Narrative Review Concerning the Clinical Spectrum of Ophthalmological Impairments in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
3. Pathogenesis of Parkinson’s Disease
3.1. Pathogenesis
3.2. Mitochondrial Dysfunction in Parkinson’s Disease
3.3. Oxidative Stress in Parkinson’s Disease
3.4. Parkinson’s Disease Evidence of Oxidative Injury
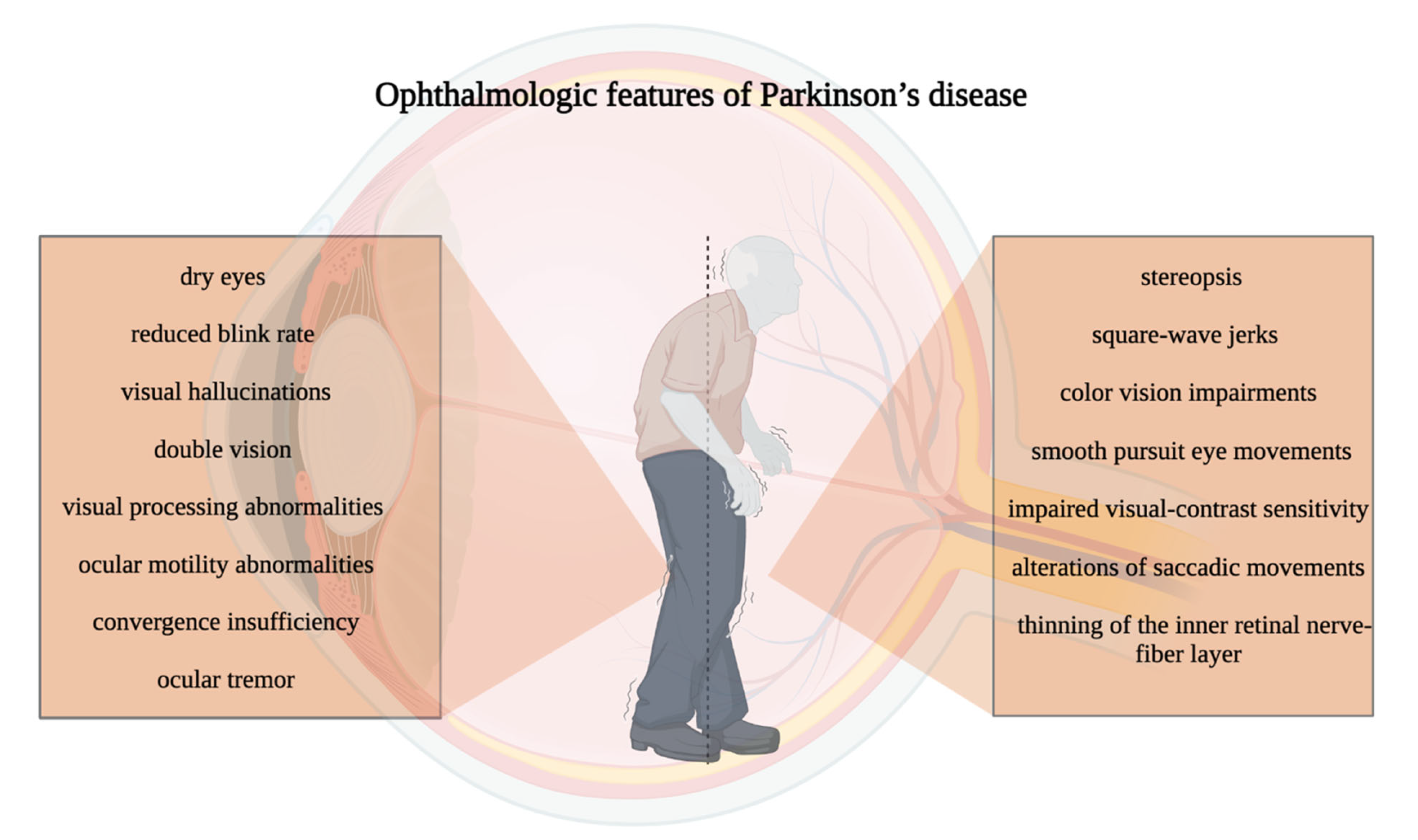
4. Ocular Manifestations in Parkinson’s Disease
4.1. Dopaminergic System and the Eye
4.2. Vision Disorders during Pd: Clinical Manifestations and Cellular and Molecular Mechanisms
4.3. Color Perception
4.4. Contrast Perception
4.5. Saccades
4.6. Effortless Pursuit of the Eyes
4.7. Square-Wave Jerks
4.8. Convergence Inadequacy
4.9. Diplopia
4.10. Blepharospasm and Blepharitis
4.11. Ocular Tremor
4.12. Reduced Blinking Rate
4.13. Stereopsis
4.14. Visual Contrast Acuity
4.15. Pupil Reactivity
4.16. Cornea in PD
4.17. Retina in PD
4.18. RNFL and OCT Changes
5. Ophthalmologic Diseases in Parkinson’s Disease
5.1. Dry-Eye Syndrome in Parkinson’s Disease
5.2. Parkinson’s Disease and Glaucoma
5.3. Parkinson’s Disease and Cataract
6. PD Visual Impairments and Quality of Life
7. Management and Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
- Bodis-Wollner, I. Retinopathy in Parkinson Disease. J. Neural Transm. 2009, 116, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Aydin, T.S.; Umit, D.; Nur, O.M.; Fatih, U.; Asena, K.; Nefise, O.Y.; Serpil, Y. Optical Coherence Tomography Findings in Parkinson’s Disease. Kaohsiung J. Med. Sci. 2018, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, T.; Wang, M.; Jin, L.; Zhao, Y.; Chen, S.; Wang, C.; Zhang, G.; Wang, Q.; Deng, Q.; et al. Pilot Study of a Novel Classroom Designed to Prevent Myopia by Increasing Children’s Exposure to Outdoor Light. PLoS ONE 2017, 12, e0181772. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.; Hankins, M.W.; Hirano, J.; Archer, S.N. Neurobiology of Retinal Dopamine in Relation to Degenerative States of the Tissue. Vis. Res. 1997, 37, 3509–3529. [Google Scholar] [CrossRef] [PubMed]
- Ortuño-Lizarán, I.; Beach, T.G.; Serrano, G.E.; Walker, D.G.; Adler, C.H.; Cuenca, N. Phosphorylated α-Synuclein in the Retina Is a Biomarker of Parkinson’s Disease Pathology Severity: P-α-Synuclein in Parkinson’s Disease Retina. Mov. Disord. 2018, 33, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Mammadova, N.; Summers, C.M.; Kokemuller, R.D.; He, Q.; Ding, S.; Baron, T.; Yu, C.; Valentine, R.J.; Sakaguchi, D.S.; Kanthasamy, A.G.; et al. Accelerated Accumulation of Retinal α-Synuclein (PSer129) and Tau, Neuroinflammation, and Autophagic Dysregulation in a Seeded Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2019, 121, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Funke, C.; Schneider, S.A.; Berg, D.; Kell, D.B. Genetics and Iron in the Systems Biology of Parkinson’s Disease and Some Related Disorders. Neurochem. Int. 2013, 62, 637–652. [Google Scholar] [CrossRef]
- Davidsdottir, S.; Cronin-Golomb, A.; Lee, A. Visual and Spatial Symptoms in Parkinson’s Disease. Vis. Res. 2005, 45, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Urwyler, P.; Nef, T.; Killen, A.; Collerton, D.; Thomas, A.; Burn, D.; McKeith, I.; Mosimann, U.P. Visual Complaints and Visual Hallucinations in Parkinson’s Disease. Park. Relat. Disord. 2014, 20, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Biouse, V.; Skibell, B.C.; Watts, R.L.; Loupe, D.N.; Drews-Botsch, C.; Newman, N.J. Ophthalmologic Features of Parkinson’s Disease. Am. J. Ophthalmol. 2004, 137, 972. [Google Scholar] [CrossRef]
- Nicoletti, V.; Palermo, G.; Del Prete, E.; Mancuso, M.; Ceravolo, R. Understanding the Multiple Role of Mitochondria in Parkinson’s Disease and Related Disorders: Lesson from Genetics and Protein-Interaction Network. Front. Cell Dev. Biol. 2021, 9, 636506. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Mitochondria, Oxidative Damage, and Inflammation in Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2003, 991, 120–131. [Google Scholar] [CrossRef]
- Muqit, M.M.K.; Gandhi, S.; Wood, N.W. Mitochondria in Parkinson Disease: Back in Fashion with a Little Help from Genetics: Back in Fashion with a Little Help from Genetics. Arch. Neurol. 2006, 63, 649–654. [Google Scholar] [CrossRef]
- Greene, J.G.; Dingledine, R.; Greenamyre, J.T. Gene Expression Profiling of Rat Midbrain Dopamine Neurons: Implications for Selective Vulnerability in Parkinsonism. Neurobiol. Dis. 2005, 18, 19–31. [Google Scholar] [CrossRef]
