Hypomyelinating Leukodystrophy 8 (HLD8)-Associated Mutation of POLR3B Leads to Defective Oligodendroglial Morphological Differentiation Whose Effect Is Reversed by Ibuprofen
Abstract
:1. Introduction
2. Material and Methods
2.1. Plasmid Constructions and Primary and Secondary Antibodies
2.2. Cell Culture
2.3. Cell Differentiation
2.4. Cell Transfection
2.5. Confocal Microscopic Images
2.6. Polyacrylamide Gel Electrophoresis and Immunoblotting
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
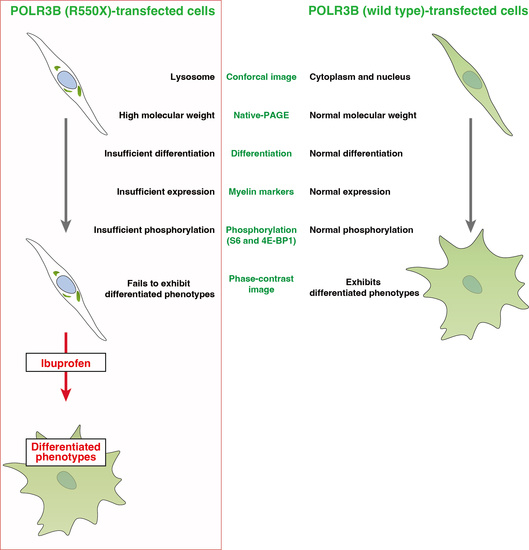
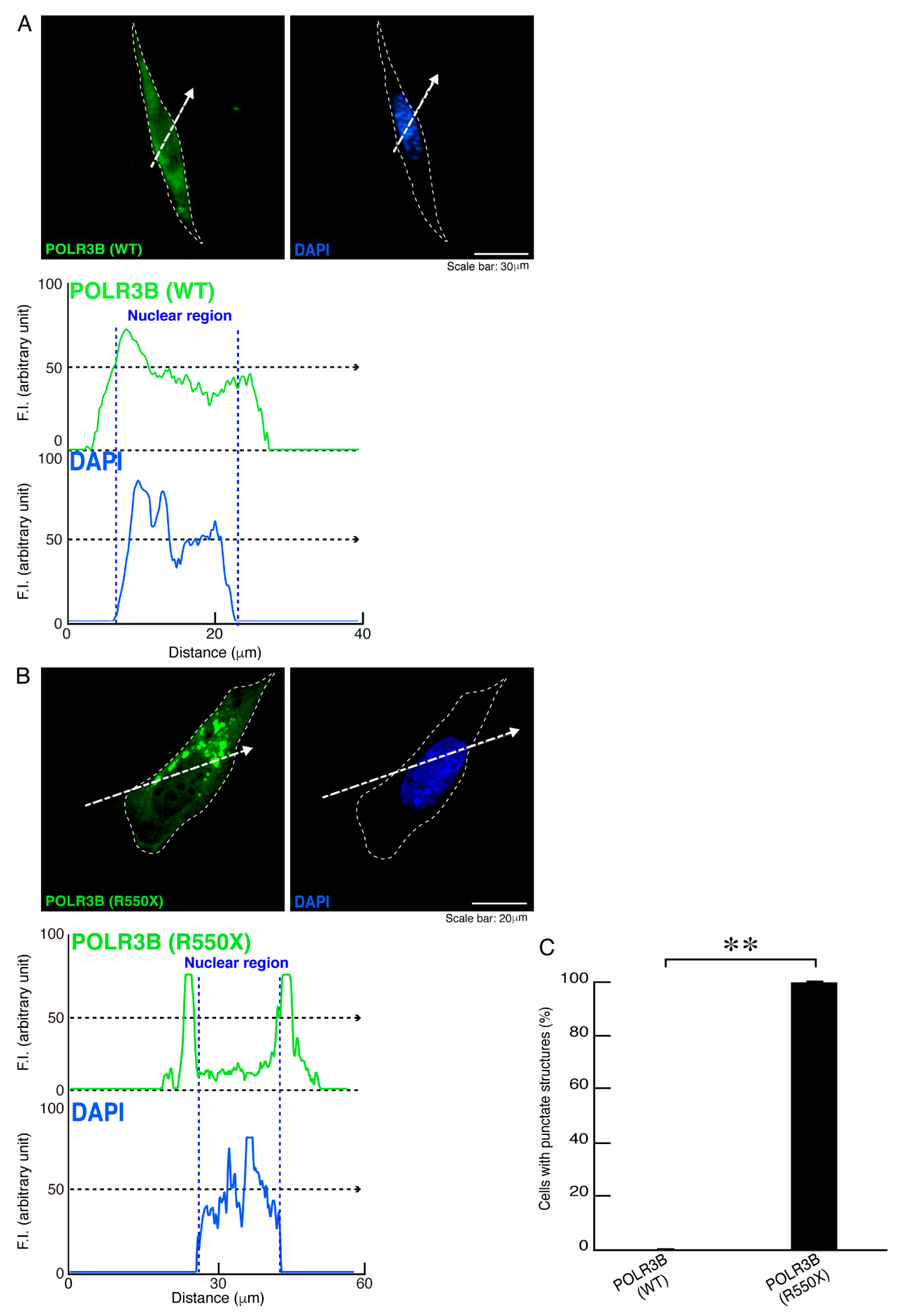
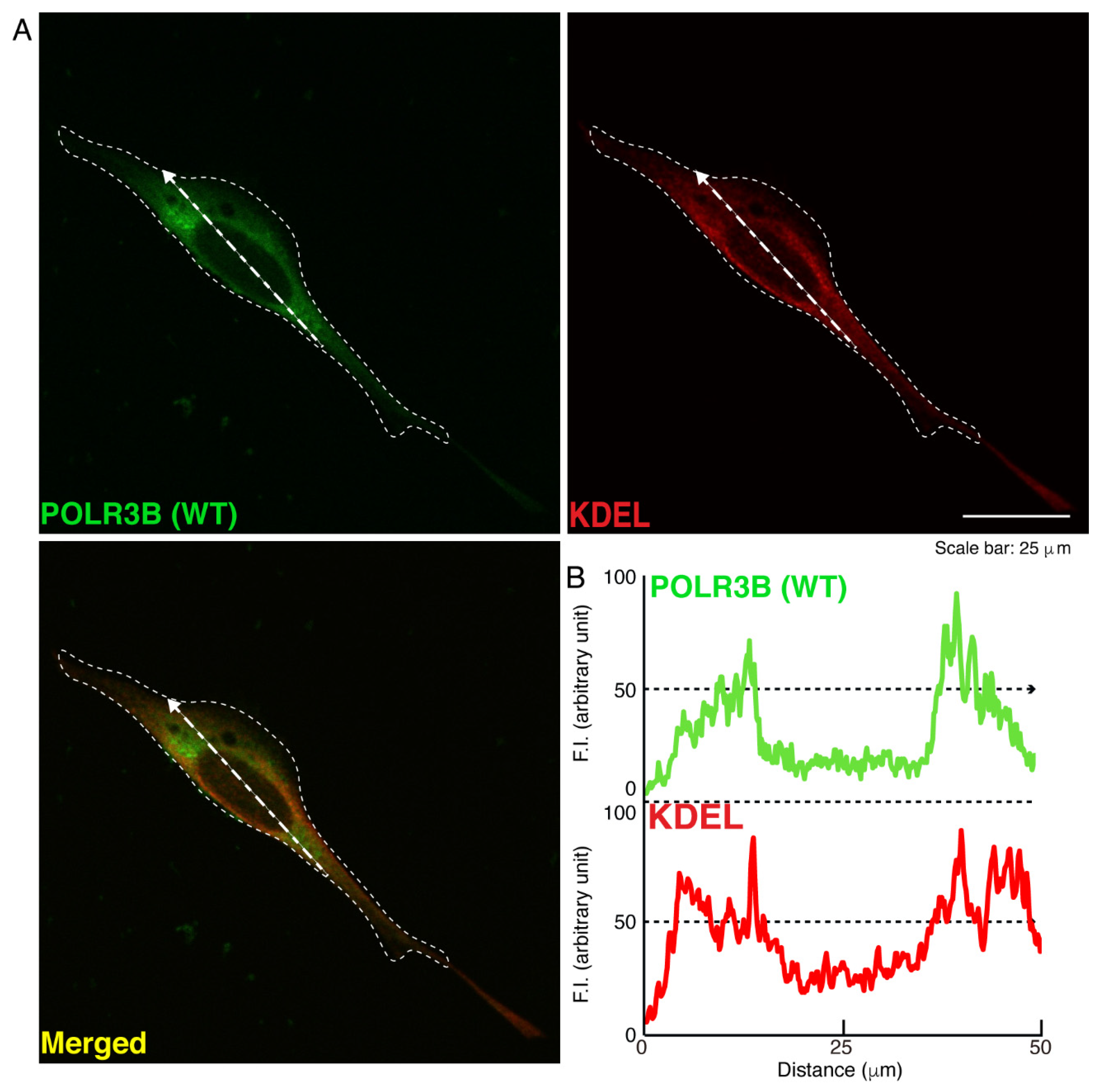
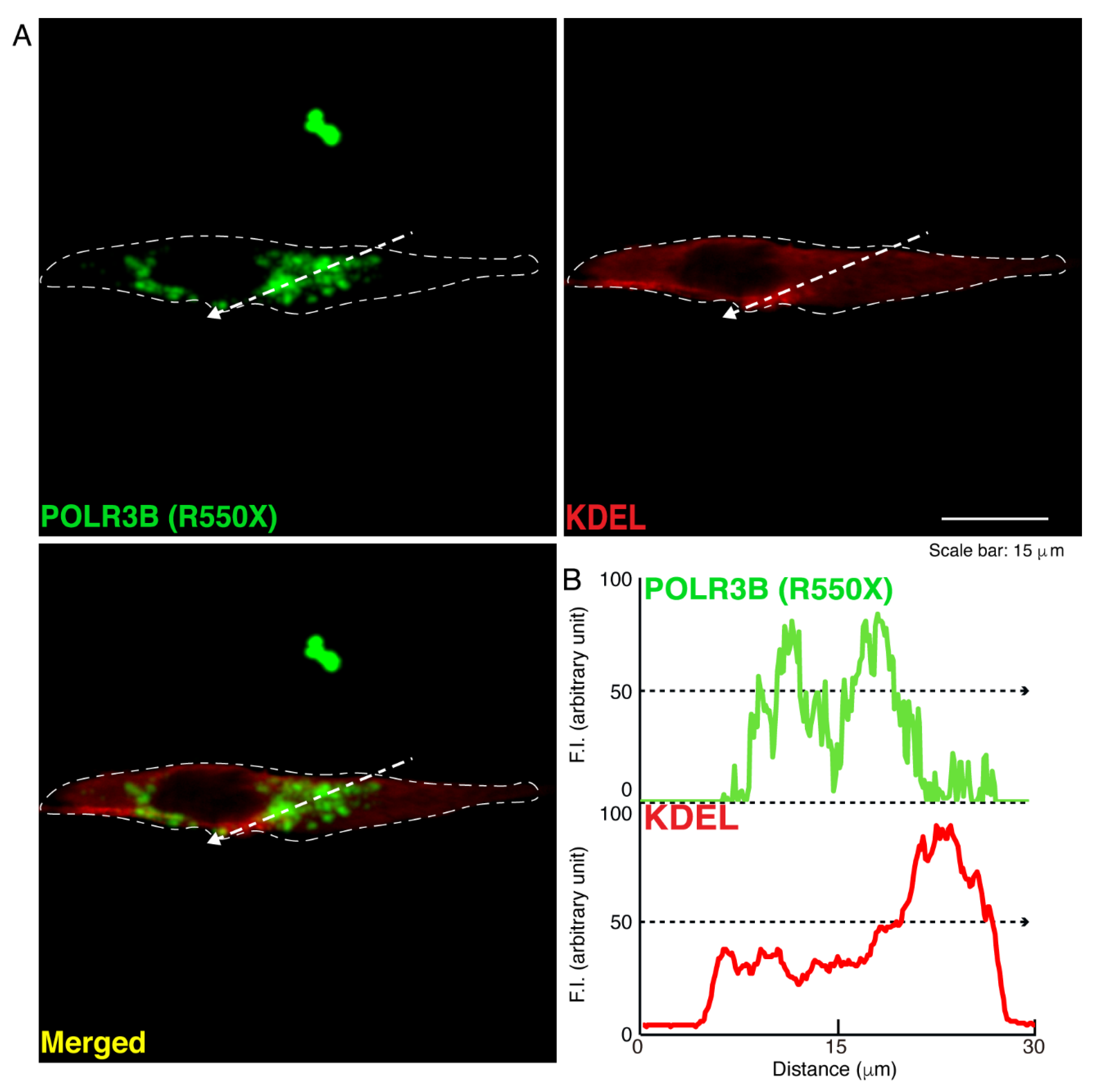
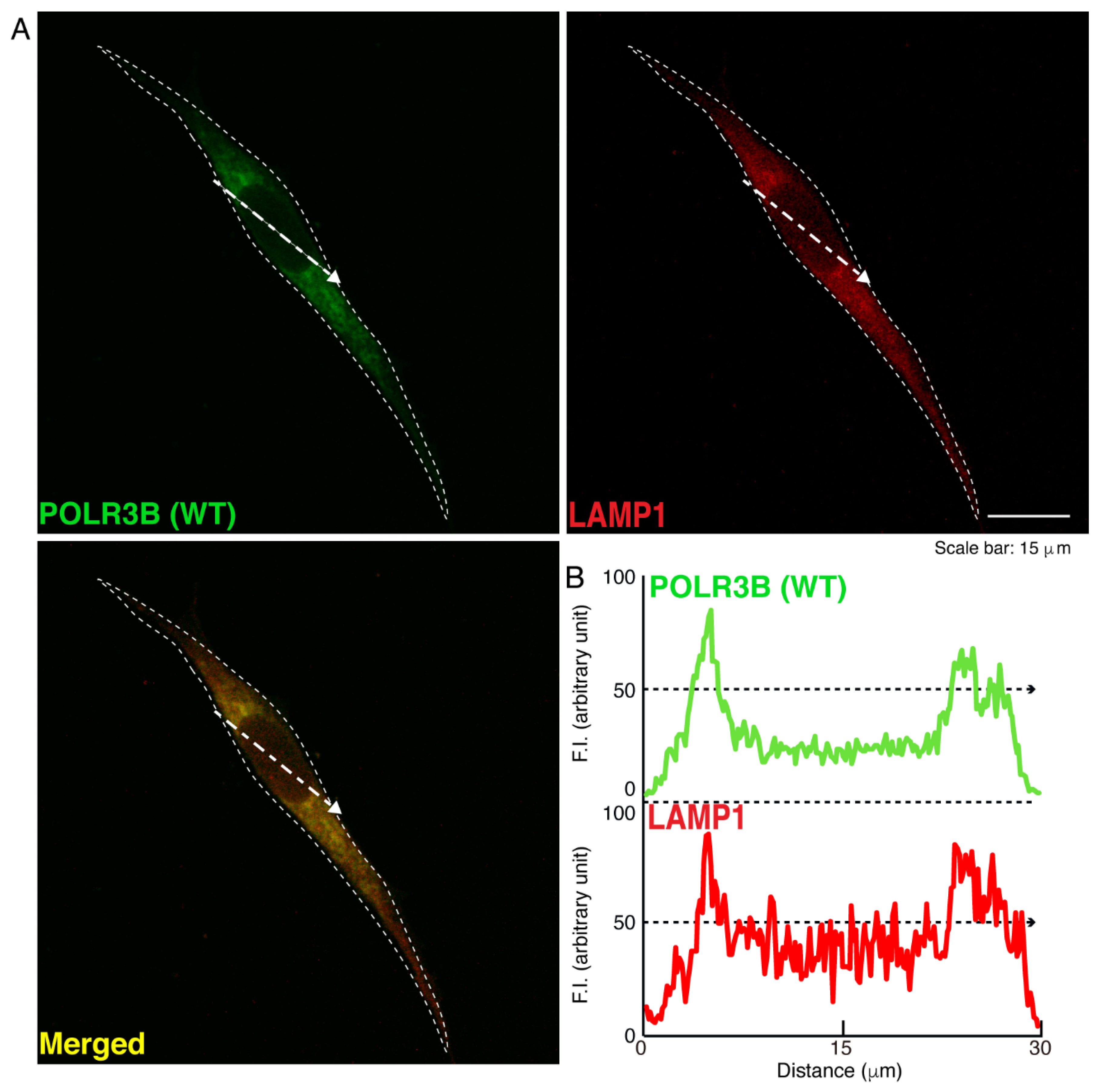
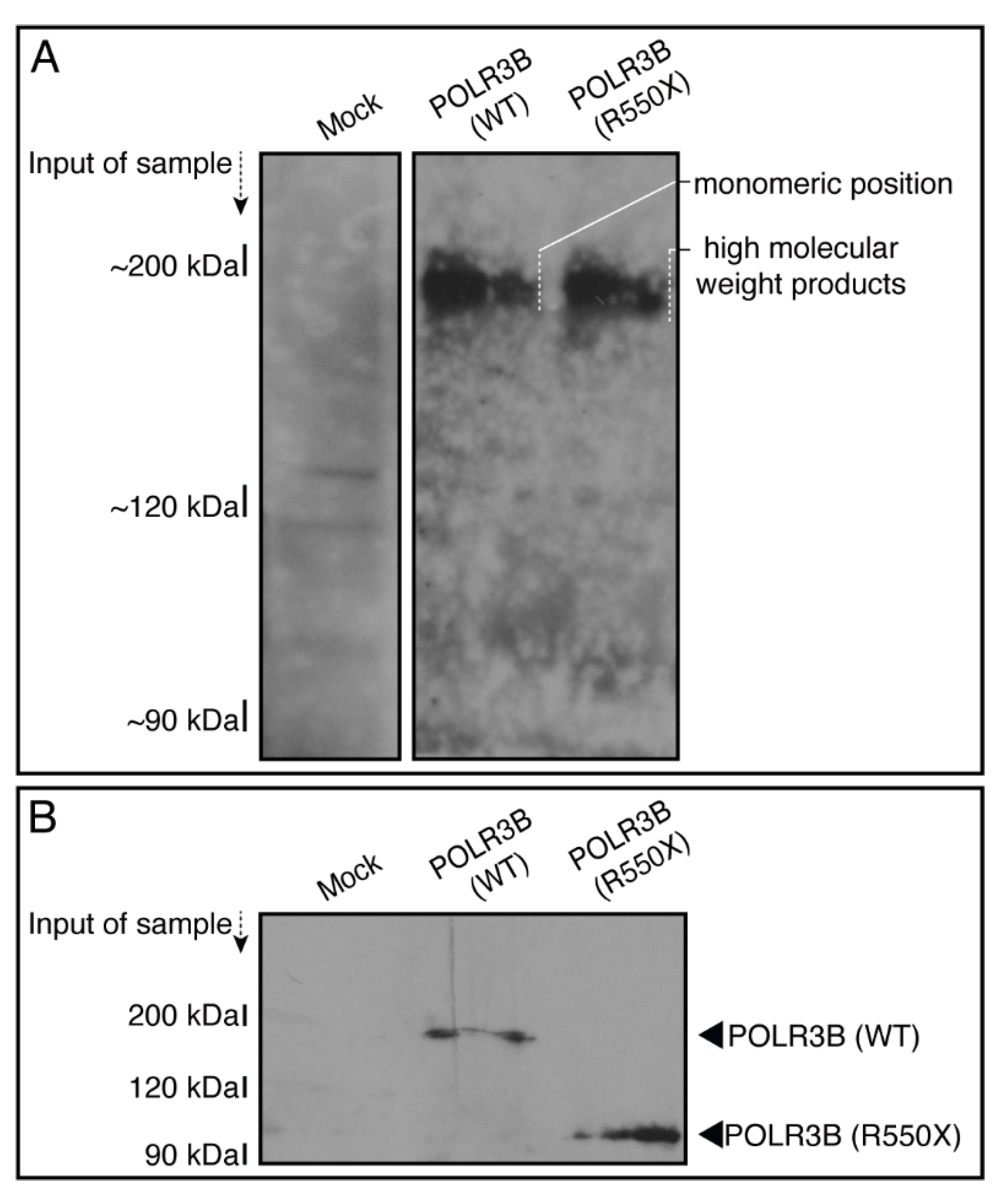
3.1. The R550X Mutant Proteins of POLR3B Are Aggregated in Lysosomes in FBD-102b Cells
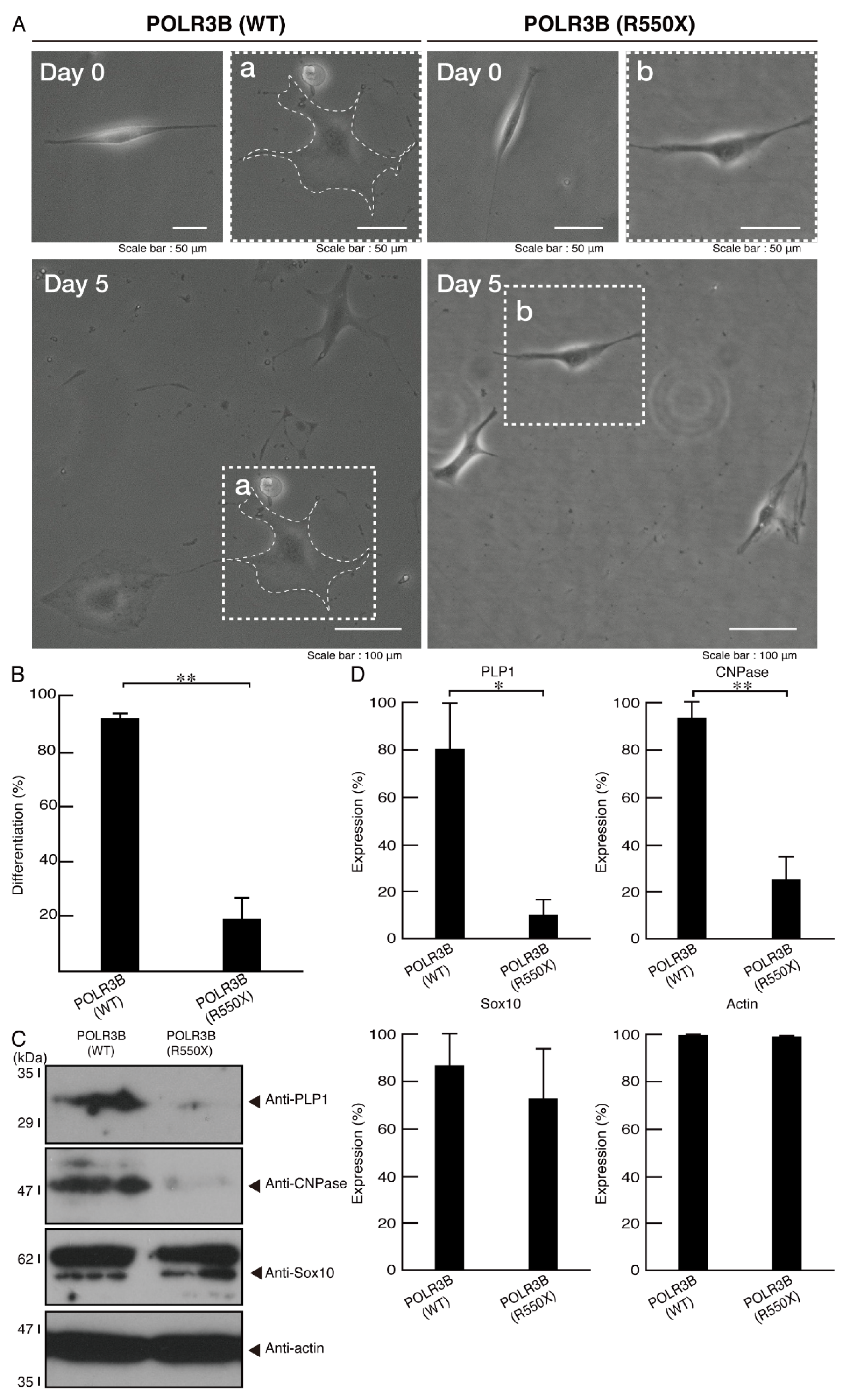
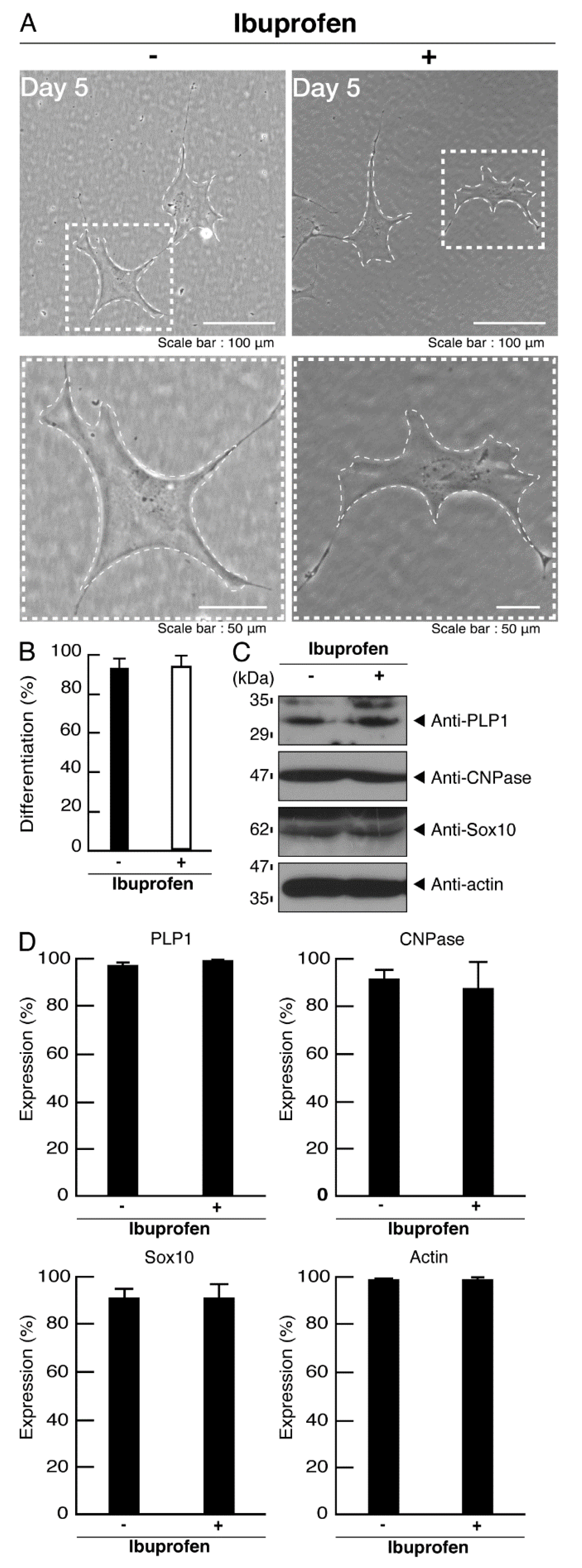
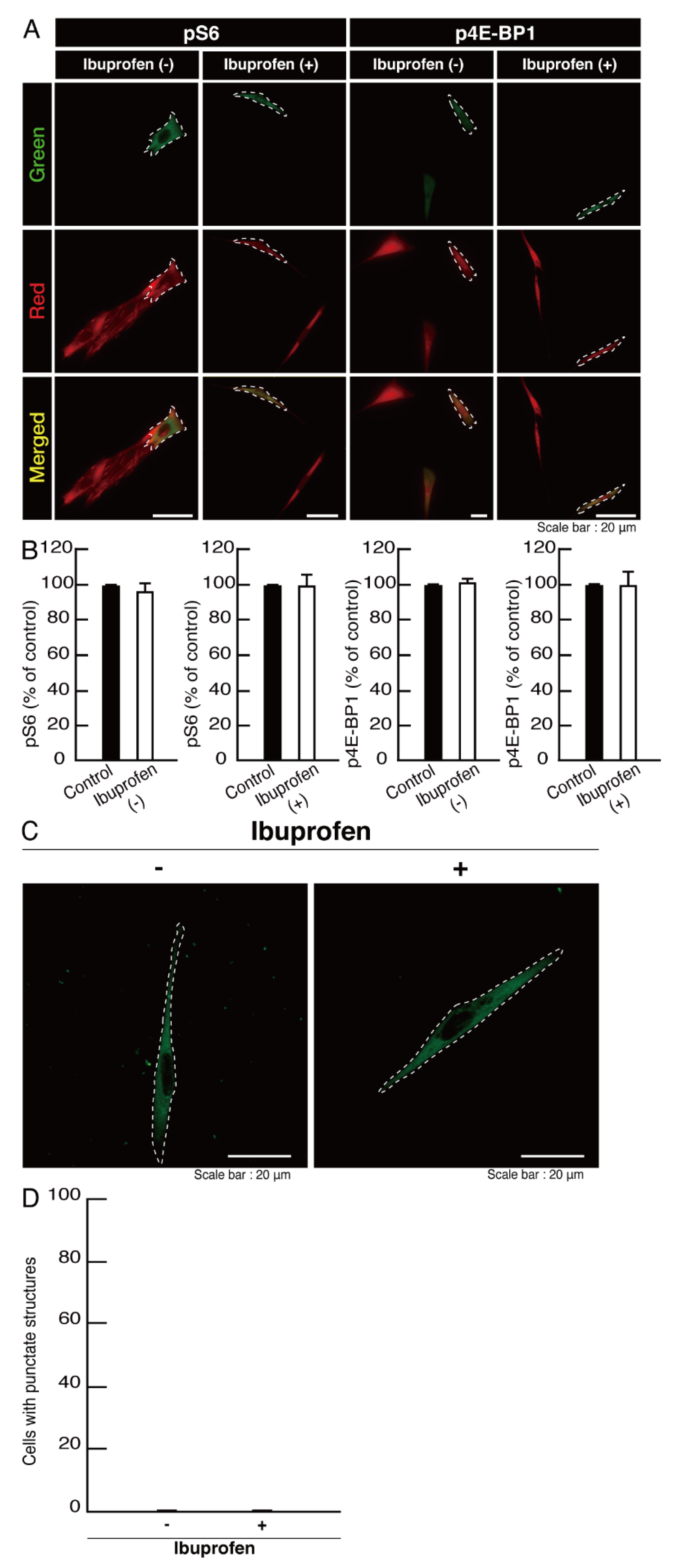
3.2. Cells Expressing the R550X Mutant Proteins of POLR3B Fail to Exhibit Differentiation Phenotypes
