3D Imaging of Striatal Transplants in a Small Animal Model of Huntington’s Disease
Abstract
1. Introduction
2. Materials and Methods
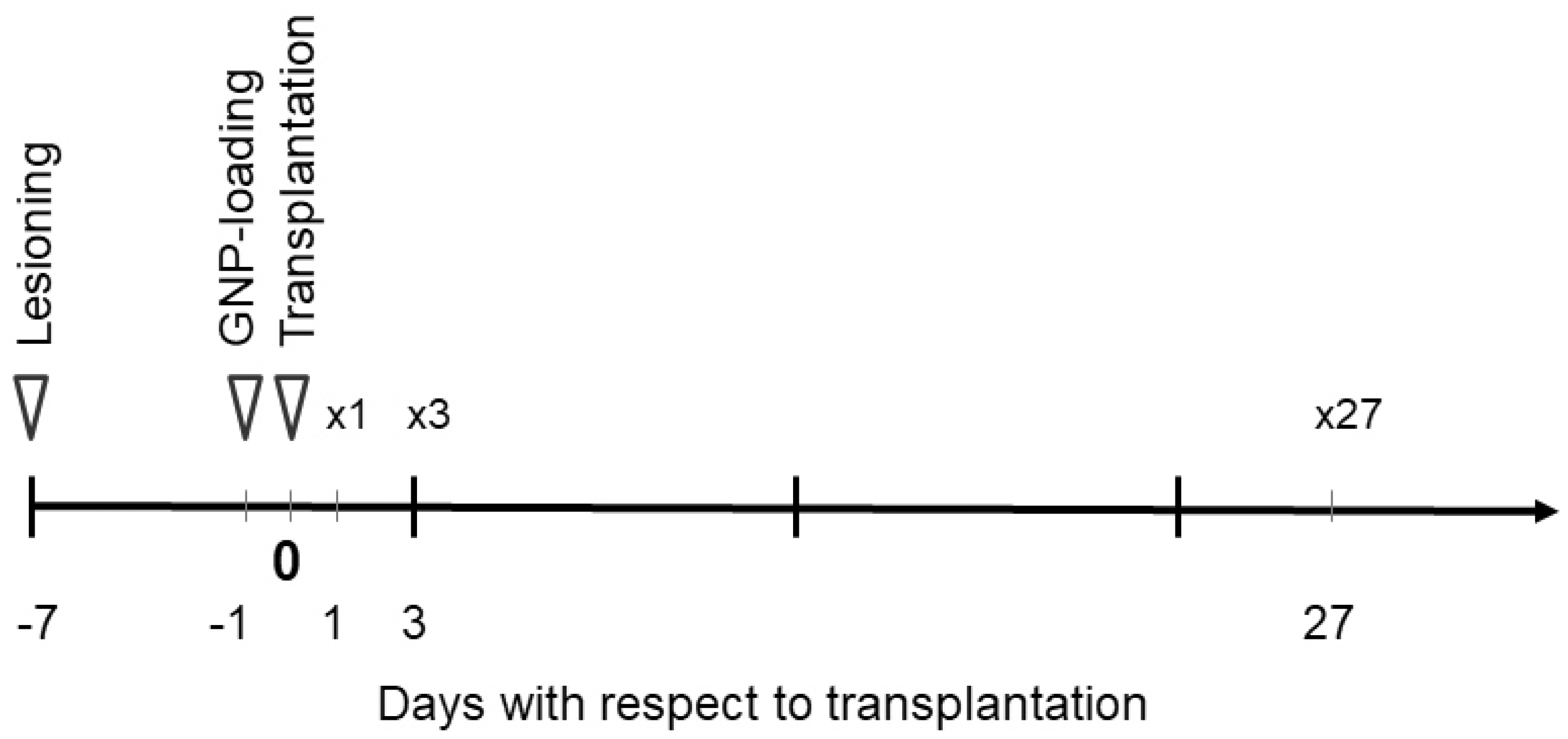
2.1. Striatal Lesioning in the Rat Model
2.2. Dissection of Rat Embryonic Whole Ganglionic Eminences (wGEs)
2.3. Tissue Culture and GNP Exposure
2.4. Transplantation in the Rat Model
2.5. Synchrotron Radiation-Based CT Image Acquisition
2.6. MR Image Acquisition
2.7. Immunohistochemistry
3. Results
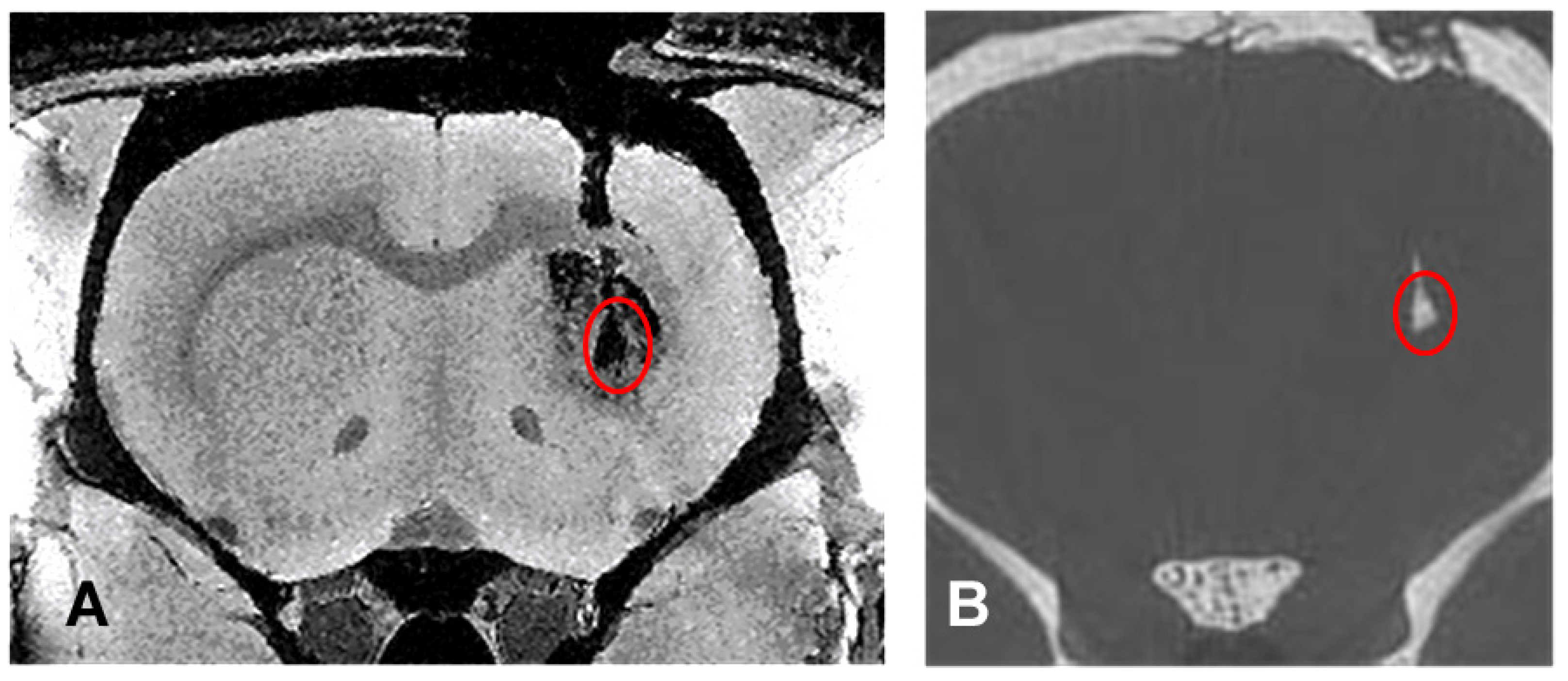
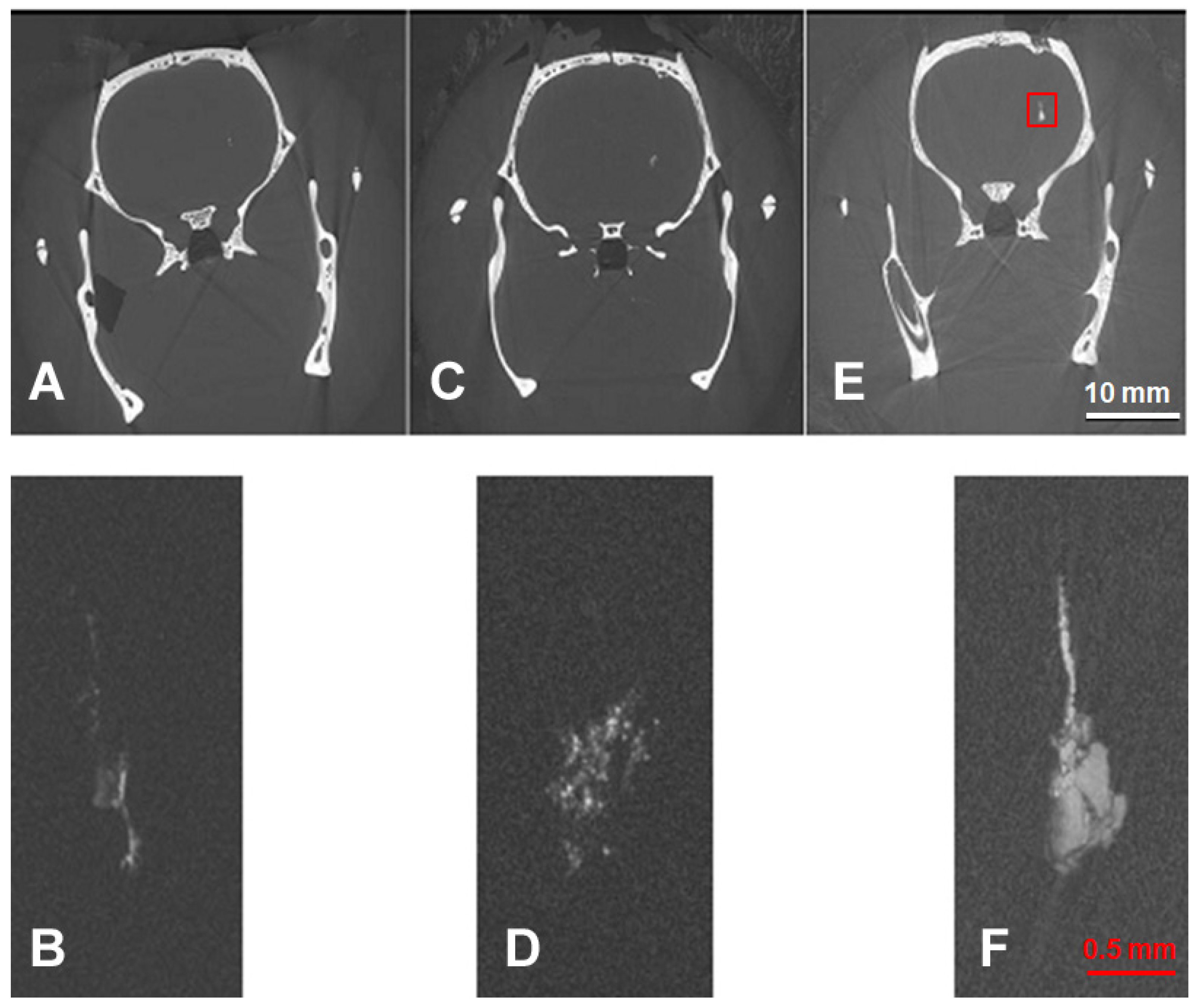
3.1. Comparison of Imaging Modalities
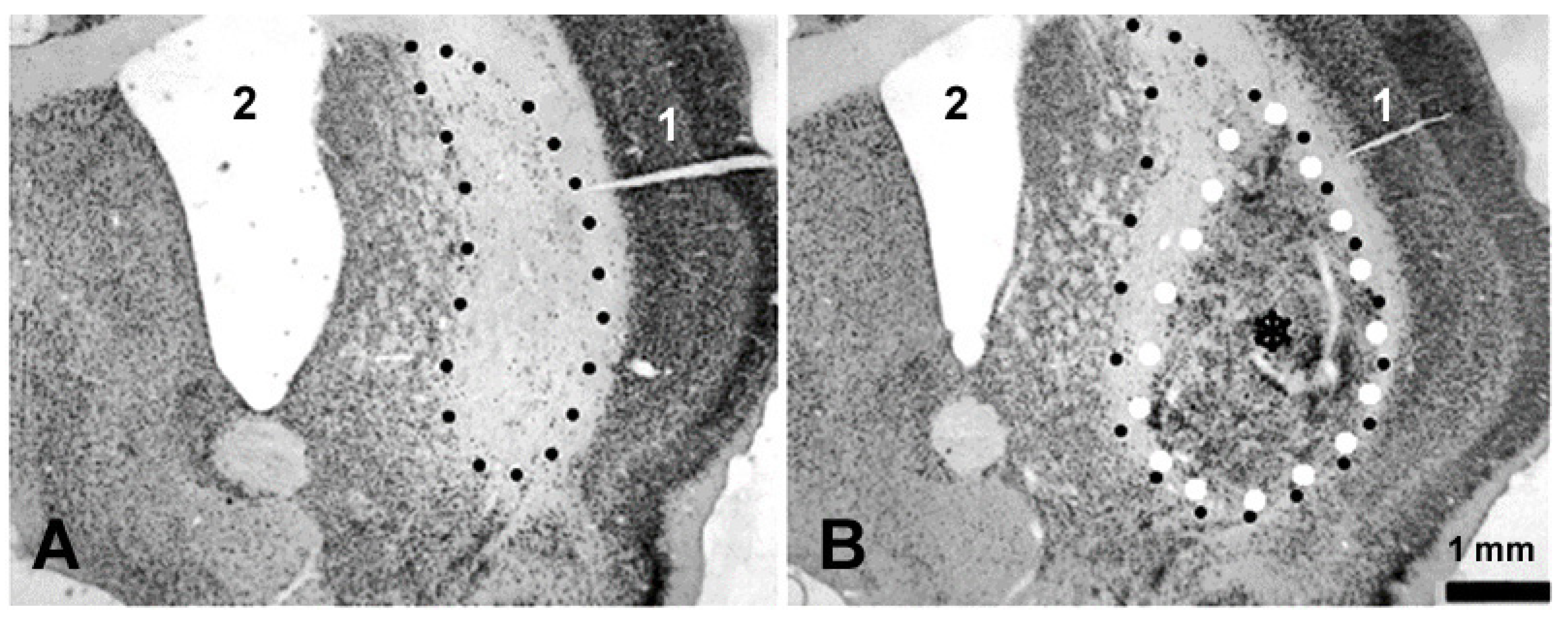
3.2. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachoud-Lévi, A.C.; Massart, R.; Rosser, A. Cell therapy in Huntington’s disease: Taking stock of past studies to move the field forward. Stem Cells 2021, 39, 144–155. [Google Scholar] [CrossRef]
- Dunnett, S.B.; Rosser, A.E. Clinical translation of cell transplantation in the brain. Curr. Opin. Organ Transplant. 2011, 16, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Björklund, A. Cell replacement therapy: Helping the brain to repair itself. NeuroRx 2004, 1, 379–381. [Google Scholar] [CrossRef]