- Jayaram, S.; Krishnamurthy, P.T. Role of Microgliosis, Oxidative Stress and Associated Neuroinflammation in the Pathogenesis of Parkinson’s Disease: The Therapeutic Role of Nrf2 Activators. Neurochem. Int. 2021, 145, 105014. [Google Scholar] [CrossRef]
- Pinho, B.R.; Reis, S.D.; Hartley, R.C.; Murphy, M.P.; Oliveira, J.M.A. Mitochondrial Superoxide Generation Induces a Parkinsonian Phenotype in Zebrafish and Huntingtin Aggregation in Human Cells. Free Radic. Biol. Med. 2019, 130, 318–327. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Epitranscriptome Analysis of Oxidative Stressed Retinal Epithelial Cells Depicted a Possible RNA Editing Landscape of Retinal Degeneration. Antioxidants 2022, 11, 1967. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Marino, S.; Alafaci, C.; D’Angelo, R.; Sidoti, A. Vis-à-Vis: A Focus on Genetic Features of Cerebral Cavernous Malformations and Brain Arteriovenous Malformations Pathogenesis. Neurol. Sci. 2019, 40, 243–251. [Google Scholar] [CrossRef]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’Angelo, R.; Sidoti, A. The Impact of Modifier Genes on Cone-Rod Dystrophy Heterogeneity: An Explorative Familial Pilot Study and a Hypothesis on Neurotransmission Impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; Esposito, T.; Alafaci, C.; D’Angelo, R.; Sidoti, A. Transcriptome Analysis Provides New Molecular Signatures in Sporadic Cerebral Cavernous Malformation Endothelial Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165956. [Google Scholar] [CrossRef]
- Aborode, A.T.; Pustake, M.; Awuah, W.A.; Alwerdani, M.; Shah, P.; Yarlagadda, R.; Ahmad, S.; Silva Correia, I.F.; Chandra, A.; Nansubuga, E.P.; et al. Targeting Oxidative Stress Mechanisms to Treat Alzheimer’s and Parkinson’s Disease: A Critical Review. Oxid. Med. Cell. Longev. 2022, 2022, 7934442. [Google Scholar] [CrossRef]
- Huang, B.; Zhenxin, Y.; Chen, S.; Tan, Z.; Zong, Z.; Zhang, H.; Xiong, X. The Innate and Adaptive Immune Cells in Alzheimer’s and Parkinson’s Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 1315248. [Google Scholar] [CrossRef]
- Bender, A.; Krishnan, K.J.; Morris, C.M.; Taylor, G.A.; Reeve, A.K.; Perry, R.H.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; et al. High Levels of Mitochondrial DNA Deletions in Substantia Nigra Neurons in Aging and Parkinson Disease. Nat. Genet. 2006, 38, 515–517. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative Stress Factors in Parkinson’s Disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Jurja, S.; Coman, M.; Hîncu, M.C. The Ultraviolet Influence upon Soft Eye Tissues. Rom. J. Morphol. Embryol. 2017, 58, 45–52. [Google Scholar]
- Mo, J.-S.; Kim, M.-Y.; Ann, E.-J.; Hong, J.-A.; Park, H.-S. DJ-1 Modulates UV-Induced Oxidative Stress Signaling through the Suppression of MEKK1 and Cell Death. Cell Death Differ. 2008, 15, 1030–1041. [Google Scholar] [CrossRef]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD+ in Regulating Cellular and Metabolic Signaling Pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef]
- Pîrvu, A.S.; Andrei, A.M.; Stănciulescu, E.C.; Baniță, I.M.; Pisoschi, C.G.; Jurja, S.; Ciuluvica, R. NAD+ Metabolism and Retinal Degeneration (Review). Exp. Ther. Med. 2021, 22, 670. [Google Scholar] [CrossRef]
- Cicchetti, F.; Lapointe, N.; Roberge-Tremblay, A.; Saint-Pierre, M.; Jimenez, L.; Ficke, B.W.; Gross, R.E. Systemic Exposure to Paraquat and Maneb Models Early Parkinson’s Disease in Young Adult Rats. Neurobiol. Dis. 2005, 20, 360–371. [Google Scholar] [CrossRef]
- Yemula, N.; Dietrich, C.; Dostal, V.; Hornberger, M. Parkinson’s Disease and the Gut: Symptoms, Nutrition, and Microbiota. J. Park. Dis. 2021, 11, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, T.N.; Logvinenko, A.A.; Poleshchuk, V.V.; Muzychuk, O.A.; Shabalina, A.A.; Illarioshkin, S.N. Lipid Peroxidation Products in the Blood Plasma of Patients with Parkinson’s Disease as Possible Biomarkers of Different Stages of the Disease. Neurochem. J. 2019, 13, 391–395. [Google Scholar] [CrossRef]
- Jiang, Y.-N.; Guo, Y.-Z.; Lu, D.-H.; Pan, M.-H.; Liu, H.-Z.; Jiao, G.-L.; Bi, W.; Kurihara, H.; Li, Y.-F.; Duan, W.-J.; et al. Tianma Gouteng Granules Decreases the Susceptibility of Parkinson’s Disease by Inhibiting ALOX15-Mediated Lipid Peroxidation. J. Ethnopharmacol. 2020, 256, 112824. [Google Scholar] [CrossRef]
- Gonçalves, R.P.; Elias, D.B.D.; Magalhães, H.I.F.; de Souza, J.H. Study of Correlation of Nitrite Levels with Malonaldehyde and the Prognosis of Patients with Sickle Cell Disease on Hydroxyurea, Ceará-Brazil. J. Clin. Lab. Anal. 2011, 25, 369–373. [Google Scholar] [CrossRef]
