Relevance of Medullary Vein Sign in Neurosarcoidosis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General Results
3.2. CSF Findings
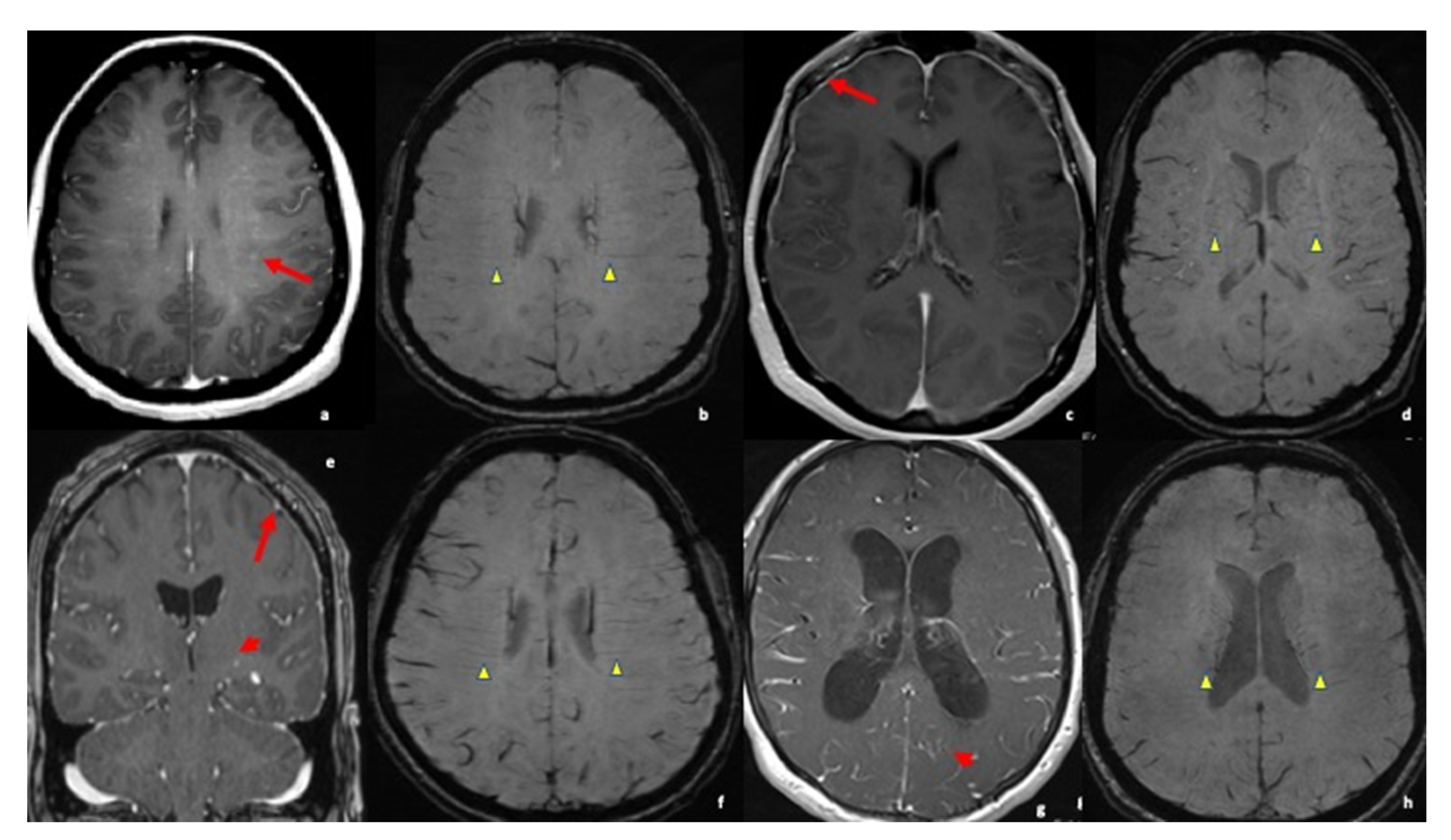
3.3. MRI Findings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviation
| MRI | Magnetic Resonance Imaging |
| CSF | Cerebrospinal fluid |
| EVD | External Ventricular drain |
| ACE | Angiotensin converting enzymes |
| LP | Lumbar Puncture |
| TTE | Transthoracic Echocardiography |
| ICP | Intracranial Pressure |
| CNS | Central Nervous System |
| NMO | Neuromyelitis Optica |
| MS | Multiple Sclerosis |
| CN | Cranial Nerve |
| SWI | Susceptibility weighted imaging |
| MRI | Magnetic resonance imaging) |
| OSA | obstructive Sleep apnea |
| GERD | Gastroesophageal reflux disease |
| IVDU | Intravenous drug use |
| SE | spin-echo |
| MP-RAGE | magnetization prepared rapid gradient echo |
| MIP | maximum intensity projection |
| MinIP | minimum intensity projection |
References
- Bradshaw, M.J.; Pawate, S.; Koth, L.L.; Cho, T.A.; Gelfand, J.M. Neurosarcoidosis: Pathophysiology, Diagnosis, and Treatment. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1084. [Google Scholar] [CrossRef]
- Lord, J.; Paz Soldan, M.; Galli, J.; Salzman, K.; Kresser, J.; Bacharach, R.; DeWitt, L.; Klein, J.; Rose, J.; Greenlee, J.; et al. Neurosarcoidosis. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e743. [Google Scholar] [CrossRef]
- Ibitoye, R.; Wilkins, A.; Scolding, N. Neurosarcoidosis: A clinical approach to diagnosis and management. J. Neurol. 2016, 264, 1023–1028. [Google Scholar] [CrossRef]
- Krumholz, A.; Stern, B.J. Neurologic manifestations of sarcoidosis. Handb. Clin. Neurol. 2014, 119, 305–333. [Google Scholar] [CrossRef]
- Bathla, G.; Abdel-Wahed, L.; Agarwal, A.; Cho, T.; Gupta, S.; Jones, K.; Priya, S.; Soni, N.; Wasserman, B. Vascular Involvement in Neurosarcoidosis. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1063. [Google Scholar] [CrossRef]
- Ungprasert, P.; Matteson, E.L. Neurosarcoidosis. Rheum. Dis. Clin. N. Am. 2017, 43, 593–606. [Google Scholar] [CrossRef]
- Stern, B.J.; Royal, W., 3rd; Gelfand, J.M.; Clifford, D.B.; Tavee, J.; Pawate, S.; Berger, J.R.; Aksamit, A.J.; Krumholz, A.; Pardo, C.A.; et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018, 75, 1546–1553. [Google Scholar] [CrossRef]
- Bridel, C.; Courvoisier, D.S.; Vuilleumier, N.; Lalive, P.H. Cerebrospinal fluid angiotensin-converting enzyme for diagnosis of neurosarcoidosis. J. Neuroimmunol. 2015, 285, 1–3. [Google Scholar] [CrossRef]
- Gibbons, E.; Whittam, D.; Jacob, A.; Huda, S. Images of the month 1: Trident sign and neurosarcoidosis. Clin. Med. 2021, 21, e667–e668. [Google Scholar] [CrossRef]
- Fritz, D.; van de Beek, D.; Brouwer, M.C. Clinical features, treatment and outcome in neurosarcoidosis: Systematic review and meta-analysis. BMC Neurol. 2016, 16, 220. [Google Scholar] [CrossRef]
