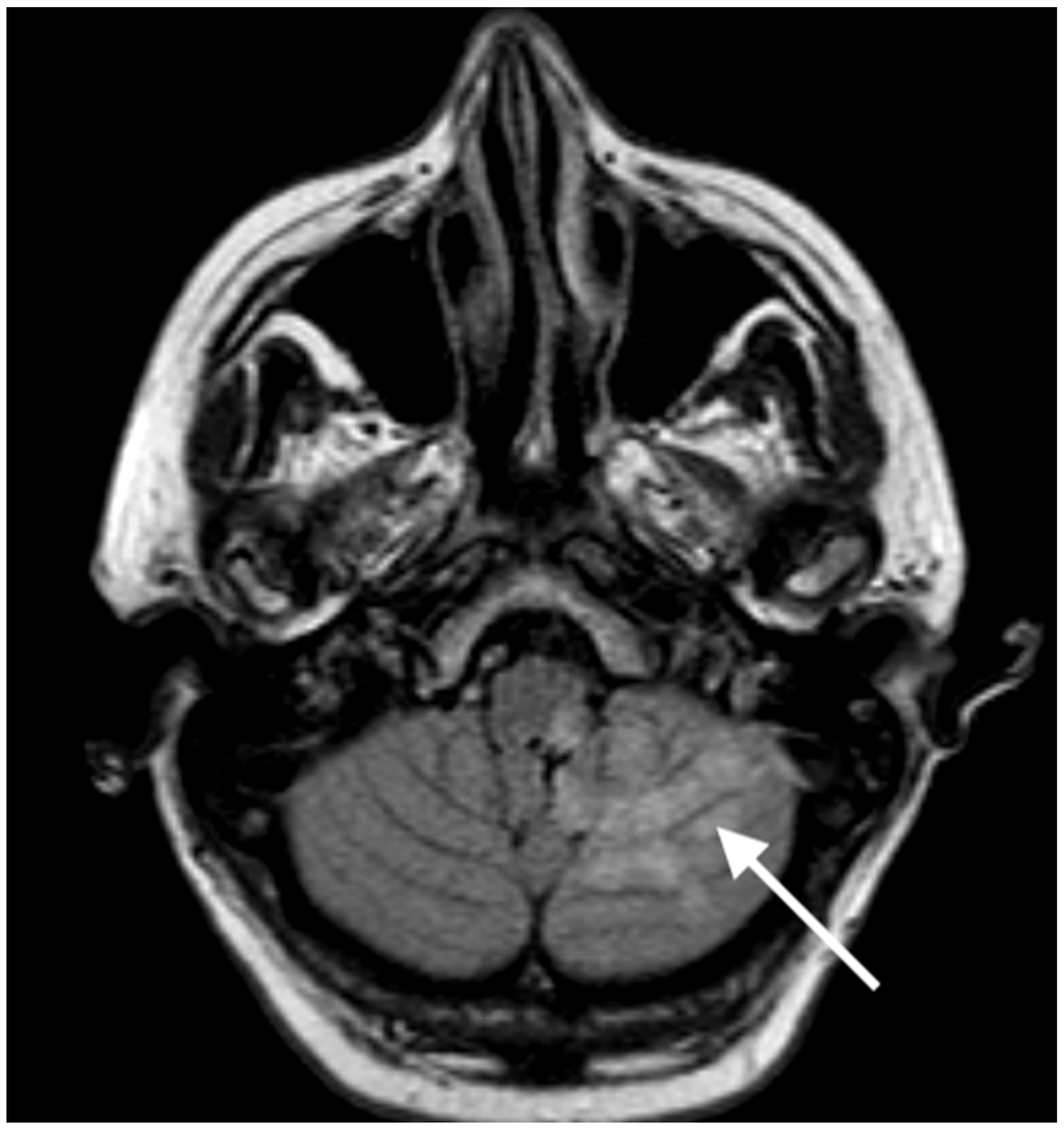
2. Case Report
3. Review of the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alrohimi, A.; Purdy, K.; Alqarni, M.; Alotaibi, G.; Blevins, G.; Butcher, K.; Rempel, J.; Wu, C.; Sun, H.L.; Khan, K. The Clinical Conundrum of Managing Ischemic Stroke in Patients with Immune Thrombocytopenia. Can. J. Neurol. Sci. 2021, 48, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Palmaro, A.; Montastruc, J.L.; Godeau, B.; Lapeyre-Mestre, M.; Sailler, L. Epidemiology of incident immune thrombocytopenia: A nationwide population-based study in France. Blood 2014, 124, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Semple, J.W. Recent advances in the mechanisms and treatment of immune thrombocytopenia. eBioMedicine 2022, 76, 103820. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J.; Callaghan, T.; Martlew, V. Thromboembolic events are not uncommon in 319 patients with immune thrombocytopenia. Br. J. Haematol. 2010, 150, 496–497. [Google Scholar] [PubMed]
- Rhee, H.Y.; Choi, H.Y.; Kim, S.B.; Shin, W.C. Recurrent ischemic stroke in a patient with idiopathic thrombocytopenic purpura. J. Thromb. Thrombolysis 2010, 30, 229–232. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A 349 Guideline for Healthcare Professionals from the American Heart Association/American 350 Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Tomich, C.; Debruxelles, S.; Delmas, Y.; Sagnier, S.; Poli, M.; Olindo, S.; Renou, P.; Rouanet, F.; Sibon, I. Immune-Thrombotic Thrombocytopenic Purpura is a Rare Cause of Ischemic Stroke in Young Adults: Case Reports and Literature Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 3163–3171. [Google Scholar] [CrossRef]
- Mihalov, J.; Timárová, G. A Seeming Paradox: Ischemic Stroke in the Context of Idiopathic Thrombocytopenic Purpura. Clin. Appl. Thromb. Hemost. 2016, 22, 115–120. [Google Scholar] [CrossRef]
- Chandan, J.S.; Thomas, T.; Lee, S.; Marshall, T.; Willis, B.; Nirantharakumar, K.; Gill, P. The association between idiopathic thrombocytopenic purpura and cardiovascular disease: A retrospective cohort study. J. Thromb. Haemost. 2018, 16, 474–480. [Google Scholar] [CrossRef]
- Hindi, Z.; Onteddu, N.; Ching, C.A.; Khaled, A.A. Vertebral Artery Thrombosis in Chronic Idiopathic Thrombocytopenic Purpura. Case Rep. Hematol. 2017, 2017, 3184346. [Google Scholar] [CrossRef]
- Hayashi, T.; Akioka, N.; Kashiwazaki, D.; Kuwayama, N.; Kuroda, S. Ischemic stroke in pediatric moyamoya disease associated with immune thrombocytopenia—A case report. Childs Nerv. Syst. 2015, 31, 991–996. [Google Scholar] [CrossRef]
- Ichijo, M.; Ishibashi, S.; Ohkubo, T.; Nomura, S.; Sanjo, N.; Yokota, T.; Mizusawa, H. Elevated platelet microparticle levels after acute ischemic stroke with concurrent idiopathic thrombocytopenic purpura. J. Stroke Cerebrovasc. Dis. 2014, 23, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Theeler, B.J.; Ney, J.P. A patient with idiopathic thrombocytopenic purpura presenting with an acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2008, 17, 244–245. [Google Scholar] [CrossRef]