- Meoni, G.; Tenori, L.; Schade, S.; Licari, C.; Pirazzini, C.; Bacalini, M.G.; Garagnani, P.; Turano, P.; PROPAG-AGEING Consortium; Trenkwalder, C.; et al. Metabolite and Lipoprotein Profiles Reveal Sex-Related Oxidative Stress Imbalance in de Novo Drug-Naive Parkinson’s Disease Patients. NPJ Park. Dis. 2022, 8, 14. [Google Scholar] [CrossRef]
- He, Y.; Yu, Z.; Chen, S. Alpha-Synuclein Nitration and Its Implications in Parkinson’s Disease. ACS Chem. Neurosci. 2019, 10, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Hmila, I.; Vaikath, N.N.; Majbour, N.K.; Erskine, D.; Sudhakaran, I.P.; Gupta, V.; Ghanem, S.S.; Islam, Z.; Emara, M.M.; Abdesselem, H.B.; et al. Novel Engineered Nanobodies Specific for N-Terminal Region of Alpha-Synuclein Recognize Lewy-Body Pathology and Inhibit in-Vitro Seeded Aggregation and Toxicity. FEBS J. 2022, 289, 4657–4673. [Google Scholar] [CrossRef]
- Reiszadeh Jahromi, S.; Ramesh, S.R.; Finkelstein, D.I.; Haddadi, M. A-Synuclein E46K Mutation and Involvement of Oxidative Stress in a Drosophila Model of Parkinson’s Disease. Park. Dis. 2021, 2021, 6621507. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative Damage in Nucleic Acids and Parkinson’s Disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Diederich, N.J.; Pieri, V.; Hipp, G.; Rufra, O.; Blyth, S.; Vaillant, M. Discriminative Power of Different Nonmotor Signs in Early Parkinson’s Disease. A Case-Control Study: Nonmotor Signs in Early Parkinson’s Disease. Mov. Disord. 2010, 25, 882–887. [Google Scholar] [CrossRef]
- Guo, L.; Normando, E.M.; Shah, P.A.; De Groef, L.; Cordeiro, M.F. Oculo-Visual Abnormalities in Parkinson’s Disease: Possible Value as Biomarkers: Ocular Markers in PD. Mov. Disord. 2018, 33, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.M.; Kluger, B.M.; Corcos, D.M.; Pelak, V.S.; Gisbert, R.; McRae, C.; Atkinson, C.C.; Schenkman, M. Patient Perceptions of Visual, Vestibular, and Oculomotor Deficits in People with Parkinson’s Disease. Physiother. Theory Pract. 2020, 36, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Martin-Bastida, A.; Murueta-Goyena, A.; Gabilondo, I.; Cuenca, N.; Piccini, P.; Jeon, B. Multimodal Brain and Retinal Imaging of Dopaminergic Degeneration in Parkinson Disease. Nat. Rev. Neurol. 2022, 18, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Harnois, C.; Di Paolo, T. Decreased Dopamine in the Retinas of Patients with Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2473–2475. [Google Scholar]
- Ucak, T.; Alagoz, A.; Cakir, B.; Celik, E.; Bozkurt, E.; Alagoz, G. Analysis of the Retinal Nerve Fiber and Ganglion Cell—Inner Plexiform Layer by Optical Coherence Tomography in Parkinson’s Patients. Park. Relat. Disord. 2016, 31, 59–64. [Google Scholar] [CrossRef]
- Beach, T.G.; Carew, J.; Serrano, G.; Adler, C.H.; Shill, H.A.; Sue, L.I.; Sabbagh, M.N.; Akiyama, H.; Cuenca, N. Arizona Parkinson’s Disease Consortium Phosphorylated α-Synuclein-Immunoreactive Retinal Neuronal Elements in Parkinson’s Disease Subjects. Neurosci. Lett. 2014, 571, 34–38. [Google Scholar] [CrossRef]
- Koh, S.-B.; Suh, S.-I.; Kim, S.-H.; Kim, J.H. Stereopsis and Extrastriate Cortical Atrophy in Parkinson’s Disease: A Voxel-Based Morphometric Study. Neuroreport 2013, 24, 229–232. [Google Scholar] [CrossRef]
- Ramírez-Ruiz, B.; Martí, M.-J.; Tolosa, E.; Falcón, C.; Bargalló, N.; Valldeoriola, F.; Junqué, C. Brain Response to Complex Visual Stimuli in Parkinson’s Patients with Hallucinations: A Functional Magnetic Resonance Imaging Study: FMRI Response in PD with Visual Hallucinations. Mov. Disord. 2008, 23, 2335–2343. [Google Scholar] [CrossRef]
- Arrigo, A.; Calamuneri, A.; Milardi, D.; Mormina, E.; Rania, L.; Postorino, E.; Marino, S.; Di Lorenzo, G.; Anastasi, G.P.; Ghilardi, M.F.; et al. Visual System Involvement in Patients with Newly Diagnosed Parkinson Disease. Radiology 2017, 285, 885–895. [Google Scholar] [CrossRef]
- Sharpe, J.A. Neurophysiology and Neuroanatomy of Smooth Pursuit: Lesion Studies. Brain Cogn. 2008, 68, 241–254. [Google Scholar] [CrossRef]
- Rieger, J.W.; Kim, A.; Argyelan, M.; Farber, M.; Glazman, S.; Liebeskind, M.; Meyer, T.; Bodis-Wollner, I. Cortical Functional Anatomy of Voluntary Saccades in Parkinson Disease. Clin. EEG Neurosci. 2008, 39, 169–174. [Google Scholar] [CrossRef]
- Halliday, G.M.; Del Tredici, K.; Braak, H. Critical Appraisal of Brain Pathology Staging Related to Presymptomatic and Symptomatic Cases of Sporadic Parkinson’s Disease. J. Neural Transm. Suppl. 2006, 70, 99–103. [Google Scholar] [CrossRef]
