Antipsychotic Polypharmacy-Related Cardiovascular Morbidity and Mortality: A Comprehensive Review
Abstract
:1. Introduction
2. Cardiac Issues and Antipsychotics
2.1. QTc Interval
2.2. Risk Factors
2.3. Typical Antipsychotics
2.4. Atypical Antipsychotics
3. Interactions between Antipsychotics and Non-Psychotropic Medications
3.1. Opioids
3.2. Antibiotics
3.3. Other Antimicrobials
3.4. Illicit Drugs
4. Polypharmacy
5. Clinical Studies on Cardiac Events
5.1. Animal Studies
5.2. Phase 1
5.3. Phase 2
5.4. Phase 3
5.5. Phase 4
5.6. Other Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arciniegas, D.B. Psychosis. Continuum 2015, 21, 715–736. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R. Antipsychotics in the treatment of schizophrenia: An overview. J. Clin. Psychiatry 2011, 72 (Suppl. S1), 4–8. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, J.A.; First, M.B. Psychotic Disorders. N. Engl. J. Med. 2018, 379, 270–280. [Google Scholar] [CrossRef]
- Hany, M.; Rehman, B.; Azhar, Y.; Chapman, J. Schizophrenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Svensson, T.H. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1145–1158. [Google Scholar] [CrossRef]
- Willner, K.; Vasan, S.; Abdijadid, S. Atypical Antipsychotic Agents. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Andersson, K.-E.; Campeau, L.; Olshansky, B. Cardiac effects of muscarinic receptor antagonists used for voiding dysfunction. Br. J. Clin. Pharm. 2011, 72, 186–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, D.P.; Homoud, M.K.; Estes, N.A.M. Prediction and Prevention of Sudden Cardiac Death. Card Electrophysiol. Clin. 2017, 9, 631–638. [Google Scholar] [CrossRef]
- Sideris, D.A. High blood pressure and ventricular arrhythmias. Eur. Heart J. 1993, 14, 1548–1553. [Google Scholar] [CrossRef]
- Aidietis, A.; Laucevicius, A.; Marinskis, G. Hypertension and cardiac arrhythmias. Curr. Pharm. Des. 2007, 13, 2545–2555. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Coca, A.; Kahan, T.; Boriani, G.; Manolis, A.S.; Olsen, M.H.; Oto, A.; Potpara, T.S.; Steffel, J.; Marín, F.; et al. Hypertension and cardiac arrhythmias: Executive summary of a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Eur. Heart J.—Cardiovasc. Pharmacother. 2017, 3, 235–250. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, A.; Guàrdia, A.; Monreal, J.A. Peri- and Post-Menopausal Women with Schizophrenia and Related Disorders Are a Population with Specific Needs: A Narrative Review of Current Theories. J. Pers. Med. 2021, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Masand, P.S. Side effects of antipsychotics in the elderly. J. Clin. Psychiatry 2000, 61 (Suppl. S8), 43–49; discussion 50–51. [Google Scholar]
- Yamaguchi, T.; Sumida, T.S.; Nomura, S.; Satoh, M.; Higo, T.; Ito, M.; Ko, T.; Fujita, K.; Sweet, M.E.; Sanbe, A.; et al. Cardiac dopamine D1 receptor triggers ventricular arrhythmia in chronic heart failure. Nat. Commun. 2020, 11, 4364. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Effect of alpha 1-adrenergic receptor antagonists on susceptibility to malignant arrhythmias: Protection from ventricular fibrillation. J. Cardiovasc. Pharm. 1994, 24, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kuriachan, V.P.; Sumner, G.L.; Mitchell, L.B. Sudden cardiac death. Curr. Probl. Cardiol. 2015, 40, 133–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hou, W.; Xu, Y.; Ji, F.; Wang, G.; Chen, C.; Lin, C.; Lin, X.; Li, J.; Zhuo, C.; et al. Antipsychotic drugs and sudden cardiac death: A literature review of the challenges in the prediction, management, and future steps. Psychiatry Res. 2019, 281, 112598. [Google Scholar] [CrossRef]