- Pan, L.; Leng, H.; Huang, Y.; Xia, N.; Jin, L.; Zhang, H.T. Ischemic stroke/transient ischemic attack in adults with primary immune thrombocytopenia: A meta-analysis. Neurol. Sci. 2021, 42, 2013–2020. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, S.H. Ischemic stroke associated with immune thrombocytopenia: Lesion patterns and characteristics. Neurol. Sci. 2014, 35, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, A.; Fareed, J.; Thethi, I.; Morales-Vidal, S.; Schneck, M.J.; Shafer, D. Ischemic stroke in the setting of chronic immune thrombocytopenia in an elderly patient—A therapeutic dilemma. Clin. Appl. Thromb. Hemost. 2012, 18, 324–326. [Google Scholar] [CrossRef]
- Rong, X.; Jiang, L.; Qu, M.; Yang, S.; Wang, K.; Jiang, L. Risk factors and characteristics of ischemic stroke in patients with immune thrombocytopenia: A retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2022, 31, 106693. [Google Scholar] [CrossRef]
- Rungjirajittranon, T.; Owattanapanich, W. A serious thrombotic event in a patient with immune thrombocytopenia requiring intravenous immunoglobulin: A case report. J. Med. Case Rep. 2019, 13, 25. [Google Scholar] [CrossRef]
- Wang, W.-T.; Li, Y.-Y.; Lin, W.-C.; Chen, J.-Y.; Lan, K.-M.; Sun, C.-K.; Hung, K.-C. Bilateral visual loss and cerebral infarction after spleen embolization in a trauma patient with idiopathic thrombocytopenic purpura: A case report. Medicine 2018, 97, e0332. [Google Scholar] [CrossRef]
- Zhao, H.; Lian, Y.; Zhang, H.; Xie, N.; Gao, Y.; Wang, Z.; Zhang, Y. Ischemic stroke associated with immune thrombocytopenia. J. Thromb. Thrombolysis 2015, 40, 156–160. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Giagounidis, A.; Viallard, J.-F.; Godeau, B.; Pabinger, I.; Cines, D.; Liebman, H.; Wang, X.; Woodard, P. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: A pooled analysis of 13 clinical trials. Eur. J. Haematol. 2013, 91, 423–436. [Google Scholar] [CrossRef]
- Tjepkema, M.; Amini, S.; Schipperus, M. Risk of thrombosis with thrombopoietin receptor agonists for ITP patients: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2022, 171, 103581. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Wasser, J.; Rodeghiero, F.; Chong, B.H.; Steurer, M.; Provan, D.; Lyons, R.; Garcia-Chavez, J.; Carpenter, N.; Wang, X.; et al. Safety and efficacy of romiplostim in splenectomized and nonsplenectomized patients with primary immune thrombocytopenia. Haematologica 2017, 102, 1342–1351. [Google Scholar] [CrossRef]