- Picillo, M.; Palladino, R.; Barone, P.; Erro, R.; Colosimo, C.; Marconi, R.; Morgante, L.; Antonini, A.; the PRIAMO Study Group. The PRIAMO Study: Urinary Dysfunction as a Marker of Disease Progression in Early Parkinson’s Disease. Eur. J. Neurol. 2017, 24, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, T.A.; Chesnokova, N.B.; Nodel, M.R.; Kim, A.R.; Ugrumov, M.V. Molecular Mechanisms and Clinical Manifestations of Catecholamine Dysfunction in the Eye in Parkinson’s Disease as a Basis for Developing Early Diagnosis. Acta Nat. 2020, 12, 52–62. [Google Scholar] [CrossRef]
- Hamedani, A.G.; Gold, D.R. Eyelid Dysfunction in Neurodegenerative, Neurogenetic, and Neurometabolic Disease. Front. Neurol. 2017, 8, 329. [Google Scholar] [CrossRef]
- Piro, A.; Tagarelli, A.; Nicoletti, G.; Fletcher, R.; Quattrone, A. Color Vision Impairment in Parkinson’s Disease. J. Parkinsons Dis. 2014, 4, 317–319. [Google Scholar] [CrossRef]
- Piro, A.; Tagarelli, A.; Nicoletti, G.; Lupo, A.; Fletcher, R.; Quattrone, A. Parkinson’s Disease, Parkinsonism, de Novo Parkinson’s Disease, Essential Tremor: A Color Vision Preliminary Analysis. Park. Relat. Disord. 2016, 22, e42–e43. [Google Scholar] [CrossRef]
- Li, D.-K.; Liu, F.-T.; Chen, K.; Bu, L.-L.; Yang, K.; Chen, C.; Liu, Z.-Y.; Tang, Y.-L.; Zhao, J.; Sun, Y.-M.; et al. Depressive Symptoms Are Associated with Color Vision but Not Olfactory Function in Patients with Parkinson’s Disease. J. Neuropsychiatr. Clin. Neurosci. 2018, 30, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.U.; Zimmermann, H.G.; Oberwahrenbrock, T.; Isensee, J.; Müller, T.; Paul, F. Self-Perception and Determinants of Color Vision in Parkinson’s Disease. J. Neural Transm. 2018, 125, 145–152. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.E.; Massicotte-Marquez, J.; Montplaisir, J. Potential Early Markers of Parkinson Disease in Idiopathic REM Sleep Behavior Disorder. Neurology 2006, 66, 845–851. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.-F.; Bertrand, J.-A.; Génier Marchand, D.; Montplaisir, J.Y. Parkinson Risk in Idiopathic REM Sleep Behavior Disorder: Preparing for Neuroprotective Trials: Preparing for Neuroprotective Trials. Neurology 2015, 84, 1104–1113. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Romenets, S.R.; Anang, J.B.M.; Latreille, V.; Gagnon, J.-F.; Postuma, R.B. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison with Other Phenotypes: A Prospective Cohort Comparison with Other Phenotypes. JAMA Neurol. 2015, 72, 863–873. [Google Scholar] [CrossRef]
- Lubbe, S.; Morris, H.R. Recent Advances in Parkinson’s Disease Genetics. J. Neurol. 2014, 261, 259–266. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Mejia-Santana, H.; Mirelman, A.; Saunders-Pullman, R.; Raymond, D.; Palmese, C.; Caccappolo, E.; Ozelius, L.; Orr-Urtreger, A.; Clark, L.; et al. Neuropsychological Performance in LRRK2 G2019S Carriers with Parkinson’s Disease. Park. Relat. Disord. 2015, 21, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.; Palidis, D.J.; Spering, M.; McKeown, M.J. Visual Contrast Sensitivity in Early-Stage Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5696–5704. [Google Scholar] [CrossRef]
- Savitt, J.; Aouchiche, R. Management of Visual Dysfunction in Patients with Parkinson’s Disease. J. Park. Dis. 2020, 10, S49–S56. [Google Scholar] [CrossRef]
- Hong, S.B.; Ahn, J.; Yoo, D.; Shin, J.Y.; Jeon, B.; Lee, J.-Y. Contrast Sensitivity Impairment in Drug-Naïve Parkinson’s Disease Patients Associates with Early Cognitive Decline. Neurol. Sci. 2020, 41, 1837–1842. [Google Scholar] [CrossRef]
- Hutton, J.T.; Morris, J.L.; Elias, J.W.; Varma, R.; Poston, J.N. Spatial Contrast Sensitivity Is Reduced in Bilateral Parkinson’s Disease. Neurology 1991, 41, 1200–1202. [Google Scholar] [CrossRef]
- Hutton, J.T.; Morris, J.L.; Elias, J.W. Levodopa Improves Spatial Contrast Sensitivity in Parkinson’s Disease. Arch. Neurol. 1993, 50, 721–724. [Google Scholar] [CrossRef]
- Miri, S.; Glazman, S.; Mylin, L.; Bodis-Wollner, I. A Combination of Retinal Morphology and Visual Electrophysiology Testing Increases Diagnostic Yield in Parkinson’s Disease. Park. Relat. Disord. 2016, 22 (Suppl. S1), S134–S137. [Google Scholar] [CrossRef] [PubMed]
- Miller-Patterson, C.; Hsu, J.Y.; Chahine, L.M.; Morley, J.F.; Willis, A.W. Selected Autonomic Signs and Symptoms as Risk Markers for Phenoconversion and Functional Dependence in Prodromal Parkinson’s Disease. Clin. Auton. Res. 2022, 32, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Blekher, T.; Weaver, M.; Rupp, J.; Nichols, W.C.; Hui, S.L.; Gray, J.; Yee, R.D.; Wojcieszek, J.; Foroud, T. Multiple Step Pattern as a Biomarker in Parkinson Disease. Park. Relat. Disord. 2009, 15, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.; Murueta-Goyena, A.; Teijeira-Portas, S.; Acera, M.; Del Pino, R.; Sáez-Atxukarro, O.; Diez-Cirarda, M.; Tijero, B.; Fernández-Valle, T.; Gómez Esteban, J.C.; et al. Impact of Visual Impairment on Vision-Related Quality of Life in Parkinson’s Disease. J. Parkinsons Dis. 2022, 12, 1633–1643. [Google Scholar] [CrossRef]