- Terushkin, V.; Stern, B.J.; Judson, M.A.; Hagiwara, M.; Pramanik, B.; Sanchez, M.; Prystowsky, S. Neurosarcoidosis: Presentations and management. Neurologist 2010, 16, 2–15, Erratum in Neurologist 2010, 16, 140. [Google Scholar] [CrossRef]
- Baughman, R.P.; Valeyre, D.; Korsten, P.; Mathioudakis, A.G.; Wuyts, W.A.; Wells, A.; Rottoli, P.; Nunes, H.; Lower, E.E.; Judson, M.A.; et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur. Respir. J. 2021, 58, 2004079. [Google Scholar] [CrossRef]
- Haacke, E.M.; Ye, Y. The role of susceptibility weighted imaging in functional MRI. Neuroimage 2012, 62, 923–929. [Google Scholar] [CrossRef]
- Letourneau-Guillon, L.; Krings, T. Simultaneous arteriovenous shunting and venous congestion identification in dural arteriovenous fistulas using susceptibility-weighted imaging: Initial experience. AJNR Am. J. Neuroradiol. 2012, 33, 301–307. [Google Scholar] [CrossRef]
- Sakthivel, P.; Bruder, D. Mechanism of granuloma formation in sarcoidosis. Curr. Opin. Hematol. 2017, 24, 59–65. [Google Scholar] [CrossRef]
- Mirfakhraee, M.; Crofford, M.J.; Guinto, F.C., Jr.; Nauta, H.J.; Weedn, V.W. Virchow-Robin space: A path of spread in neurosarcoidosis. Radiology 1986, 158, 715–720. [Google Scholar] [CrossRef]
- Wei, J.; Yin, H.; Wang, L.; Cui, L.; Wang, R. Systemic autoimmune diseases complicated with hydrocephalus: Pathogenesis and management. Neurosurg. Rev. 2019, 42, 255–261. [Google Scholar] [CrossRef]
- Switlyk, M.D.; Niehusmann, P.; Sprauten, M.; Magelssen, H.; Aarhus, M.; Rasmussen, F.Ø.; Knutstad, K.; Brandal, P. Neurosarcoidosis resembling multiple meningiomas: A misleading presentation of the disease and diagnostic challenge. Acta Radiol. Open 2021, 10, 20584601211036550. [Google Scholar] [CrossRef]
- Zamora, C.; Hung, S.C.; Tomingas, C.; Atkinson, C.; Castillo, M. Engorgement of Deep Medullary Veins in Neurosarcoidosis: A Common-Yet-Underrecognized Cerebrovascular Finding on SWI. AJNR Am. J. Neuroradiol. 2018, 39, 2045–2050. [Google Scholar] [CrossRef]
- Nakagawa, I.; Taoka, T.; Wada, T.; Nakagawa, H.; Sakamoto, M.; Kichikawa, K.; Hironaka, Y.; Motoyama, Y.; Park, Y.S.; Nakase, H. The use of susceptibility-weighted imaging as an indicator of retrograde leptomeningeal venous drainage and venous congestion with dural arteriovenous fistula: Diagnosis and follow-up after treatment. Neurosurgery 2013, 72, 47–54, discussion 55. [Google Scholar] [CrossRef]
- Sarbu, N.; Shih, R.Y.; Jones, R.V.; Horkayne-Szakaly, I.; Oleaga, L.; Smirniotopoulos, J.G. White Matter Diseases with Radiologic-Pathologic Correlation. Radiographics 2016, 36, 1426–1447, Update in Radiographics 2020, 40, E4–E7. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, H.F.; Zheng, Y.; Wu, L.; Wu, Z.Y.; Ding, M.P. Diffuse intracranial calcification, deep medullary vein engorgement, and symmetric white matter involvement in a patient with systemic lupus erythematosus. CNS Neurosci. Ther. 2020, 26, 278–280. [Google Scholar] [CrossRef]