- Palandri, F.; Rossi, E.; Bartoletti, D.; Ferretti, A.; Ruggeri, M.; Lucchini, E.; Carrai, V.; Barcellini, W.; Patriarca, A.; Rivolti, E.; et al. Real-world use of thrombopoietin receptor agonists in older patients with primary immune thrombocytopenia. Blood 2021, 138, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Gernsheimer, T.B.; George, J.N.; Aledort, L.M.; Tarantino, M.D.; Sunkara, U.; Guo, D.M.; Nichol, J.L. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP). J. Thromb. Haemost. 2010, 8, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.M.; Saleh, M.N.; Khelif, A.; Salama, A.; Portella, M.S.O.; Burgess, P.; Bussel, J.B. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: Final results of the EXTEND study. Blood 2017, 130, 2527–2536, Erratum in Blood 2018, 131, 709. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Pramanik, S.; Jandial, A.; Sahu, K.K.; Sandal, R.; Ahuja, A.; Yanamandra, U.; Kumar, R.; Kapoor, R.; Verma, T.; et al. Real-world experience of eltrombopag in immune thrombocytopenia. Am. J. Blood Res. 2020, 10, 240–251. [Google Scholar]
- Idowu, M.; Reddy, P. Atypical thrombotic thrombocytopenic purpura in a middle-aged woman who presented with a recurrent stroke. Am. J. Hematol. 2013, 88, 237–239. [Google Scholar] [CrossRef]
- Sevy, A.; Doche, E.; Squarcioni, C.; Poullin, P.; Serratrice, J.; Nicoli, F.; Weiller, P.-J. Stroke in a young patient treated by alteplase heralding an acquired thrombotic thrombocytopenic purpura. J. Clin. Apher. 2011, 26, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Shulman, K. Rituximab induces remission of cerebral ischemia caused by thrombotic thrombocytopenic purpura. Eur. J. Haematol. 2003, 70, 183–185. [Google Scholar] [CrossRef]
- Gensicke, H.; Al Sultan, A.S.; Strbian, D.; Hametner, C.; Zinkstok, S.M.; Moulin, S.; Bill, O.; Zini, A.; Padjen, V.; Kägi, G.; et al. Thrombolysis in Stroke Patients (TRISP) Collaborators. Intravenous thrombolysis and platelet count. Neurology 2018, 90, e690–e697, Erratum in Neurology 2018, 91, 852. [Google Scholar] [CrossRef] [PubMed]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, W.N.; Emberson, J.; Lees, K.R.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: A secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016, 15, 925–933. [Google Scholar] [CrossRef]
- Lerario, M.P.; Grotta, J.C.; Merkler, A.E.; Omran, S.S.; Chen, M.L.; Parikh, N.S.; Yaghi, S.; Murthy, S.; Navi, B.B.; Kamel, H. Association between intravenous thrombolysis and anaphylaxis among medicare benefeciaries with acute ischemic stroke. Stroke 2019, 50, 3283–3285. [Google Scholar] [CrossRef]
- Wölbing, F.; Fischer, J.; Köberle, M.; Kaesler, S.; Biedermann, T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy 2013, 68, 1085–1092. [Google Scholar] [CrossRef]
- Chiang, M.R.; Wei, C.C.; Muo, C.S.; Fu, L.S.; Li, T.C.; Kao, C.H. Association of primary immune thrombocytopenia and common allergic diseases among children. Pediatr. Res. 2015, 77, 597–601. [Google Scholar] [CrossRef]
- Bahoush, G.; Poorasgari, A.; Nojomi, M. Relationship of primary immune thrombocytopenic purpura and atopia among children: A case control study. Sci. Rep. 2020, 10, 11717. [Google Scholar] [CrossRef]