- Precious, S.V.; Zietlow, R.; Dunnett, S.B.; Kelly, C.M.; Rosser, A.E. Is there a place for human fetal-derived stem cells for cell replacement therapy in Huntington’s disease? Neurochem. Int. 2017, 106, 114–121. [Google Scholar] [CrossRef]
- Ebrahimi, M.J.; Aliaghaei, A.; Boroujeni, M.E.; Khodagholi, F.; Meftahi, G.; Abdollahifar, M.A.; Ahmadi, H.; Danyali, S.; Daftari, M.; Sadeghi, Y. Human Umbilical Cord Matrix Stem Cells Reverse Oxidative Stress-Induced Cell Death and Ameliorate Motor Function and Striatal Atrophy in Rat Model of Huntington Disease. Neurotox. Res. 2018, 34, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.D.; Rossignol, J.; Crane, A.T.; Davis, K.K.; Bombard, M.C.; Bavar, A.M.; Clerc, S.; Lowrance, S.A.; Song, C.; Lescaudron, L.; et al. Transplantation of umbilical cord-derived mesenchymal stem cells into the striata of R6/2 mice: Behavioral and neuropathological analysis. Stem Cell Res. Ther. 2013, 4, 130. [Google Scholar] [CrossRef]
- Adil, M.M.; Gaj, T.; Rao, A.T.; Kulkarni, R.U.; Fuentes, C.M.; Ramadoss, G.N.; Ekman, F.K.; Miller, E.W.; Schaffer, D.V. hPSC-Derived Striatal Cells Generated Using a Scalable 3D Hydrogel Promote Recovery in a Huntington Disease Mouse Model. Stem Cell Rep. 2018, 10, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharaibeh, A.; Culver, R.; Stewart, A.N.; Srinageshwar, B.; Spelde, K.; Frollo, L.; Kolli, N.; Story, D.; Paladugu, L.; Anwar, S.; et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Transplantations Reduced Behavioral Deficits and Ameliorated Neuropathological Changes in YAC128 Mouse Model of Huntington’s Disease. Front. Neurosci. 2017, 11, 628. [Google Scholar] [CrossRef]
- Hauser, R.A.; Furtado, S.; Cimino, C.R.; Delgado, H.; Eichler, S.; Schwartz, S.; Scott, D.; Nauert, G.M.; Soety, E.; Sossi, V.; et al. Bilateral human fetal striatal transplantation in Huntington’s disease. Neurology 2002, 58, 687–695. [Google Scholar] [CrossRef]
- Kopyov, O.V.; Jacques, S.; Lieberman, A.; Duma, C.M.; Eagle, K.S. Safety of intrastriatal neurotransplantation for Huntington’s disease patients. Exp. Neurol. 1998, 149, 97–108. [Google Scholar] [CrossRef]
- Keene, C.D.; Chang, R.C.; Leverenz, J.B.; Kopyov, O.; Perlman, S.; Hevner, R.F.; Born, D.E.; Bird, T.D.; Montine, T.J. A patient with Huntington’s disease and long-surviving fetal neural transplants that developed mass lesions. Acta Neuropathol. 2009, 117, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.; Dunnett, S.B. Mouse Models of Huntington’s Disease. In Behavioral Neurobiology of Huntington’s Disease and Parkinson’s Disease; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2015; Volume 22, pp. 101–133. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Butland, S.L.; Pouladi, M.A.; Hayden, M.R. Mouse models of Huntington disease: Variations on a theme. Dis. Model. Mech. 2009, 2, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sanberg, P.R.; Calderon, S.F.; Giordano, M.; Tew, J.M.; Norman, A.B. The quinolinic acid model of Huntington’s disease: Locomotor abnormalities. Exp. Neurol. 1989, 105, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Beal, M.F.; Mazurek, M.F.; Malloy, J.R.; Bird, E.D.; Martin, J.B. Amino acid neurotransmitter abnormalities in Huntington’s disease and the quinolinic acid animal model of Huntington’s disease. Brain 1987, 110 Pt 6, 1657–1673. [Google Scholar] [CrossRef] [PubMed]
- Nakao, N.; Grasbon-Frodl, E.M.; Widner, H.; Brundin, P. DARPP-32-rich zones in grafts of lateral ganglionic eminence govern the extent of functional recovery in skilled paw reaching in an animal model of Huntington’s disease. Neuroscience 1996, 74, 959–970. [Google Scholar] [CrossRef]
- Jiang, W.; Büchele, F.; Papazoglou, A.; Döbrössy, M.; Nikkhah, G. Multitract microtransplantation increases the yield of DARPP-32-positive embryonic striatal cells in a rodent model of Huntington’s disease. Cell Transplant. 2011, 20, 1515–1527. [Google Scholar] [CrossRef]
- Kuhlenbeck, H. Central Nervous System of Vertebrates; Karger Medical and Scientific Publishers: New York, NY, USA, 1973; Volume 3, Part II; p. 735. ISBN 3805517327/9783805517324. [Google Scholar]
- Allen, J.S.; Damasio, H.; Grabowski, T.J. Normal neuroanatomical variation in the human brain: An MRI-volumetric study. Am. J. Phys. Anthropol. 2002, 118, 341–358. [Google Scholar] [CrossRef]
- Sahin, B.; Aslan, H.; Unal, B.; Canan, S.; Bilgic, S.; Kaplan, S.; Tumkaya, L. Brain volumes of the lamb, rat and bird do not show hemispheric asymmetry: A stereological study. Image Anal. Stereol. 2001, 20, 9–13. [Google Scholar] [CrossRef]
- Schültke, E.; Menk, R.; Pinzer, B.; Astolfo, A.; Stampanoni, M.; Arfelli, F.; Harsan, L.A.; Nikkhah, G. Single-cell resolution in high-resolution synchrotron X-ray CT imaging with gold nanoparticles. J. Synchrotron Radiat. 2014, 21 Pt 1, 242–250. [Google Scholar] [CrossRef]