- Pinkhardt, E.H.; Jürgens, R.; Lulé, D.; Heimrath, J.; Ludolph, A.C.; Becker, W.; Kassubek, J. Eye Movement Impairments in Parkinson’s Disease: Possible Role of Extradopaminergic Mechanisms. BMC Neurol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Terao, Y.; Fukuda, H.; Ugawa, Y.; Hikosaka, O. New Perspectives on the Pathophysiology of Parkinson’s Disease as Assessed by Saccade Performance: A Clinical Review. Clin. Neurophysiol. 2013, 124, 1491–1506. [Google Scholar] [CrossRef]
- Frei, K. Abnormalities of Smooth Pursuit in Parkinson’s Disease: A Systematic Review. Clin. Park. Relat. Disord. 2021, 4, 100085. [Google Scholar] [CrossRef]
- Lencer, R.; Trillenberg, P. Neurophysiology and Neuroanatomy of Smooth Pursuit in Humans. Brain Cogn. 2008, 68, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.A.; Pokorny, J.J.; Liu, P. Activity of Substantia Nigra Pars Reticulata Neurons during Smooth Pursuit Eye Movements in Monkeys: Basal Ganglia and Pursuit. Eur. J. Neurosci. 2005, 22, 448–464. [Google Scholar] [CrossRef]
- Bares, M.; Brázdil, M.; Kanovský, P.; Jurák, P.; Daniel, P.; Kukleta, M.; Rektor, I. The Effect of Apomorphine Administration on Smooth Pursuit Ocular Movements in Early Parkinsonian Patients. Park. Relat. Disord. 2003, 9, 139–144. [Google Scholar] [CrossRef]
- Thomson, K.; Karouta, C.; Sabeti, F.; Anstice, N.; Leung, M.; Jong, T.; Maddess, T.; Morgan, I.G.; Game, J.; Ashby, R. The Safety and Tolerability of Levodopa Eye Drops for the Treatment of Ocular Disorders: A Randomized First-in-Human Study. Clin. Transl. Sci. 2022, 15, 2673–2684. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Sabatini, U.; Simonetta-Moreau, M.; Montastruc, J.L.; Rascol, A.; Clanet, M. Square Wave Jerks in Parkinsonian Syndromes. J. Neurol. Neurosurg. Psychiatr. 1991, 54, 599–602. [Google Scholar] [CrossRef]
- Abadi, R.V.; Gowen, E. Characteristics of Saccadic Intrusions. Vis. Res. 2004, 44, 2675–2690. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Xu-Wilson, M.; Grill, S.; Zee, D.S. “Staircase” Square-Wave Jerks in Early Parkinson’s Disease. Br. J. Ophthalmol. 2011, 95, 705–709. [Google Scholar] [CrossRef]
- Averbuch-Heller, L.; Stahl, J.S.; Hlavin, M.L.; Leigh, R.J. Square-Wave Jerks Induced by Pallidotomy in Parkinsonian Patients. Neurology 1999, 52, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Ghadban, R.; Martinez, J.M.; Diehl, N.N.; Mohney, B.G. The Incidence and Clinical Characteristics of Adult-Onset Convergence Insufficiency. Ophthalmology 2015, 122, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, B.; Lubinski, W.; Honczarenko, K.; Potemkowski, A.; Safranow, K. Ophthalmological Features of Parkinson Disease. Med. Sci. Monit. 2014, 20, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Gokden, M.S.; Tychsen, L.S.; Perlmutter, J.S. Convergence Insufficiency in Idiopathic Parkinson’s Disease Responsive to Levodopa. Strabismus 1999, 7, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Almer, Z.; Klein, K.S.; Marsh, L.; Gerstenhaber, M.; Repka, M.X. Ocular Motor and Sensory Function in Parkinson’s Disease. Ophthalmology 2012, 119, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Turcano, P.; Chen, J.J.; Bureau, B.L.; Savica, R. Early Ophthalmologic Features of Parkinson’s Disease: A Review of Preceding Clinical and Diagnostic Markers. J. Neurol. 2019, 266, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, C.; Kraft, S.; Hinkelmann, K.; Krause, S.; Gerloff, C.; Zangemeister, W.H. Visual Attention and Saccadic Oculomotor Control in Parkinson’s Disease. Eur. Neurol. 2015, 73, 283–293. [Google Scholar] [CrossRef]
- Hanuška, J.; Bonnet, C.; Rusz, J.; Sieger, T.; Jech, R.; Rivaud-Péchoux, S.; Vidailhet, M.; Gaymard, B.; Růžička, E. Fast Vergence Eye Movements Are Disrupted in Parkinson’s Disease: A Video-Oculography Study. Park. Relat. Disord. 2015, 21, 797–799. [Google Scholar] [CrossRef] [PubMed]
- MacAskill, M.R.; Anderson, T.J. Eye Movements in Neurodegenerative Diseases. Curr. Opin. Neurol. 2016, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Schindlbeck, K.A.; Schönfeld, S.; Naumann, W.; Friedrich, D.J.; Maier, A.; Rewitzer, C.; Klostermann, F.; Marzinzik, F. Characterization of Diplopia in Non-Demented Patients with Parkinson’s Disease. Park. Relat. Disord. 2017, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Gu, Z.; Cao, M.; Li, D.; Chan, P. Stereopsis Impairment Is Associated with Decreased Color Perception and Worse Motor Performance in Parkinson’s Disease. Eur. J. Med. Res. 2014, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Park, J.-H.; Kim, Y.H.; Koh, S.-B. Stereopsis in Drug Naïve Parkinson’s Disease Patients. Can. J. Neurol. Sci. 2011, 38, 299–302. [Google Scholar] [CrossRef]