- Diez-Feijóo, R.; Rodríguez-Sevilla, J.; Colomo, L.; Papaleo, N.; Maiques, J.; Gimeno, E.; Andrade-Campos, M.; Abella, E.; Merchan, B.; Calvo, X.; et al. Massive intrasplenic arterial thrombosis in a patient with chronic ITP during the development of an Evans syndrome. Thromb. Res. 2021, 208, 226–229. [Google Scholar] [CrossRef]
- Saito, M.; Morioka, M.; Izumiyama, K.; Mori, A.; Kondo, T. Severe Portal Vein Thrombosis During Eltrombopag Treatment Concomitant Splenectomy for Immune Thrombocytopenia. Cureus 2021, 13, e17478. [Google Scholar] [CrossRef]
- Dichtwald, S.; Meyer, A.; Ifrach, N. Catastrophic anti-phospholipid syndrome with Libman-Sacks endocarditis following eltrombopag therapy for immune thrombocytopenic purpura: A case report. Lupus 2021, 30, 2304–2309. [Google Scholar] [CrossRef]
- Ghumman, G.M.; Fatima, H.; Singh, G.; Khalid, T.; Ayoubi, M. Risk of Thromboembolism with Eltrombopag: A Case Report of Deep Vein Thrombosis and Bilateral Pulmonary Embolism. Cureus 2023, 15, e33615. [Google Scholar] [CrossRef]
- Mulla, C.M.; Rashidi, A.; Levitov, A.B. Extensive cerebral venous sinus thrombosis following a dose increase in eltrombopag in a patient with idiopathic thrombocytopenic purpura. Platelets 2014, 25, 144–146. [Google Scholar] [CrossRef]
- Gunes, H.; Kivrak, T. Eltrombopag Induced Thrombosis: A Case with Acute Myocardial Infarction. Curr. Drug Saf. 2016, 11, 174–176. [Google Scholar] [CrossRef]
- Iinuma, S.; Nagasawa, Y.; Sasaki, K.; Hayashi, K.; Kanno, K.; Honma, M.; Sugawara, M.; Kinouchi, M.; Obata, M.; Ishida-Yamamoto, A. Cutaneous thrombosis associated with eltrombopag treatment for immune thrombocytopenia. J. Dermatol. 2020, 47, e57–e58. [Google Scholar] [CrossRef]
- Mohamed, S.E.; Yassin, M.A. Eltrombopag Use for Treatment of Thrombocytopenia in a Patient with Chronic Liver Disease and Portal Vein Thrombosis: Case Report. Case Rep. Oncol. 2020, 13, 863–866. [Google Scholar] [CrossRef]
- Oo, Z.; Manvar, K.; Wang, J.C. Eltrombopag-Induced Thrombocytosis and Thrombosis in Patients With Antiphospholipid Syndrome and Immune Thrombocytopenic Purpura. J. Investig. Med. High Impact Case Rep. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Bosi, A.; Barcellini, W.; Fattizzo, B. Pulmonary embolism in a patient with eltrombopag-treated aplastic anaemia and paroxysmal nocturnal haemoglobinuria clone during COVID-19 pneumonia. Thromb. J. 2022, 20, 46, Erratum in Thromb. J. 2022, 20, 51. [Google Scholar] [CrossRef]
- Kawano, N.; Hasuike, S.; Iwakiri, H.; Nakamura, K.; Ozono, Y.; Kusumoto, H.; Nagata, K.; Kikuchi, I.; Yoshida, S.; Kuriyama, T.; et al. Portal vein thrombosis during eltrombopag treatment for immune thrombocytopenic purpura in a patient with liver cirrhosis due to hepatitis C viral infection. J. Clin. Exp. Hematop. 2013, 53, 151–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baumann, A.J.; Wheeler, D.S.; Varadi, G.; Feyssa, E. Severe Thrombotic Complication of Eltrombopag in a Cirrhotic Patient. ACG Case Rep. J. 2016, 3, 121–123. [Google Scholar] [CrossRef]