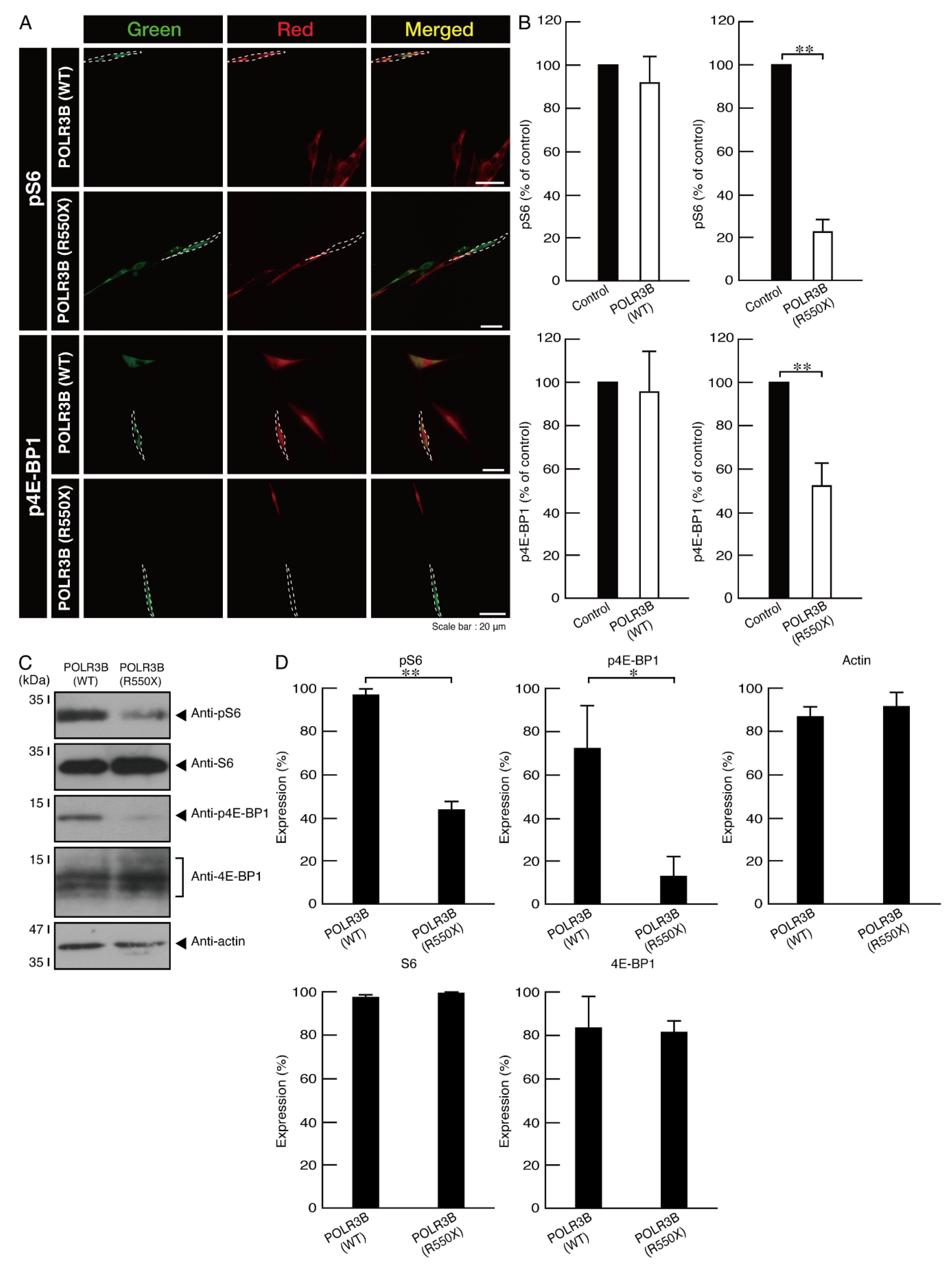
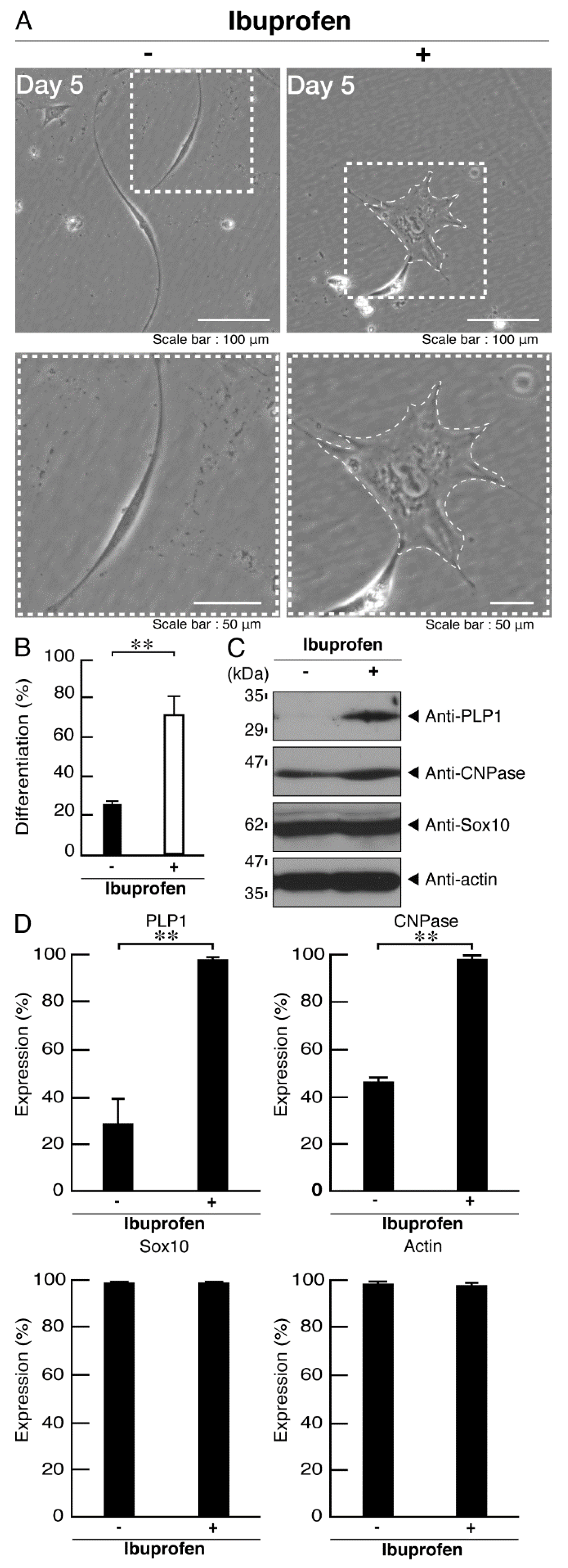
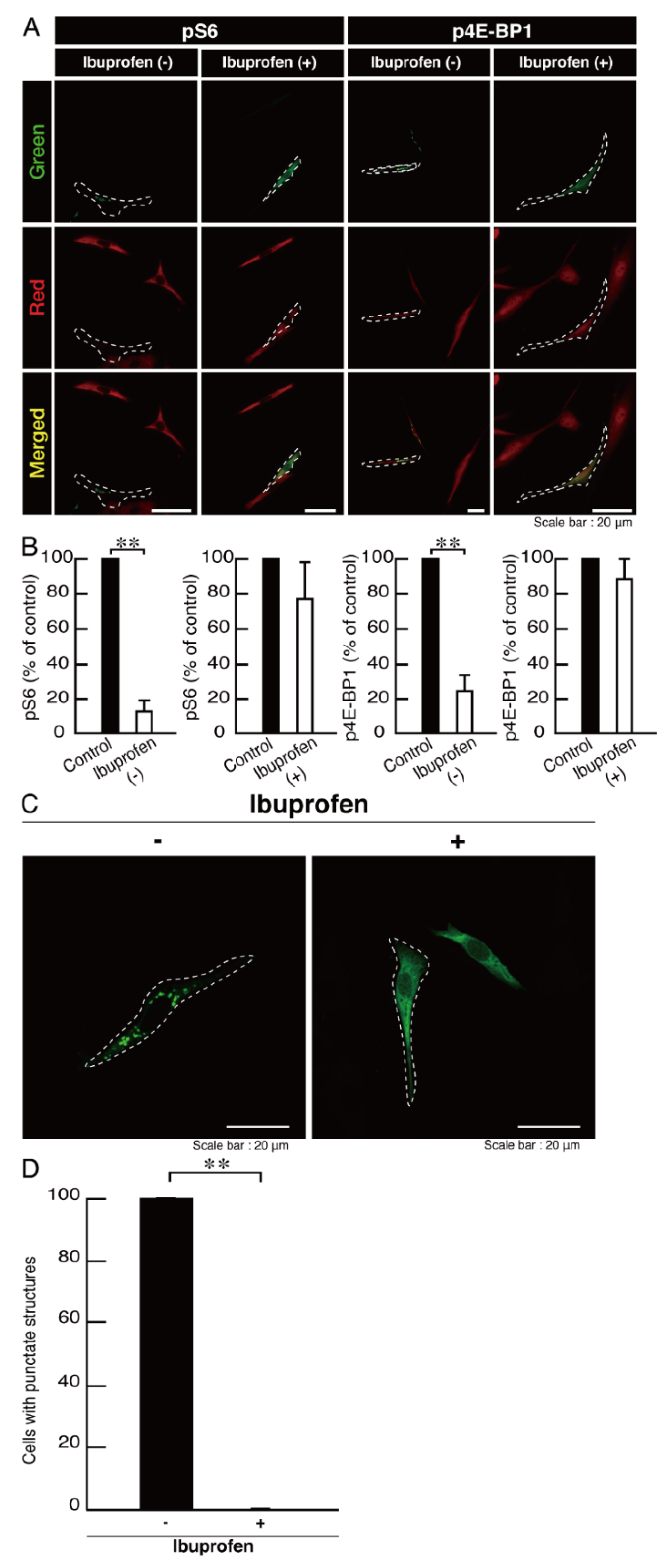
3.3. Ibuprofen Specifically Improves the Phenotypes of Cells Expressing the R550X Mutant Proteins of POLR3B
4. Discussion
5. Limitation of This Study and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernard, G.; Chouery, E.; Putorti, M.L.; Tétreault, M.; Takanohashi, A.; Carosso, G.; Clément, I.; Boespflug-Tanguy, O.; Rodriguez, D.; Delague, V.; et al. Mutations of POLR3A encoding a catalytic subunit of RNA polymerase Pol III cause a recessive hypomyelinating leukodystrophy. Am. J. Hum. Genet. 2011, 89, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Saitsu, H.; Osaka, H.; Sasaki, M.; Takanashi, J.; Hamada, K.; Yamashita, A.; Shibayama, H.; Shiina, M.; Kondo, Y.; Nishiyama, K.; et al. Mutations in POLR3A and POLR3B encoding RNA polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy. Am. J. Hum. Genet. 