- Bowen, R.C.; Koeppel, J.N.; Christensen, C.D.; Snow, K.B.; Ma, J.; Katz, B.J.; Krauss, H.R.; Landau, K.; Warner, J.E.A.; Crum, A.V.; et al. The Most Common Causes of Eye Pain at 2 Tertiary Ophthalmology and Neurology Clinics. J. Neuroophthalmol. 2018, 38, 320–327. [Google Scholar] [CrossRef]
- Milner, M.S.; Beckman, K.A.; Luchs, J.I.; Allen, Q.B.; Awdeh, R.M.; Berdahl, J.; Boland, T.S.; Buznego, C.; Gira, J.P.; Goldberg, D.F.; et al. Dysfunctional Tear Syndrome: Dry Eye Disease and Associated Tear Film Disorders—New Strategies for Diagnosis and Treatment: Dry Eye Disease and Associated Tear Film Disorders—New Strategies for Diagnosis and Treatment. Curr. Opin. Ophthalmol. 2017, 27 (Suppl. 1), 3–47. [Google Scholar] [CrossRef]
- Gitchel, G.T.; Wetzel, P.A.; Baron, M.S. Pervasive Ocular Tremor in Patients with Parkinson Disease. Arch. Neurol. 2012, 69, 1011–1017. [Google Scholar] [CrossRef]
- Duval, C. Ocular Tremor in Parkinson’s Disease: The Debate Is Not over: Ocular Tremor in Parkinson’s Disease. Mov. Disord. 2013, 28, 713–714. [Google Scholar] [CrossRef]
- Kaski, D.; Saifee, T.A.; Buckwell, D.; Bronstein, A.M. Ocular Tremor in Parkinson’s Disease Is Due to Head Oscillation. Mov. Disord. 2013, 28, 534–537. [Google Scholar] [CrossRef]
- Bologna, M.; Fasano, A.; Modugno, N.; Fabbrini, G.; Berardelli, A. Effects of Subthalamic Nucleus Deep Brain Stimulation and L-DOPA on Blinking in Parkinson’s Disease. Exp. Neurol. 2012, 235, 265–272. [Google Scholar] [CrossRef]
- Kwon, K.-Y.; Kang, S.H.; Kim, M.; Lee, H.M.; Jang, J.W.; Kim, J.Y.; Lee, S.-M.; Koh, S.-B. Nonmotor Symptoms and Cognitive Decline in de Novo Parkinson’s Disease. Can. J. Neurol. Sci. 2014, 41, 597–602. [Google Scholar] [CrossRef]
- Armstrong, R.A. Visual Signs and Symptoms of Parkinson’s Disease. Clin. Exp. Optom. 2008, 91, 129–138. [Google Scholar] [CrossRef]
- Lin, T.P.; Rigby, H.; Adler, J.S.; Hentz, J.G.; Balcer, L.J.; Galetta, S.L.; Devick, S.; Cronin, R.; Adler, C.H. Abnormal Visual Contrast Acuity in Parkinson’s Disease. J. Parkinsons Dis. 2015, 5, 125–130. [Google Scholar] [CrossRef]
- Manohar, S.G.; Husain, M. Reduced Pupillary Reward Sensitivity in Parkinson’s Disease. NPJ Park. Dis. 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Oculo-Visual Dysfunction in Parkinson’s Disease. J. Park. Dis. 2015, 5, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ferdousi, M.; Kalteniece, A.; Kass-Iliyya, L.; Petropoulos, I.N.; Malik, R.A.; Kobylecki, C.; Silverdale, M. Corneal Confocal Microscopy Detects Small Fibre Neurodegeneration in Parkinson’s Disease Using Automated Analysis. Sci. Rep. 2020, 10, 20147. [Google Scholar] [CrossRef]
- Misra, S.L.; Kersten, H.M.; Roxburgh, R.H.; Danesh-Meyer, H.V.; McGhee, C.N.J. Corneal Nerve Microstructure in Parkinson’s Disease. J. Clin. Neurosci. 2017, 39, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bitirgen, G.; Turkmen, K.; Malik, R.A.; Ozkagnici, A.; Zengin, N. Corneal Confocal Microscopy Detects Corneal Nerve Damage and Increased Dendritic Cells in Fabry Disease. Sci. Rep. 2018, 8, 12244. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Rania, L.; Calamuneri, A.; Postorino, E.I.; Mormina, E.; Gaeta, M.; Marino, S.; Di Lorenzo, G.; Quartarone, A.; Anastasi, G.; et al. Early Corneal Innervation and Trigeminal Alterations in Parkinson Disease: A Pilot Study: A Pilot Study. Cornea 2018, 37, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Shrier, E.M.; Adam, C.R.; Spund, B.; Glazman, S.; Bodis-Wollner, I. Interocular Asymmetry of Foveal Thickness in Parkinson Disease. J. Ophthalmol. 2012, 2012, 728457. [Google Scholar] [CrossRef] [PubMed]
- Bodis-Wollner, I.; Kozlowski, P.B.; Glazman, S.; Miri, S. A-Synuclein in the Inner Retina in Parkinson Disease: A-Synuclein in PD Retina. Ann. Neurol. 2014, 75, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.H.; Park, H.N.; Han, M.K.; Obertone, T.S.; Abey, J.; Aseem, F.; Thule, P.M.; Iuvone, P.M.; Pardue, M.T. Dopamine Deficiency Contributes to Early Visual Dysfunction in a Rodent Model of Type 1 Diabetes. J. Neurosci. 2014, 34, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Tsironi, E.E.; Dastiridou, A.; Katsanos, A.; Dardiotis, E.; Veliki, S.; Patramani, G.; Zacharaki, F.; Ralli, S.; Hadjigeorgiou, G.M. Perimetric and Retinal Nerve Fiber Layer Findings in Patients with Parkinson’s Disease. BMC Ophthalmol. 2012, 12, 54. [Google Scholar] [CrossRef]