- Shah, A.A.; Aftab, A.; Coverdale, J. QTc Prolongation with Antipsychotics: Is Routine ECG Monitoring Recommended? J. Psychiatr. Pract. 2014, 20, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Beach, S.R.; Celano, C.M.; Noseworthy, P.A.; Januzzi, J.L.; Huffman, J.C. QTc Prolongation, Torsades de Pointes, and Psychotropic Medications. Psychosomatics 2013, 54, 1–13. [Google Scholar] [CrossRef]
- Witchel, H.J.; Hancox, J.C.; Nutt, D.J. Psychotropic Drugs, Cardiac Arrhythmia, and Sudden Death. J. Clin. Psychopharmacol. 2003, 23, 58–77. [Google Scholar] [CrossRef]
- Fanoe, S.; Kristensen, D.; Fink-Jensen, A.; Jensen, H.K.; Toft, E.; Nielsen, J.; Videbech, P.; Pehrson, S.; Bundgaard, H. Risk of arrhythmia induced by psychotropic medications: A proposal for clinical management. Eur. Heart J. 2014, 35, 1306–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöllberger, C.; Huber, J.O.; Finsterer, J. Antipsychotic drugs and QT prolongation. Int. Clin. Psychopharmacol. 2005, 20, 243–251. [Google Scholar] [CrossRef]
- Torres, V.; Tepper, D.; Flowers, D.; Wynn, J.; Lam, S.; Keefe, D.; Miura, D.S.; Somberg, J.C. QT prolongation and the antiarrhythmic efficacy of amiodarone. J. Am. Coll. Cardiol. 1986, 7, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Harrigan, E.P.; Miceli, J.J.; Anziano, R.; Watsky, E.; Reeves, K.R.; Cutler, N.R.; Sramek, J.; Shiovitz, T.; Middle, M. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J. Clin. Psychopharmacol. 2004, 24, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Chung, C.P.; Murray, K.T.; Hall, K.; Stein, C.M. Atypical Antipsychotic Drugs and the Risk of Sudden Cardiac Death. N. Engl. J. Med. 2009, 360, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Tarabová, B.; Nováková, M.; Lacinová, L. Haloperidol moderately inhibits cardiovascular L-type calcium current. Gen. Physiol. Biophys. 2009, 28, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Hert, M.; Detraux, J.; van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2012, 8, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.M.J.M.; Bleumink, G.S.; Dieleman, J.P.; van der Lei, J.; ‘t Jong, G.W.; Kingma, J.H.; Sturkenboom, M.C.J.M.; Stricker, B.H.C. Antipsychotics and the Risk of Sudden Cardiac Death. Arch. Intern. Med. 2004, 164, 1293–1297. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Ashburn, M.A. Cardiac Effects of Opioid Therapy. Pain Med. 2015, 16, S27–S31. [Google Scholar] [CrossRef] [Green Version]
- Kao, D.P.; Haigney, M.C.P.; Mehler, P.S.; Krantz, M.J. Arrhythmia associated with buprenorphine and methadone reported to the food and drug administration. Addiction 2015, 110, 1468–1475. [Google Scholar] [CrossRef]
- Valentine, E.A.; Kaye, A.D.; Abadie, J.V.; Kaye, A.M. Drug-Induced QT Prolongation. In Essentials of Pharmacology for Anesthesia, Pain Medicine, and Critical Care; Kaye, A.D., Kaye, A.M., Urman, R.D., Eds.; Springer: New York, NY, USA, 2015; pp. 753–766. ISBN 978-1-4614-8948-1. [Google Scholar]
- Price, L.C.; Wobeter, B.; Delate, T.; Kurz, D.; Shanahan, R. Methadone for Pain and the Risk of Adverse Cardiac Outcomes. J. Pain Symptom Manag. 2014, 48, 333–342.e1. [Google Scholar] [CrossRef]