2011, 89, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tétreault, M.; Choquet, K.; Orcesi, S.; Tonduti, D.; Balottin, U.; Teichmann, M.; Fribourg, S.; Schiffmann, R.; Brais, B.; Vanderver, A.; et al. Recessive mutations in POLR3B, encoding the second largest subunit of Pol III, cause a rare hypomyelinating leukodystrophy. Am. J. Hum. Genet. 2011, 89, 652–655. [Google Scholar] [CrossRef] [Green Version]
- Daoud, H.; Tétreault, M.; Gibson, W.; Guerrero, K.; Cohen, A.; Gburek-Augustat, J.; Synofzik, M.; Brais, B.; Stevens, C.A.; Sanchez-Carpintero, R.; et al. Mutations in POLR3A and POLR3B are a major cause of hypomyelinating leukodystrophies with or without dental abnormalities and/or hypogonadotropic hypogonadism. J. Med. Genet. 2013, 50, 194–197. [Google Scholar] [CrossRef]
- Garbern, J.; Cambi, F.; Shy, M.; Kamholz, J. The molecular pathogenesis of Pelizaeus-Merzbacher disease. Arch. Neurol. 1999, 56, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Pelizaeus-Merzbacher disease: Molecular and cellular pathologies and associated phenotypes. Adv. Exp. Med. Biol. 2019, 1190, 201–216. [Google Scholar] [PubMed]