- Astolfo, A.; Schültke, E.; Menk, R.H.; Hall, C.; Kirch, R.; Stebel, M.; Harsan, L.; Juurlink, B.H.J.; Arfelli, F. In-vivo visualizatio, n of gold loaded cells in mice using x-ray computed tomography. Nanomedicine 2013, 9, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Döbrössy, M.D.; Dunnett, S.B. Training specificity, graft development and graft-mediated functional recovery in a rodent model of Huntington’s disease. Neuroscience 2005, 132, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni, M.; Groso, A.; Isenegger, A.; Mikuljan, G.; Chen, Q.; Meister, D.; Lange, M.; Betemps, R.; Henein, S.; Abela, R. TOMCAT: A beamline for TOmographic Microscopy and Coherent rAdiology experimenTs. In Synchrotron Radiation Instrumentation, Proceedings of the Ninth International Conference on Synchrotron Radiation Instrumentation, Daegu, Republic of Korea, 28 May–2 June 2006; Choi, J.-Y., Rah, S., Eds.; AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2007; Volume 879, pp. 848–851. [Google Scholar] [CrossRef]
- Hintermüller, C.; Marone, F.; Isenegger, A.; Stampanoni, M. Image processing pipeline for synchrotron-radiation-based tomographic microscopy. J. Synchrotron Radiat. 2010, 17, 550–559. [Google Scholar] [CrossRef]
- Available online: http://www.osirix-viewer.com (accessed on 1 January 2013).
- Available online: http://anusf.anu.edu.au/Vizlab/drishti/ (accessed on 1 January 2013).