- Tran, P.N.; Sheng, J.; Randolph, A.L.; Baron, C.A.; Thiebaud, N.; Ren, M.; Wu, M.; Johannesen, L.; Volpe, D.A.; Patel, D.; et al. Mechanisms of QT prolongation by buprenorphine cannot be explained by direct hERG channel block. PLoS ONE 2020, 15, e0241362. [Google Scholar] [CrossRef]
- Baker, J.R.; Best, A.M.; Pade, P.A.; McCance-Katz, E.F. Effect of Buprenorphine and Antiretroviral Agents on the QT Interval in Opioid-Dependent Patients. Ann. Pharm. 2006, 40, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.K.; Yuan, J.; Li, M.; Sutton, S.S.; Rao, G.A.; Jacob, S.; Bennett, C.L. Cardiac risks associated with antibiotics: Azithromycin and levofloxacin. Expert Opin. Drug Saf. 2015, 14, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, G.A.; Mann, J.R.; Shoaibi, A.; Bennett, C.L.; Nahhas, G.; Sutton, S.S.; Jacob, S.; Strayer, S.M. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann. Fam. Med. 2014, 12, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strle, F.; Maraspin, V. Is azithromycin treatment associated with prolongation of the Q-Tc interval? Wien Klin. Wochenschr. 2002, 114, 396–399. [Google Scholar]
- Cheng, Y.-J.; Nie, X.-Y.; Chen, X.-M.; Lin, X.-X.; Tang, K.; Zeng, W.-T.; Mei, W.-Y.; Liu, L.-J.; Long, M.; Yao, F.-J.; et al. The Role of Macrolide Antibiotics in Increasing Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 66, 2173–2184. [Google Scholar] [CrossRef] [Green Version]
- Makaryus, A.N.; Byrns, K.; Makaryus, M.N.; Natarajan, U.; Singer, C.; Goldner, B. Effect of ciprofloxacin and levofloxacin on the QT interval: Is this a significant "clinical" event? South. Med. J. 2006, 99, 52–57. [Google Scholar] [CrossRef]
- Effects of Three Fluoroquinolones on QT Interval in Healthy Adults after Single Doses-Noel-2003-Clinical Pharmacology & Therapeutics—Wiley Online Library. Available online: https://ascpt.onlinelibrary.wiley.com/doi/full/10.1016/S0009-9236%2803%2900009-2?casa_token=XQCtPtt4Tp4AAAAA%3A7TWvQJbQl7EW-iRmai4f6fZuBwXAJuR5WcUKqNdtONM90WnLI7xFJNN8ACmsNfq_9hSxPOOpq7QizA (accessed on 19 April 2021).
- Postma, D.F.; Spitoni, C.; van Werkhoven, C.H.; van Elden, L.J.R.; Oosterheert, J.J.; Bonten, M.J.M. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: Post-hoc analysis of a cluster-randomized trial. BMC Infect. Dis. 2019, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Drug Metabolism—The Importance of Cytochrome P450 3A4. Available online: https://www.medsafe.govt.nz/profs/puarticles/march2014drugmetabolismcytochromep4503a4.htm (accessed on 20 December 2021).
- van der Weide, J.; Hinrichs, J.W. The Influence of Cytochrome P450 Pharmacogenetics on Disposition of Common Antidepressant and Antipsychotic Medications. Clin. Biochem. Rev. 2006, 27, 17–25. [Google Scholar]
- Arranz, M.J.; Gonzalez-Rodriguez, A.; Perez-Blanco, J.; Penadés, R.; Gutierrez, B.; Ibañez, L.; Arias, B.; Brunet, M.; Cervilla, J.; Salazar, J.; et al. A pharmacogenetic intervention for the improvement of the safety profile of antipsychotic treatments. Transl. Psychiatry 2019, 9, 177. [Google Scholar] [CrossRef]
- Cohen, I.V.; Makunts, T.; Moumedjian, T.; Issa, M.A.; Abagyan, R. Cardiac adverse events associated with chloroquine and hydroxychloroquine exposure in 20 years of drug safety surveillance reports. Sci. Rep. 2020, 10, 19199. [Google Scholar] [CrossRef]
- Anson, B.D.; Weaver, J.G.; Ackerman, M.J.; Akinsete, O.; Henry, K.; January, C.T.; Badley, A.D. Blockade of HERG channels by HIV protease inhibitors. Lancet 2005, 365, 682–686. [Google Scholar] [CrossRef]