- Ribelayga, C.; Cao, Y.; Mangel, S.C. The Circadian Clock in the Retina Controls Rod-Cone Coupling. Neuron 2008, 59, 790–801. [Google Scholar] [CrossRef]
- Slotnick, S.; Ding, Y.; Glazman, S.; Durbin, M.; Miri, S.; Selesnick, I.; Sherman, J.; Bodis-Wollner, I. A Novel Retinal Biomarker for Parkinson’s Disease: Quantifying the Foveal Pit with Optical Coherence Tomography: OCT Biomarker for Parkinson’s Disease. Mov. Disord. 2015, 30, 1692–1695. [Google Scholar] [CrossRef]
- Bambo, M.P.; Garcia-Martin, E.; Satue, M.; Perez-Olivan, S.; Alayon, S.; Gonzalez-Hernandez, M.; Polo, V.; Larrosa, J.M.; Gonzalez-De la Rosa, M. Measuring Hemoglobin Levels in the Optic Disc of Parkinson’s Disease Patients Using New Colorimetric Analysis Software. Park. Dis. 2014, 2014, 946540. [Google Scholar] [CrossRef]
- Inzelberg, R.; Ramirez, J.A.; Nisipeanu, P.; Ophir, A. Retinal Nerve Fiber Layer Thinning in Parkinson Disease. Vis. Res. 2004, 44, 2793–2797. [Google Scholar] [CrossRef]
- La Morgia, C.; Barboni, P.; Rizzo, G.; Carbonelli, M.; Savini, G.; Scaglione, C.; Capellari, S.; Bonazza, S.; Giannoccaro, M.P.; Calandra-Buonaura, G.; et al. Loss of Temporal Retinal Nerve Fibers in Parkinson Disease: A Mitochondrial Pattern?: Loss of Temporal Retinal Nerve Fibers in PD. Eur. J. Neurol. 2013, 20, 198–201. [Google Scholar] [CrossRef]
- Roth, N.M.; Saidha, S.; Zimmermann, H.; Brandt, A.U.; Isensee, J.; Benkhellouf-Rutkowska, A.; Dornauer, M.; Kühn, A.A.; Müller, T.; Calabresi, P.A.; et al. Photoreceptor Layer Thinning in Idiopathic Parkinson’s Disease: Photoreceptors in Parkinson’s Disease. Mov. Disord. 2014, 29, 1163–1170. [Google Scholar] [CrossRef]
- Archibald, N.K.; Clarke, M.P.; Mosimann, U.P.; Burn, D.J. Visual Symptoms in Parkinson’s Disease and Parkinson’s Disease Dementia. Mov. Disord. 2011, 26, 2387–2395. [Google Scholar] [CrossRef]
- Albrecht, P.; Müller, A.-K.; Südmeyer, M.; Ferrea, S.; Ringelstein, M.; Cohn, E.; Aktas, O.; Dietlein, T.; Lappas, A.; Foerster, A.; et al. Optical Coherence Tomography in Parkinsonian Syndromes. PLoS ONE 2012, 7, e34891. [Google Scholar] [CrossRef] [PubMed]
- Satue, M.; Obis, J.; Rodrigo, M.J.; Otin, S.; Fuertes, M.I.; Vilades, E.; Gracia, H.; Ara, J.R.; Alarcia, R.; Polo, V.; et al. Optical Coherence Tomography as a Biomarker for Diagnosis, Progression, and Prognosis of Neurodegenerative Diseases. J. Ophthalmol. 2016, 2016, 8503859. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Larrosa, J.M.; Polo, V.; Satue, M.; Marques, M.L.; Alarcia, R.; Seral, M.; Fuertes, I.; Otin, S.; Pablo, L.E. Distribution of Retinal Layer Atrophy in Patients with Parkinson Disease and Association with Disease Severity and Duration. Am. J. Ophthalmol. 2014, 157, 470–478.e2. [Google Scholar] [CrossRef] [PubMed]
- Chrysou, A.; Jansonius, N.M.; van Laar, T. Retinal Layers in Parkinson’s Disease: A Meta-Analysis of Spectral-Domain Optical Coherence Tomography Studies. Park. Relat. Disord. 2019, 64, 40–49. [Google Scholar] [CrossRef]
- Ponirakis, G.; Al Hamad, H.; Sankaranarayanan, A.; Khan, A.; Chandran, M.; Ramadan, M.; Tosino, R.; Gawhale, P.; Alobaidi, M.; AlSulaiti, E.; et al. P4-339: Association of Corneal Nerve Fiber Measures with Cognitive Function in Dementia. Alzheimers Dement. 2019, 15, P1427–P1428. [Google Scholar] [CrossRef]
- Tamer, C.; Melek, I.M.; Duman, T.; Oksüz, H. Tear Film Tests in Parkinson’s Disease Patients. Ophthalmology 2005, 112, 1795. [Google Scholar] [CrossRef]
- Demirci, S.; Gunes, A.; Koyuncuoglu, H.R.; Tok, L.; Tok, O. Evaluation of Corneal Parameters in Patients with Parkinson’s Disease. Neurol. Sci. 2016, 37, 1247–1252. [Google Scholar] [CrossRef]
- Huang, F.-C.; Tseng, S.-H.; Shih, M.-H.; Chen, F.K. Effect of Artificial Tears on Corneal Surface Regularity, Contrast Sensitivity, and Glare Disability in Dry Eyes. Ophthalmology 2002, 109, 1934–1940. [Google Scholar] [CrossRef]
- Green, K.E.; Rastall, D.; Eggenberger, E. Treatment of Blepharospasm/Hemifacial Spasm. Curr. Treat. Options Neurol. 2017, 19, 41. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Lai, S.-W.; Lin, C.-L.; Liao, K.-F.; Chang-Ou, K.-C. Increased Risk of Parkinson’s Disease in Cataract Patients: A Population-Based Cohort Study. Park. Relat. Disord. 2015, 21, 68–71. [Google Scholar] [CrossRef]
- Klettner, A.; Richert, E.; Kuhlenbäumer, G.; Nölle, B.; Bhatia, K.P.; Deuschl, G.; Roider, J.; Schneider, S.A. Alpha Synuclein and Crystallin Expression in Human Lens in Parkinson’s Disease: Alpha-Synuclein and Crystallins in PD Cataract Lens. Mov. Disord. 2016, 31, 600–601. [Google Scholar] [CrossRef]
