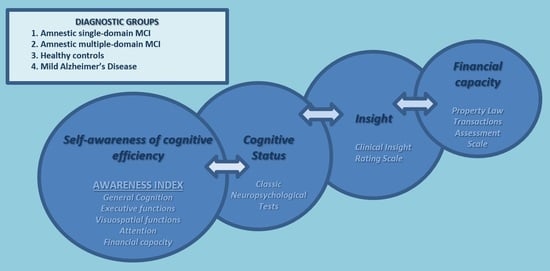
Self-Awareness of Cognitive Efficiency, Cognitive Status, Insight, and Financial Capacity in Patients with Mild AD, aMCI, and Healthy Controls: An Intriguing Liaison with Clinical Implications?
Abstract
1. Introduction
2. Method
3. Results
4. Discussion
5. Conclusions
6. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, R.G.; Mograbi, D.C. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex 2013, 49, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.P.; Kunik, M.E.; Doody, R.; Snow, A.L. Self-reported awareness of performance in dementia. Cogn. Brain Res. 2005, 25, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, S.; Kontaxopoulou, D.; Beratis, I.N.; Andronas, N.; Economou, A.; Yannis, G.; Papanicolaou, A.; Papageorgiou, S.G. Self-awareness of cognitive efficiency: Differences between healthy elderly and patients with mild cognitive impairment (MCI). J. Clin. Exp. Neuropsychol. 2016, 38, 1144–1157. [Google Scholar] [CrossRef]
- Okonkwo, O.C.; Griffith, H.R.; Vance, D.E.; Marson, D.C.; Ball, K.K.; Wadley, V.G. Awareness of functional difficulties in mild cognitive impairment: A multidomain assessment approach. J. Am. Geriatr. Soc. 2009, 57, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Orfei, M.D.; Blundo, C.; Celia, E.; Casini, A.R.; Caltagirone, C.; Spalletta, G.; Varsi, A.E. Anosognosia in mild cognitive impairment and mild Alzheimer’s disease: Frequency and neuropsychological correlates. Am. J. Geriatr. Psychiatry 2010, 18, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Stokholm, J.; Gade, A.; Andersen, B.B.; Hejl, A.M.; Waldemar, G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: Do MCI patients have impaired insight? Dement. Geriatr. Cogn. Disord. 2004, 17, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Piras, F.; Piras, F.; Orfei, M.D.; Caltagirone, C.; Spalletta, G. Self-awareness in mild cognitive impairment: Quantitative evidence from systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 61, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, O.; Vallotti, B.; Frisoni, G.B.; Geroldi, C.; Bianchetti, A.; Pasqualetti, P.; Trabucchi, M. Insight in dementia: When does it occur? Evidence for a nonlinear relationship between insight and cognitive status. J. Gerontol. B Psychol. Sci. Soc. Sci. 1999, 54, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. Vascular dementia, depression, and financial capacity assessment. Alzheimer Dis. Assoc. Disord. 2021, 35, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. Mild Alzheimer Disease, financial capacity, and the role of depression: Eyes wide shut? Alzheimer Dis. Assoc. Disord. 2021, 35, 360–362. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Depression and financial capacity assessment in Parkinson’s disease with dementia: Overlooking an important factor? Psychiatriki 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. Unraveling Ariadne’s thread into the labyrinth of aMCI: Depression and financial capacity. Alzheimer Dis. Assoc. Disord. 2021, 35, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Stamovlasis, D.; Tsolaki, M. Exploring the role of cognitive factors in a new instrument for elders’ financial capacity assessment. J. Alzheimers Dis. 2018, 62, 1579–1594. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Frontotemporal dementia and financial capacity: Facing the Cerberus of overestimation or underestimation? Australas. Psychiatry 2022, 30, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. A neglected drama for elders: Discrepancy between self-perception and objective performance regarding financial capacity in patients with cognitive deficits. Psychol. Thought 2015, 8, 142–147. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Questions about dementia with Lewy Bodies, personal beliefs and real performance for financial capacity tasks. Eur. Psychiatry 2016, 33, S469–S470. [Google Scholar] [CrossRef]
- Zamboni, G.; Drazich, E.; McCulloch, E.; Filippini, N.; Mackay, C.E.; Jenkinson, M.; Tracey, I.; Wilcock, G.K. Neuroanatomy of impaired self-awareness in Alzheimer’s disease and mild cognitive impairment. Cortex 2013, 49, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Pannu, J.K.; Kaszniak, A.W. Metamemory experiments in neurological populations: A review. Neuropsychol. Rev. 2005, 15, 105–130. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the etiological links behind neurodegenerative diseases: Inflammatory cytokines and bioactive kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. Financial capacity of patients with mild Alzheimer’s Disease: What neurologists need to know about where the impairment lies. Neurol. Int. 2022, 14, 90–98. [Google Scholar]
- Giannouli, V.; Tsolaki, M. Are left angular gyrus and amygdala volumes important for financial capacity in mild cognitive impairment? Hell. J. Nucl. Med. 2019, 22, 160–164. [Google Scholar]
- Stoeckel, L.E.; Stewart, C.C.; Griffith, H.R.; Triebel, K.; Okonkwo, O.C.; den Hollander, J.A.; Martin, R.C.; Belue, K.; Copeland, J.N.; Harrell, L.E.; et al. MRI volume of the medial frontal cortex predicts financial capacity in patients with mild Alzheimer’s disease. Brain Imaging Behav. 2013, 7, 282–292. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. Is negative affect associated with deficits in financial capacity in nondepressed older adults? A preliminary study. J. Affect Disord. Rep. 2022, 10, 100391. [Google Scholar] [CrossRef]
- Warren, S.L.; Reid, E.; Whitfield, P.; Moustafa, A.A. Subjective memory complaints as a predictor of mild cognitive impairment and Alzheimer’s disease. Discover. Psychol. 2021, 2, 13. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Financial capacity and illiteracy: Does education matter in amnstic mild cognitive impairment? J. Alzheimers Dis. Rep. 2021, 5, 715–719. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Liberating older adults from the bonds of vascular risk factors: What is their impact on financial capacity in amnestic mild cognitive impairment? Psychiatry Clin. Neurosci. 2022, 76, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Tsolaki, M. Is depression or apathy playing a key role in predicting financial capacity in Parkinson’s disease with dementia and frontotemporal dementia? Brain Sci. 2021, 11, 785. [Google Scholar] [CrossRef]
- Giannouli, V.; Stamovlasis, D.; Tsolaki, M. Longitudinal study of depression on amnestic mild cognitive impairment and financial capacity. Clin. Gerontol. 2022, 45, 708–714. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Tsolaki, M.; Iacovides, A.; Yesavage, J.; O’hara, R.; Kazis, A.; Ierodiakonou, C. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging Clin. Exp. Res. 1999, 11, 367–372. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Legal capacity of the elderly in Greece. Hel. J. Nucl. Med. 2014, 17, 2–6. [Google Scholar]