- Hunt, K.; Hughes, C.A.; Hills-Nieminen, C. Protease inhibitor-associated QT interval prolongation. Ann Pharm. 2011, 45, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, V.M. Chemical Cardiomyopathies: The Negative Effects of Medications and Nonprescribed Drugs on the Heart. Am. J. Med. 2011, 124, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devlin, R.J.; Henry, J.A. Clinical review: Major consequences of illicit drug consumption. Crit. Care 2008, 12, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozturk, H.M.; Yetkin, E.; Ozturk, S. Synthetic Cannabinoids and Cardiac Arrhythmia Risk: Review of the Literature. Cardiovasc. Toxicol. 2019, 19, 191–197. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Qin, X.; Yang, Q.; Fan, H.; Li, J.; Song, X.; Xu, S.; Guo, W.; Deng, W.; et al. Antipsychotics and risk of natural death in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2019, 15, 1863–1871. [Google Scholar] [CrossRef] [Green Version]
- Baandrup, L.; Gasse, C.; Jensen, V.D.; Glenthoj, B.Y.; Nordentoft, M.; Lublin, H.; Fink-Jensen, A.; Lindhardt, A.; Mortensen, P.B. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: A population-based nested case-control study. J. Clin. Psychiatry 2010, 71, 103–108. [Google Scholar] [CrossRef]
- Fleischhacker, W.W.; Uchida, H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int. J. Neuropsychopharmacol. 2014, 17, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Kasteridis, P.; Ride, J.; Gutacker, N.; Aylott, L.; Dare, C.; Doran, T.; Gilbody, S.; Goddard, M.; Gravelle, H.; Kendrick, T.; et al. Association Between Antipsychotic Polypharmacy and Outcomes for People with Serious Mental Illness in England. Psychiatr. Serv. 2019, 70, 650–656. [Google Scholar] [CrossRef]
- Kadra, G.; Stewart, R.; Shetty, H.; MacCabe, J.H.; Chang, C.-K.; Taylor, D.; Hayes, R.D. Long-term antipsychotic polypharmacy prescribing in secondary mental health care and the risk of mortality. Acta Psychiatr. Scand. 2018, 138, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Elliott, A.; Mørk, T.J.; Højlund, M.; Christensen, T.; Jeppesen, R.; Madsen, N.; Viuff, A.G.; Hjorth, P.; Nielsen, J.C.; Munk-Jørgensen, P. QTc interval in patients with schizophrenia receiving antipsychotic treatment as monotherapy or polypharmacy. CNS Spectr. 2018, 23, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, M.; Geguchadze, R.; Guntur, A.R.; Nevola, K.; Le, P.T.; Barlow, D.; Rue, M.; Vary, C.P.H.; Lary, C.W.; Motyl, K.J.; et al. Exploring mechanisms of increased cardiovascular disease risk with antipsychotic medications: Risperidone alters the cardiac proteomic signature in mice. Pharm. Res. 2020, 152, 104589. [Google Scholar] [CrossRef] [PubMed]
- Miceli, J.J.; Tensfeldt, T.G.; Shiovitz, T.; Anziano, R.J.; O’Gorman, C.; Harrigan, R.H. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: A randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin. Ther. 2010, 32, 472–491. [Google Scholar] [CrossRef]
- PubChem Iloperidone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/71360 (accessed on 20 December 2021).
- Ziprasidone (Geodon) Pharmacokinetics—Psychopharmacology Institute. Available online: https://psychopharmacologyinstitute.com/publication/ziprasidone-geodon-pharmacokinetics-2166 (accessed on 20 December 2021).
- Winter, H.R.; Earley, W.R.; Hamer-Maansson, J.E.; Davis, P.C.; Smith, M.A. Steady-state pharmacokinetic, safety, and tolerability profiles of quetiapine, norquetiapine, and other quetiapine metabolites in pediatric and adult patients with psychotic disorders. J. Child. Adolesc. Psychopharmacol. 2008, 18, 81–98. [Google Scholar] [CrossRef]