- Wolf, N.I.; Ffrench-Constant, C.; van der Knaap, M.S. Hypomyelinating leukodystrophies-unravelling myelin biology. Nat. Rev. Neurol. 2021, 17, 88–103. [Google Scholar] [CrossRef]
- Dhaunchak, A.S.; Colman, D.R.; Nave, K.A. Misalignment of PLP/DM20 transmembrane domains determines protein misfolding in Pelizaeus-Merzbacher disease. J. Neurosci. 2011, 31, 14961–14971. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.; Lyons, D.A. Axonal selection and myelin sheath generation in the central nervous system. Curr. Opin. Cell Biol. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Morton, P.D.; Ishibashi, N.; Jonas, R.A.; Gallo, V. Congenital cardiac anomalies and white matter injury. Trends Neurosci. 2015, 38, 353–563. [Google Scholar] [CrossRef] [Green Version]
- Saab, A.S.; Nave, K.A. Myelin dynamics: Protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 2017, 47, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rub, M.; Miller, R.H. Emerging cellular and molecular strategies for enhancing central nervous system (CNS) remyelination. Brain Sci. 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, N.; Watanabe, N.; Iibe, N.; Tatsumi, Y.; Hattori, K.; Takeuchi, Y.; Oizumi, H.; Ohbuchi, K.; Torii, T.; Miyamoto, Y.; et al. Hypomyelinating leukodystrophy-associated mutation of RARS leads it to the lysosome, inhibiting oligodendroglial morphological differentiation. Biochem. Biophys. Rep. 2019, 20, 100705. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Hattori, K.; Takeuchi, Y.; Yamawaki, M.; Watanabe, N.; Matsumoto, N.; Homma, K.; Miyamoto, Y.; Yamauchi, J. Effects of HLD-associated POLR1C mutant proteins on cellular localization and differentiation. Mol. Genet. Metab. Rep. 2018, 17, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Miyamoto, Y.; Hattori, K.; Ito, A.; Harada, H.; Oizumi, H.; Ohbuchi, K.; Mizoguchi, K.; Yamauchi, J. PP1C and PP2A are p70S6K phosphatases whose inhibition ameliorates HLD12-associated inhibition of oligodendroglial cell morphological differentiation. Biomedicines 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Tago, K.; Memezawa, S.; Ochiai, A.; Sawaguchi, S.; Kato, Y.; Sato, T.; Tomizuka, K.; Ooizumi, H.; Ohbuchi, K.; et