- Bockeria, L.; Bogin, V.; Bockeria, O.; Le, T.; Alekyan, B.; Woods, E.J.; Brown, A.A.; Ichim, T.E.; Patel, A.N. Endometrial regenerative cells for treatment of heart failure: A new stem cell enters the clinic. J. Translat. Med. 2013, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal stem cells prevent hydrocephalus after severe Intraventricular haemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef]
- Giusto, E.; Donegà, M.; Cossetti, C.; Pluchino, S. Neuro-immune interactions of neural stem cell transplants: From animal disease models to human trials. Exp. Neurol. 2013, 260, 19–32. [Google Scholar] [CrossRef]
- van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal Stem Cell Transplantation Attenuates Brain Injury after Neonatal Stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef]
- Björklund, A. Cell replacement strategies for neurodegenerative disorders. Novartis Found. Symp. 2000, 231, 7–15, discussion 16–20. [Google Scholar] [CrossRef]
- Seminatore, C.; Polentes, J.; Ellman, D.; Kozubenko, N.; Itier, V.; Tine, S.; Tritschler, L.; Brenot, M.; Guidou, E.; Blondeau, J.; et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke 2010, 41, 153–159. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef]
- Lu, S.S.; Liu, S.; Zu, Q.Q.; Xu, X.Q.; Yu, J.; Wang, J.W.; Zhang, Y.; Shi, H.B. In vivo MR imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PLoS ONE 2013, 8, e54963. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Björklund, A.; Campbell, K. Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience 1998, 84, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Bronckaers, A.; Vande Velde, G.; Willems, G.; Cadenas de Llano-Pérula, M. In vivo micro-computerized tomography tracking of human periodontal ligament stem cells labeled with gold nanocomplexes. Adv. Healthc. Mater. 2022, 11, e2101133. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Bao, H.; Chen, Z.; Li, X.; Liu, X.; Wang, W.; Huang, J.; Zhang, Z. Enhanced and long-term CT imaging tracking of transplanted stem cells labeled with temperature-responsive gold nanoparticles. J. Mater. Chem. B 2021, 9, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Pinzer, B.R.; Cacquevel, M.; Modregger, P.; McDonald, S.A.; Bensadoun, J.C.; Thüring, T.; Aebischer, P.; Stampanoni, M. Imaging brain amyloid deposition using grating-based differential phase contrast tomography. NeuroImage 2012, 61, 1336–1346. [Google Scholar] [CrossRef]
- Conner, L.T.; Srinageshwar, B.; Bakke, J.L.; Dunbar, G.L.; Rossignol, J. Advances in stem cell and other therapies for Huntington’s disease: An update. Brain Res. Bull. 2023, 199, 110673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schültke, E.; Pinzer, B.R.; Stampanoni, M.; Harsan, L.; Döbrössy, M. 3D Imaging of Striatal Transplants in a Small Animal Model of Huntington’s Disease. Neurol. Int. 2023, 15, 896-907. https://doi.org/10.3390/neurolint15030057
Schültke E, Pinzer BR, Stampanoni M, Harsan L, Döbrössy M. 3D Imaging of Striatal Transplants in a Small Animal Model of Huntington’s Disease. Neurology International. 2023; 15(3):896-907. https://doi.org/10.3390/neurolint15030057
Chicago/Turabian StyleSchültke, Elisabeth, Bernd R. Pinzer, Marco Stampanoni, Laura Harsan, and Mátè Döbrössy. 2023. "3D Imaging of Striatal Transplants in a Small Animal Model of Huntington’s Disease" Neurology International 15, no. 3: 896-907. https://doi.org/10.3390/neurolint15030057
APA StyleSchültke, E., Pinzer, B. R., Stampanoni, M., Harsan, L., & Döbrössy, M. (2023). 3D Imaging of Striatal Transplants in a Small Animal Model of Huntington’s Disease. Neurology International, 15(3), 896-907. https://doi.org/10.3390/neurolint15030057