- Wadhwa, R.R.; Cascella, M. Steady State Concentration; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Potkin, S.G.; Preskorn, S.; Hochfeld, M.; Meng, X. A Thorough QTc Study of 3 Doses of Iloperidone Including Metabolic Inhibition Via CYP2D6 and/or CYP3A4 and a Comparison to Quetiapine and Ziprasidone. J. Clin. Psychopharmacol. 2013, 33, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hough, D.W.; Natarajan, J.; Vandebosch, A.; Rossenu, S.; Kramer, M.; Eerdekens, M. Evaluation of the effect of paliperidone extended release and quetiapine on corrected QT intervals: A randomized, double-blind, placebo-controlled study. Int. Clin. Psychopharmacol. 2011, 26, 25–34. [Google Scholar] [CrossRef]
- Strom, B.L.; Faich, G.A.; Reynolds, R.F.; Eng, S.M.; D’Angostino, R.B.; Ruskin, J.N.; Kane, J.M. The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC): Design and Baseline Subject Characteristics. J. Clin. Psychiatry 2008, 69, 114–121. [Google Scholar] [CrossRef]
- Geier, J.L.; Karayal, O.N.; Lewis, M.; Camm, J.A.; Keane, M.; Kremer, C.M.E.; Kolluri, S.; Reynolds, R.; Eng, S.; Strom, B.L. Methodological challenges in the coding and adjudication of sudden deaths in a large simple trial with observational follow-up: The ziprasidone observational study of cardiac outcomes (ZODIAC): CODING AND ADJUDICATION OF SUDDEN DEATH IN ZODIAC. Pharm. Drug Saf. 2011, 20, 1192–1198. [Google Scholar] [CrossRef]
- Strom, B.L.; Eng, S.M.; Faich, G.; Reynolds, R.F.; D’Agostino, R.B.; Ruskin, J.; Kane, J.M. Comparative Mortality Associated with Ziprasidone and Olanzapine in Real-World Use Among 18,154 Patients with Schizophrenia: The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC). AJP 2011, 168, 193–201. [Google Scholar] [CrossRef]
- Cassella, J.V.; Spyker, D.A.; Yeung, P.P. A randomized, placebo-controlled repeat-dose thorough QT study of inhaled loxapine in healthy volunteers. CP 2015, 53, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tsai, Y.; Tsai, H. Antipsychotic Drugs and the Risk of Ventricular Arrhythmia and/or Sudden Cardiac Death: A Nation-wide Case-Crossover Study. JAHA 2015, 4, e001568. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Rossbach, W.; Muller, M.J.; Muller-Siecheneder, F.; Ru, H.; Dittmann, R.W. Heart rate variability during sleep in patients with schizophrenia treated with olanzapine. Int. Clin. Psychopharmacol. 2004, 19, 325–330. [Google Scholar] [CrossRef] [PubMed]
| Antipsychotic | Class | Cardiac Issues | Mechanism |
|---|---|---|---|
| Thioridazine | First Generation | Highest risk of QTc prolongation Increase of 30–35 ms | Very potent blocker of IKr channels, resulting in delayed repolarization |
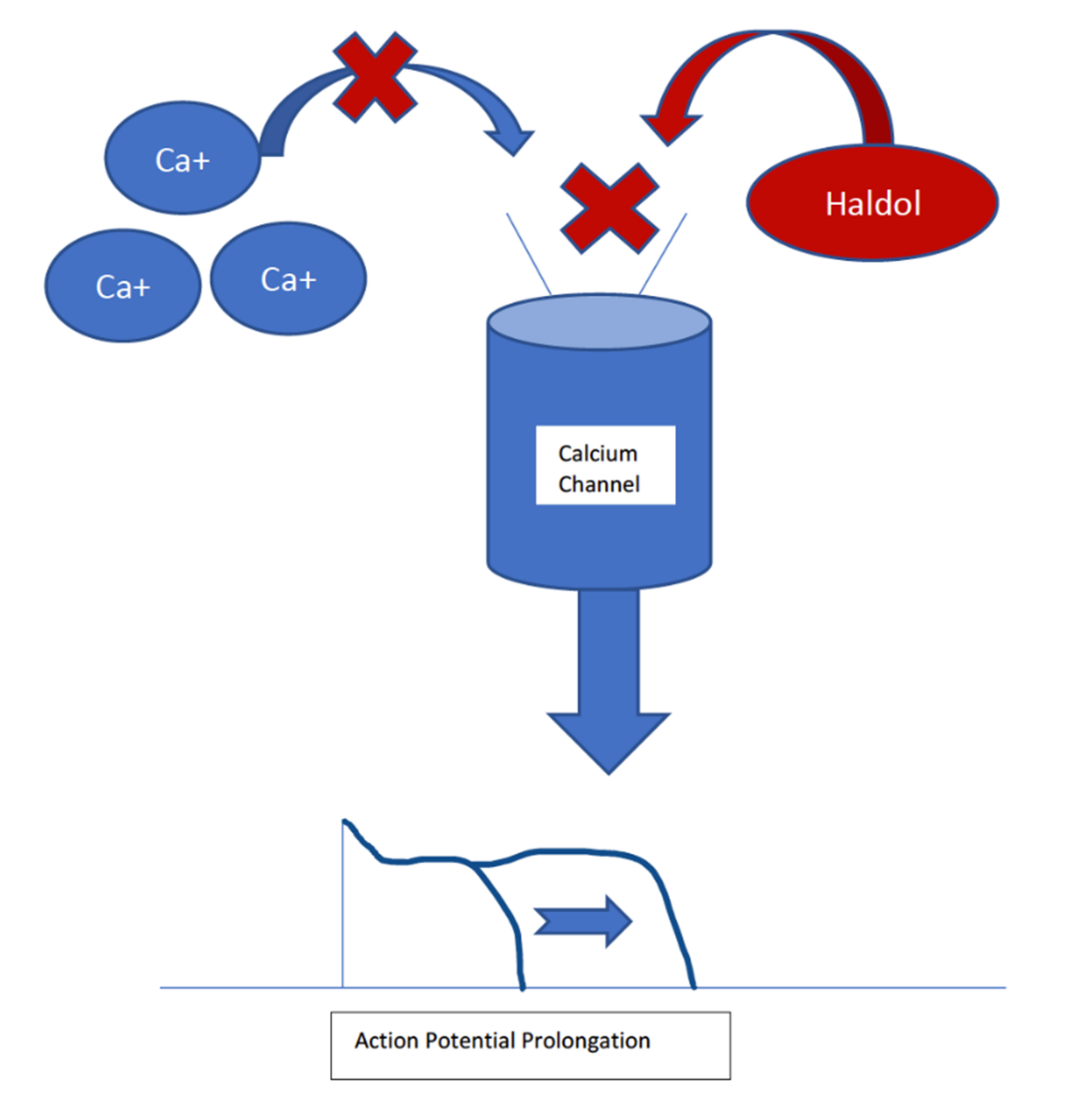
| Haloperidol | First Generation | QTc prolongation of 4–7 ms Still has the highest risk of sudden cardiac death | Can block IKr channels, but it is a less potent inhibitor than thioridazine Can block L-type calcium channels, causing a shortening of the action potential |
| Ziprasidone | Second Generation | Mean QTc prolongation of 9.6–20.3 ms | Blockade of IKr channels with a similar potency as haloperidol |
| Olanzapine | Second Generation | Mean QTc increase of 6.8 ms | Actions on IKr channels |
| Risperidone | Second Generation | Mean QTc increase of 9.1 ms | Actions on IKr channels with a similar potency as haloperidol |
| Author (Year) | Groups Studied and Intervention | Results and Key Findings | Conclusions |