- Ginat, D.T.; Dhillon, G.; Almast, J. Magnetic resonance imaging of neurosarcoidosis. J. Clin. Imaging Sci. 2011, 1, 15. [Google Scholar] [CrossRef]
- Jagadeesan, B.D.; Delgado Almandoz, J.E.; Moran, C.J.; Benzinger, T.L. Accuracy of susceptibility-weighted imaging for the detection of arteriovenous shunting in vascular malformations of the brain. Stroke 2011, 42, 87–92. [Google Scholar] [CrossRef]
- Miyasaka, T.; Taoka, T.; Nakagawa, H.; Wada, T.; Takayama, K.; Myochin, K.; Sakamoto, M.; Ochi, T.; Akashi, T.; Kichikawa, K. Application of susceptibility weighted imaging (SWI) for evaluation of draining veins of arteriovenous malformation: Utility of magnitude images. Neuroradiology 2012, 54, 1221–1227. [Google Scholar] [CrossRef]
| Case | Age/Gender | Year of Diagnosis | Onset/Presentation | CSF Analysis | MRI Results | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|
| 1 * | 56 y.o Female | 2015 | Confusion, thought blocking, leg weakness | WBC: 50 70% lymphocytes; 30% mononuclear Protein: 57 mg/dL | Small focus of restricted diffusion along left lateral medulla Medullary vein sign + | Outpatient follow-up Risk factor reduction | Resolution of acute confusion Residual facial numbness and leg weakness No progression |
| 2 * | 34 y.o. female | 2013 | Intractable headache Weakness Nausea/Vomiting Vision change | CSF ACE: normal Serum ACE: 137 U/L Glucose: 44 mg/dL WBC: 10 Lymphocytes: 79% PMNs: 10% Monos: 7% | Nonspecific markedly abnormal appearance of nodular enhancement and thickening along the pial surface in posterior fossa and basal cisterns and along tentorium and interhemispheric fissure. Developing hydrocephalus Medullary Vein sign + | IV solumedrol 1 g for 3 days Followed by 40 mg prednisone PO Methotrexate 10 mg weekly VP Shunt | No show to several appts. Headaches/vomiting unresolved but vision problems improved |
| 3 * | 62 y.o. Female | 2015 | Double vision, facial paresthesia, proprioception/balance deficits | CSF ACE: normal CSF Glucose: 59 mg/dL Protein: 41 mg/dL WBC: 5 | Diffuses dural thickening with marked dural enhancement in parasellar region Medullary vein sign + Mild leptomeningeal enhancement of optic nerves | 1000 solumedrol for four days, followed by PO steroid 60 mg for one month Methotrexate Rituximab Q6 months | Continued headaches after cessation of steroids. Visual problems after cessation of steroids. Was restarted on a steroid taper. Subsequently weaned off steroids and started on Methotrexate in 2017, which improved headaches and visual symptoms |