al. The infantile leukoencephalopathy-associated mutation of C11ORF73/HIKESHI proteins generates de novo interactive activity with Filamin A, inhibiting oligodendroglial cell morphological differentiation. Medicines 2021, 8, 9. [Google Scholar] [CrossRef]
- Sawaguchi, S.; Goto, M.; Kato, Y.; Tanaka, M.; Tago, K.; Oizumi, H.; Ohbuchi, K.; Mizoguchi, K.; Miyamoto, Y.; Yamauchi, J. Hypomyelinating leukodystrophy 15 (HLD15)-associated mutation of EPRS1 leads to its polymeric aggregation in Rab7-positive vesicle structures, inhibiting oligodendroglial cell morphological differentiation. Polymers 2021, 13, 1074. [Google Scholar] [CrossRef]
- Ochiai, A.; Sawaguchi, S.; Memezawa, S.; Seki, Y.; Morimoto, T.; Oizumi, H.; Ohbuchi, K.; Yamamoto, M.; Mizoguchi, K.; Miyamoto, Y.; et al. Knockdown of Golgi stress-responsive caspase-2 ameliorates HLD17-associated AIMP2 mutant-mediated inhibition of oligodendroglial cell morphological differentiation. Neurochem. Res. 2021, in press. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017, 171, 642–654. [Google Scholar] [CrossRef]