|---|---|---|---|
| Miceli et al., 2010 | Phase 1, randomized, single-blind, parallel-group comparison of QTc interval change following intramuscular injection of 125% greater than daily recommended dose of ziprasidone and haloperidol among 58 patients with schizoaffective disorder or schizophrenia | No QTc interval exceeded 480 msec in either group, and no changes from baseline exceeded 60 ms AEs were mostly mild-to-moderate in severity | At supratherapeutic doses, both drugs were well tolerated and QTc interval changes were clinically modest in both drugs |
| Potkin et al., 2013 | Phase 2, multicenter, randomized, open-label study evaluating iloperidone with respect to QTc interval changes in comparison to quetiapine and ziprasidone in the context of metabolic inhibition among 188 patients with schizoaffective disorder or schizophrenia | Without metabolic inhibition, mean changes in QTc in the iloperidone 8 mg BID group were comparable to the ziprasidone group The mean QTc for 24 mg QD iloperidone alone was 15 ms Mean QTc increased as concentration of iloperidone increased, yet none were clinically significant, or ≥500 ms Concentration-independent tachycardia occurred without further sequelae and was mostly mild in those taking iloperidone | The authors suggested that QTc interval prolongation may not be the optimal measurement for assessing risk of fatal arrhythmias Baseline QTc intervals in this study did not predict increased QTc with the antipsychotic drugs used in this study |
| Hough et al., 2011 | Phase 3, randomized, double-blind, placebo-controlled study evaluating the effect of paliperidone and quetiapine on QTc intervals in 109 patients with schizoaffective disorder (21%) or schizophrenia (79%) | Paliperidone extended-release treatment increased the mean QTc intervals to a modest and similar degree as quetiapine | The authors concluded that paliperidone is tolerable at the maximum recommended daily dose of 12 mg and is non-inferior to quetiapine |
| Strom et al., 2011 | The ZODIAC trial Phase 4 trial where either ziprasidone or olanzapine treatment was initiated | There was a greater risk of all-cause hospitalizations in the ziprasidone group, but no differences were noted in the risk of hospitalization due to myocardial infarction, arrhythmia, or arrhythmia reported during hospitalization between the treatment groups | Authors noted that while QTc interval prolongation is associated ziprasidone, evidence from their study demonstrates that it does not have a great deal of clinical significance with respect to cardiovascular events or death when compared to olanzapine |
| Cassella et al., 2015 | Phase 4, randomized, double-blind, active, and placebo-controlled crossover study evaluating the effect of inhaled loxapine on QTc in 60 healthy subjects Moxifloxacin served as a positive control to validate the results | Two doses of inhaled loxapine 10 mg administered 2 h apart were well tolerated and did not cause threshold QTc prolongation | The authors suggested that inhaled loxapine may be safer than haloperidol to treat agitation in emergent situations. |