- Ott, B.R.; Lafleche, G.; Whelihan, W.M.; Buongiorno, G.W.; Albert, M.S.; Fogel, B.S. Impaired awareness of deficits in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 1996, 10, 68–76. [Google Scholar] [CrossRef]
- Ready, R.E.; Ott, B.R.; Grace, J. Insight and cognitive impairment: Effects on quality-of-life reports from mild cognitive impairment and Alzheimer’s disease patients. Am. J. Alzheimer’s Dis. Other Demen. 2006, 21, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Marková, I.S.; Berrios, G.E. Awareness and insight in psychopathology: An essential distinction? Theory Psychol. 2011, 21, 421–437. [Google Scholar] [CrossRef]
- Nobili, F.; Mazzei, D.; Dessi, B.; Morbelli, S.; Brugnolo, A.; Barbieri, P.; Girtler, R.; Sambuceti, G.; Rodriguez, G.; Pagani, M. Unawareness of memory deficit in amnestic MCI: FDG-PET findings. J. Alzheimer’s Dis. 2010, 22, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Clare, L.; Woods, R.T. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: A systematic review. Dement. Geriatr. Cogn. Disord. 2009, 28, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Tremont, G.; Alosco, M.L. Relationship between cognition and awareness of deficit in mild cognitive impairment. Int. J. Geriatr. Psychiatry 2011, 26, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Galeone, F.; Pappalardo, S.; Chieffi, S.; Iavarone, A.; Carlomagno, S. Anosognosia for memory deficit in amnestic mild cognitive impairment and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2011, 26, 695–701. [Google Scholar] [CrossRef]
- Wood, S.; Lichtenberg, P.A. Financial capacity and financial exploitation of older adults: Research findings, policy recommendations and clinical implications. Clin. Gerontol. 2017, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, P.A.; Duffy, M. Psychological assessment and psychotherapy in long-term care. Clin. Psychol. Sci. Pract. 2000, 7, 317. [Google Scholar] [CrossRef]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, e14122. [Google Scholar] [CrossRef] [PubMed]
| Cognitive Domains and Tests | M | SD | |
|---|---|---|---|
| Diagnostic Groups | |||
| General cognitive state MMSE | aMCI-SD | 27.63 | 1.29 |
| aMCI-MD | 26.39 | 1.44 | |
| HC | 29.13 | 0.43 | |
| mild AD | 22.81 | 3.55 | |
| Insight CIR scale | aMCI-SD | 1.97 | 0.68 |
| aMCI-MD | 2.41 | 0.59 | |
| HC | 0.00 | 0.00 | |
| mild AD | 3.42 | 1.03 | |
| Financial capacity LCPLTAS | aMCI-SD | 185.56 | 21.13 |
| aMCI-MD | 144.21 | 73.22 | |
| HC | 211.33 | 1.21 | |
| mild AD | 117.77 | 34.64 | |
| Attention | |||
| Trail Making Part A (s) | aMCI-SD | 66.48 | 34.43 |
| aMCI-MD | 71.63 | 44.78 | |
| HC | 52.06 | 6.89 | |
| mild AD | 214.39 | 78.84 | |
| Learning and memory | |||
| RAVLT immediate1 | aMCI-SD | 5.77 | 1.12 |
| aMCI-MD | 5.73 | .97 | |
| HC | 10.50 | 3.58 | |
| mild AD | 2.63 | 1.22 | |
| RAVLT 5 | aMCI-SD | 9.31 | 3.23 |
| aMCI-MD | 7.76 | 3.54 | |
| HC | 13.13 | 0.73 | |
| mild AD | 2.55 | 1.69 | |
| RAVLT delayed recall | aMCI-SD | 11.02 | 2.53 |
| aMCI-MD | 8.52 | 2.55 | |
| HC | 14.03 | 16.30 | |
| mild AD | 1.67 | 0.47 | |
| Visuospatial perception and memory | |||
| Rey copy | aMCI-SD | 30.06 | 7.76 |
| aMCI-MD | 25.85 | 8.17 | |
| HC | 32.70 | 5.00 | |
| mild AD | 13.67 | 1.47 | |