- Landi, N.; Frost, S.J.; Menc, W.E.; Sandak, R.; Pugh, K.R. Neurobiological Bases of Reading Comprehension: Insights from Neuroimaging Studies of Word Level and Text Level Processing in Skilled and Impaired Readers. Read. Writ. Q. 2013, 29, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Kudlicka, A.; Clare, L.; Hindle, J.V. Executive Functions in Parkinson’s Disease: Systematic Review and Meta-Analysis. Mov. Disord. 2011, 26, 2305–2315. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive Impairment in Parkinson’s Disease: The Dual Syndrome Hypothesis. Neurodegener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef]
- Clifton, C., Jr.; Staub, A.; Rayner, K. Eye movements in reading words and sentences. In Eye Movements; Elsevier: Amsterdam, The Netherlands, 2007; pp. 341–371. ISBN 9780080449807. [Google Scholar]
- Fernández, G.; Mandolesi, P.; Rotstein, N.P.; Colombo, O.; Agamennoni, O.; Politi, L.E. Eye Movement Alterations during Reading in Patients with Early Alzheimer Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8345–8352. [Google Scholar] [CrossRef]
- Vitório, R.; Baptista, A.M. Visual control of locomotion in people with Parkinson’s disease. In Locomotion and Posture in Older Adults; Springer International Publishing: Cham, Switzerland, 2017; pp. 115–128. ISBN 9783319489797. [Google Scholar]
- Wood, B.H.; Bilclough, J.A.; Bowron, A.; Walker, R.W. Incidence and Prediction of Falls in Parkinson’s Disease: A Prospective Multidisciplinary Study. J. Neurol. Neurosurg. Psychiatr. 2002, 72, 721–725. [Google Scholar] [CrossRef]
- Ekker, M.S.; Janssen, S.; Seppi, K.; Poewe, W.; de Vries, N.M.; Theelen, T.; Nonnekes, J.; Bloem, B.R. Ocular and Visual Disorders in Parkinson’s Disease: Common but Frequently Overlooked. Park. Relat. Disord. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Nowacka, B.; Lubiński, W.; Karczewicz, D. Ophthalmological and Electrophysiological Features of Parkinson’s Disease. Klin. Ocz. 2010, 112, 247–252. [Google Scholar]
- Kawada, T.; Anang, J.B.M.; Postuma, R. Predictors of Dementia in Parkinson Disease: A Prospective Cohort Study. Neurology 2015, 84, 1285. [Google Scholar] [CrossRef]
- Bulens, C.; Meerwaldt, J.D.; Van der Wildt, G.J.; Van Deursen, J.B. Effect of Levodopa Treatment on Contrast Sensitivity in Parkinson’s Disease. Ann. Neurol. 1987, 22, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Kokmen, E.; Smith, G.E.; Ivnik, R.J.; Parisi, J.E.; Olson, E.J.; Petersen, R.C. REM Sleep Behavior Disorder and Degenerative Dementia: An Association Likely Reflecting Lewy Body Disease: An Association Likely Reflecting Lewy Body Disease. Neurology 1998, 51, 363–370. [Google Scholar] [CrossRef]
- Birch, J.; Kolle, R.U.; Kunkel, M.; Paulus, W.; Upadhyay, P. Acquired Colour Deficiency in Patients with Parkinson’s Disease. Vis. Res. 1998, 38, 3421–3426. [Google Scholar] [CrossRef]
- Jurja, S.; Hîncu, M.; Dobrescu, M.A.; Golu, A.E.; Bălăşoiu, A.T.; Coman, M. Ocular Cells and Light: Harmony or Conflict? Rom. J. Morphol. Embryol. 2014, 55, 257–261. [Google Scholar]
- Nagino, K.; Sung, J.; Oyama, G.; Hayano, M.; Hattori, N.; Okumura, Y.; Fujio, K.; Akasaki, Y.; Huang, T.; Midorikawa-Inomata, A.; et al. Prevalence and Characteristics of Dry Eye Disease in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 18348. [Google Scholar] [CrossRef]
- Jurja, S.; Pirjol, T.N.; Costea, D.O.; Pirjol BS, N. Correlation Between Effectiveness and Antioxidant Activity of Some Anti Cataract Eye Drops. Rev. Chim. (Buchar.) 2016, 67, 1004–1007. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuparu, A.Z.; Jurja, S.; Stuparu, A.F.; Axelerad, A. Narrative Review Concerning the Clinical Spectrum of Ophthalmological Impairments in Parkinson’s Disease. Neurol. Int. 2023, 15, 140-161. https://doi.org/10.3390/neurolint15010012
Stuparu AZ, Jurja S, Stuparu AF, Axelerad A. Narrative Review Concerning the Clinical Spectrum of Ophthalmological Impairments in Parkinson’s Disease. Neurology International. 2023; 15(1):140-161. https://doi.org/10.3390/neurolint15010012
Chicago/Turabian StyleStuparu, Alina Zorina, Sanda Jurja, Alexandru Floris Stuparu, and Any Axelerad. 2023. "Narrative Review Concerning the Clinical Spectrum of Ophthalmological Impairments in Parkinson’s Disease" Neurology International 15, no. 1: 140-161. https://doi.org/10.3390/neurolint15010012
APA StyleStuparu, A. Z., Jurja, S., Stuparu, A. F., & Axelerad, A. (2023). Narrative Review Concerning the Clinical Spectrum of Ophthalmological Impairments in Parkinson’s Disease. Neurology International, 15(1), 140-161. https://doi.org/10.3390/neurolint15010012