| Harrigan et al., 2004 | A prospective, randomized, parallel-group study evaluating QTc prolongation associated with monotherapy with the following drugs: haloperidol (n = 32), ziprasidone (n = 35), quetiapine (n = 29), olanzapine (n = 28), risperidone (n = 28), thioridazine (n = 31), followed by co-administration of drug and metabolic inhibitor | QTc prolongation was sub-clinical (<500 ms) during monotherapy and co-administration with CYP450 inhibitors The greatest change in mean baseline-corrected QTc interval was observed with thioridazine (30 ms) and least with olanzapine (1.7 ms) | Inhibition of the CYP450 pathway did not result in large increases in Cmax or QTc intervals, suggesting that an unidentified metabolic pathway plays a role in the metabolism of these antipsychotic agents |
| Wu et al., 2015 | A large (n = 17,718) Taiwanese case-crossover study performed from 2000 to 2009 assessing the risk of ventricular arrhythmia (VA) and sudden cardiac death (SCD) resulting from the use of antipsychotics | The use of antipsychotics was associated with a 1.53-fold increased risk of VA/ SCD VA/SCD risk was slightly higher among patients taking first-generation antipsychotics (FGA) than second-generation antipsychotics (SGA) and female gender Antipsychotics with human ether-à-go-go-related gene (hERG) potassium channel blocking activity were associated with 1.24-fold greater risk of VA/SCD | The authors concluded that SGA may be safer than FGA with respect to VA and SCD risks, and, importantly, they must be prescribed carefully during the first phase of treatment |
| Mann et al., 2004 | Men with schizophrenia (n = 10) completed an open-label sub-chronic olanzapine treatment, and were monitored during their sleep for heart rate variability, QTc interval, and EEG activity | Baseline sleep stages matched conditions in healthy subjects with no schizophrenia During olanzapine treatment, a small but significant shift towards enhanced sympathetic tone was observed | Olanzapine may increase heart rate, but it reduces heart rate variability in patients with schizophrenia compared to control treatment |
| Elliot et al., 2018 | Observational study of patients with schizophrenia in Denmark 65 patients in the study had EKGs available for analysis for QTc Interval | No differences were seen between monotherapy and polypharmacological treatment 65% overall had a prolonged QTc Women had a longer QTc interval than men on polypharmacological treatment than on monotherapy | When polypharmacy is used, women may be more at risk of a prolonged QTc interval |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edinoff, A.N.; Ellis, E.D.; Nussdorf, L.M.; Hill, T.W.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Antipsychotic Polypharmacy-Related Cardiovascular Morbidity and Mortality: A Comprehensive Review. Neurol. Int. 2022, 14, 294-309. https://doi.org/10.3390/neurolint14010024
Edinoff AN, Ellis ED, Nussdorf LM, Hill TW, Cornett EM, Kaye AM, Kaye AD. Antipsychotic Polypharmacy-Related Cardiovascular Morbidity and Mortality: A Comprehensive Review. Neurology International. 2022; 14(1):294-309. https://doi.org/10.3390/neurolint14010024
Chicago/Turabian StyleEdinoff, Amber N., Emily D. Ellis, Laura M. Nussdorf, Taylor W. Hill, Elyse M. Cornett, Adam M. Kaye, and Alan D. Kaye. 2022. "Antipsychotic Polypharmacy-Related Cardiovascular Morbidity and Mortality: A Comprehensive Review" Neurology International 14, no. 1: 294-309. https://doi.org/10.3390/neurolint14010024
APA StyleEdinoff, A. N., Ellis, E. D., Nussdorf, L. M., Hill, T. W., Cornett, E. M., Kaye, A. M., & Kaye, A. D. (2022). Antipsychotic Polypharmacy-Related Cardiovascular Morbidity and Mortality: A Comprehensive Review. Neurology International, 14(1), 294-309. https://doi.org/10.3390/neurolint14010024