| Rey immediate recall | aMCI-SD | 17.26 | 6.40 |
| aMCI-MD | 16.15 | 8.42 | |
| HC | 22.25 | 5.84 | |
| mild AD | 9.50 | 1.55 | |
| Rey Delayed recall | aMCI-SD | 16.31 | 26.30 |
| aMCI-MD | 14.75 | 7.35 | |
| HC | 19.70 | 5.32 | |
| mild AD | 5.88 | 1.74 | |
| Executive functions | |||
| Trail Making Part B (sec) | aMCI-SD | 121.55 | 83.47 |
| aMCI-MD | 134.90 | 95.53 | |
| HC | 108.77 | 34.41 | |
| mild AD | 212.23 | 234.87 | |
| Self-estimations | |||
| Estimations of financial capacity | aMCI-SD | 18.29 | 25.58 |
| aMCI-MD | 24.25 | 32.88 | |
| HC | 3.66 | 16.50 | |
| mild AD | 36.66 | 45.96 | |
| Estimations of general cognition | aMCI-SD | 19.75 | 26.55 |
| aMCI-MD | 25.50 | 32.81 | |
| HC | 3.66 | 16.50 | |
| mild AD | 35.00 | 45.91 | |
| Estimations of attention | aMCI-SD | 18.29 | 26.35 |
| aMCI-MD | 25.00 | 33.66 | |
| HC | 4.00 | 16.52 | |
| mild AD | 34.66 | 47.03 | |
| Estimations of learning | aMCI-SD | 19.75 | 24.23 |
| aMCI-MD | 26.25 | 32.00 | |
| HC | 3.66 | 16.50 | |
| mild AD | 34.66 | 46.36 | |
| Estimations of visual perception | aMCI-SD | 18.04 | 26.19 |
| aMCI-MD | 24.50 | 33.20 | |
| HC | 4.33 | 16.75 | |
| mild AD | 31.72 | 47.36 | |
| Estimations of executive functions | aMCI-SD | 19.02 | 25.37 |
| aMCI-MD | 24.75 | 32.02 | |
| HC | 4.33 | 16.54 | |
| mild AD | 41.47 | 48.99 |
| AI | Mean | SD | |
|---|---|---|---|
| Diagnostic Groups | |||
| AΙ financial capacity | aMCI-SD | 59.21 | 13.22 |
| aMCI-MD | 61.67 | 16.55 | |
| HC | 51.02 | 8.25 | |
| mild AD | 68.08 | 23.00 | |
| AΙ general cognition | aMCI-SD | 59.24 | 13.29 |
| aMCI-MD | 62.26 | 16.38 | |
| HC | 51.02 | 8.25 | |
| mild AD | 67.92 | 23.52 | |
| AΙ executive functions | aMCI-SD | 59.43 | 12.76 |
| aMCI-MD | 61.92 | 15.97 | |
| HC | 52.65 | 10.56 | |
| mild AD | 72.87 | 24.66 | |
| AΙ visuospatial functions | aMCI-SD | 58.79 | 12.68 |
| aMCI-MD | 61.39 | 16.68 | |
| HC | 51.47 | 8.49 | |
| mild AD | 65.75 | 23.69 | |
| AΙ attention | aMCI-SD | 58.78 | 13.20 |
| aMCI-MD | 62.11 | 17.04 | |
| HC | 51.71 | 8.26 | |
| mild AD | 61.62 | 22.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannouli, V.; Tsolaki, M. Self-Awareness of Cognitive Efficiency, Cognitive Status, Insight, and Financial Capacity in Patients with Mild AD, aMCI, and Healthy Controls: An Intriguing Liaison with Clinical Implications? Neurol. Int. 2022, 14, 628-637. https://doi.org/10.3390/neurolint14030051
Giannouli V, Tsolaki M. Self-Awareness of Cognitive Efficiency, Cognitive Status, Insight, and Financial Capacity in Patients with Mild AD, aMCI, and Healthy Controls: An Intriguing Liaison with Clinical Implications? Neurology International. 2022; 14(3):628-637. https://doi.org/10.3390/neurolint14030051
Chicago/Turabian StyleGiannouli, Vaitsa, and Magdalini Tsolaki. 2022. "Self-Awareness of Cognitive Efficiency, Cognitive Status, Insight, and Financial Capacity in Patients with Mild AD, aMCI, and Healthy Controls: An Intriguing Liaison with Clinical Implications?" Neurology International 14, no. 3: 628-637. https://doi.org/10.3390/neurolint14030051
APA StyleGiannouli, V., & Tsolaki, M. (2022). Self-Awareness of Cognitive Efficiency, Cognitive Status, Insight, and Financial Capacity in Patients with Mild AD, aMCI, and Healthy Controls: An Intriguing Liaison with Clinical Implications? Neurology International, 14(3), 628-637. https://doi.org/10.3390/neurolint14030051