| 4 * | 32 y.o. female | 2018 | Intermittent monocular blindness and blurry vision Headache Vertigo N/V Numbness | CSF protein: 66 mg/dL CSF Glucose: 54 mg/dL WBC: 6 35% neutrophil 48% lymphs | Scattered nonenhancing parenchymal lesions in white matter of cerebral hemispheres Medullary vein sign + | 1 g Solumedrol IV TI, transitioned to PO prednisone taper 1 dose IV methotrexate transitioned to 25 mg oral once per 1 week | Improvement in N/V, headaches, and vertigo. Still some residual vision impairment treated with subcutaneous methotrexate per her ophthalmologist |
| 5 * | 33 y.o. Female | 2018 | Papilledema Neck pain New blurry vision headaches | Protein: 216 mg/dL Glucose: 38 mg/dL WBC: 7 neutrophil % 1 Lymphocyte % 70 Monocytes % 27 ACE CSF: 3.5 | Scattered bilateral cerebral white matter lesions, some with associated enhancement. Leptomeningeal enhancement Medullary Vein sign + | Prednisone taper transition to imuran | Complete resolution of symptoms |
| 6 ** | 27/y o Female | 2019 | Left upper and lower extremity weakness | None | Multiple ring-enhancing lesions on post contrast T1 images, the largest within the right basal ganglia and the pons | 1 g Solumedrol IV TI, transitioned to PO prednisone taper | stable |
| 7 ** | 48/yo Male | 2018 | of bilateral retro-orbital pain and progressive vision loss over the course of two months | CSF ACE mildly elevated at 3.7 U/L (normal up to 2.5), 12 WBC predominant lymphocytic 90%, and negative meningitis panel | Enhancing left cerebellar nodule with surrounding abnormal hyperintense signal on FLAIR extending to the cerebellar peduncle. Mass-like enhancement of the folia surrounding the nodule was also noted. MRI orbits showed bilateral smooth optic nerve sheath enhancement without abnormal signal within the optic nerves | on 1 dose of cyclophosphamide with Mesna and discharged with oral tapering prednisone | Partial improvement in vision |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberio, R.; Kramer, E.; Memon, A.B.; Reinbeau, R.; Feizi, P.; Joseph, J.; Wu, J.; Sriwastava, S. Relevance of Medullary Vein Sign in Neurosarcoidosis. Neurol. Int. 2022, 14, 638-647. https://doi.org/10.3390/neurolint14030052
Liberio R, Kramer E, Memon AB, Reinbeau R, Feizi P, Joseph J, Wu J, Sriwastava S. Relevance of Medullary Vein Sign in Neurosarcoidosis. Neurology International. 2022; 14(3):638-647. https://doi.org/10.3390/neurolint14030052
Chicago/Turabian StyleLiberio, Richard, Emily Kramer, Anza B. Memon, Ryan Reinbeau, Parissa Feizi, Joe Joseph, Janet Wu, and Shitiz Sriwastava. 2022. "Relevance of Medullary Vein Sign in Neurosarcoidosis" Neurology International 14, no. 3: 638-647. https://doi.org/10.3390/neurolint14030052
APA StyleLiberio, R., Kramer, E., Memon, A. B., Reinbeau, R., Feizi, P., Joseph, J., Wu, J., & Sriwastava, S. (2022). Relevance of Medullary Vein Sign in Neurosarcoidosis. Neurology International, 14(3), 638-647. https://doi.org/10.3390/neurolint14030052