- Pravdic, Z.; Suvajdzic-Vukovic, N.; Djurdjevic, P.; Pantic, N.; Bukumiric, Z.; Virijevic, M.; Todorovic-Tirnanic, M.; Thachil, J.; Mitrovic, M. Platelet kinetics in patients with chronic immune thrombocytopaenia treated with thrombopoietin receptor agonists. Eur. J. Haematol. 2023, 110, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, W.E.M.; Poolen, G.C.; Huisman, A.; Koene, H.R.; Fijnheer, R.; Thielen, N.; van Bladel, E.R.; van Galen, K.P.M.; Schutgens, R.E.G.; Urbanus, R.T. Evaluation of the procoagulant state in chronic immune thrombocytopenia before and after eltrombopag treatment-a prospective cohort study. J. Thromb. Haemost. 2022, 21, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Garabet, L.; Ghanima, W.; Hellum, M.; Sandset, P.M.; Bussel, J.B.; Tran, H.; Henriksson, C.E. Increased microvesicle-associated thrombin generation in patients with immune thrombocytopenia after initiation of thrombopoietin receptor agonists. Platelets 2020, 31, 322–328. [Google Scholar] [CrossRef] [PubMed]
| References | Age | Gender | Platelet Count (×109/L) | NIHSS Adm | EVT | Control CT Scan | NIHSS Dis | mRS Dis | mRS 3 Months |
|---|---|---|---|---|---|---|---|---|---|
| Alrohimi et al. [1] | 61 | F | 48 | 16 | 1 | Ischaemic stroke | 0 | 1 | 1 |
| 75 | M | 366 | 4 | 1 | ICH plus SAH | NA | 6 | 6 | |
| Tomich et al. [9] | 33 | M | 80 | 2 | 0 | NA | 0 | 0 | 0 |
| Own case report | 56 | F | 109 | 7 | 0 | Ischaemic stroke | 2 | 3 | 1 |
| Total data | 56 | f:m = 1:1 | 151 | 7.3 | 2 | NA | 2.5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frol, S.; Pretnar Oblak, J.; Šabovič, M.; Kermer, P.; Sever, M. Treatment of Acute Ischaemic Stroke and Concomitant Multiple Arterial Splanchnic Thromboses in a Patient with Immune Thrombocytopenia on Thrombopoietin Agonist: A Case Report. Neurol. Int. 2023, 15, 1191-1199. https://doi.org/10.3390/neurolint15030074
Frol S, Pretnar Oblak J, Šabovič M, Kermer P, Sever M. Treatment of Acute Ischaemic Stroke and Concomitant Multiple Arterial Splanchnic Thromboses in a Patient with Immune Thrombocytopenia on Thrombopoietin Agonist: A Case Report. Neurology International. 2023; 15(3):1191-1199. https://doi.org/10.3390/neurolint15030074
Chicago/Turabian StyleFrol, Senta, Janja Pretnar Oblak, Mišo Šabovič, Pawel Kermer, and Matjaž Sever. 2023. "Treatment of Acute Ischaemic Stroke and Concomitant Multiple Arterial Splanchnic Thromboses in a Patient with Immune Thrombocytopenia on Thrombopoietin Agonist: A Case Report" Neurology International 15, no. 3: 1191-1199. https://doi.org/10.3390/neurolint15030074
APA StyleFrol, S., Pretnar Oblak, J., Šabovič, M., Kermer, P., & Sever, M. (2023). Treatment of Acute Ischaemic Stroke and Concomitant Multiple Arterial Splanchnic Thromboses in a Patient with Immune Thrombocytopenia on Thrombopoietin Agonist: A Case Report. Neurology International, 15(3), 1191-1199. https://doi.org/10.3390/neurolint15030074