- Condon, K.J.; Sabatini, D.M. Nutrient regulation of mTORC1 at a glance. J. Cell Sci. 2019, 132, jcs222. [Google Scholar] [CrossRef]
- Tyler, W.A.; Gangoli, N.; Gokina, P.; Kim, H.A.; Covey, M.; Levison, S.W.; Wood, T.L. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J. Neurosci. 2009, 29, 6367–6678. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.P.; Flores, A.I.; Wang, F.; Macklin, W.B. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. 2009, 29, 6860–6870. [Google Scholar] [CrossRef] [PubMed]
- Bibollet-Bahena, O.; Almazan, G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J. Neurochem. 2009, 109, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.; Parker, W.E.; Orlova, K.A.; Baybis, M.; Chi, A.W.; Berg, B.D.; Birnbaum, J.F.; Estevez, J.; Okochi, K.; Sarnat, H.B.; et al. Fetal brain mTOR signaling activation in tuberous sclerosis complex. Cereb. Cortex 2014, 24, 315–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, G.G.; Scott, K.F. Limitations of drug concentrations used in cell culture studies for understanding clinical responses of NSAIDs. Inflammopharmacology 2021, 29, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Tanaka, M.; Okura, N.; Fukui, Y.; Noguchi, K.; Hayashi, Y.; Torii, T.; Ooizumi, H.; Ohbuchi, K.; Mizoguchi, K.; et al. Rare neurologic disease-associated mutations of AIMP1 are related with inhibitory neuronal differentiation which is reversed by ibuprofen. Medicines 2020, 7, 25. [Google Scholar] [CrossRef]
- Kato, Y.; Ochiai, A.; Seki, Y.; Morimoto, T.; Oizumi, H.; Ohbuchi, K.; Mizoguchi, K.; Yamamoto, M.; Sakagami, H.; Miyamoto, Y.; et al. Phospholipase D and phosphatidylinositol-4-phosphate 5-kinase 1 are involved in the regulation of oligodendrocyte morphological differentiation. Exp. Cell Res. 2021, 405, 112654. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Torii, T.; Tanoue, A.; Yamauchi, J. VCAM1 acts in parallel with CD69 and is required for the initiation of oligodendrocyte myelination. Nat. Commun. 2016, 7, 13478. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Torii, T.; Tago, K.; Tanoue, A.; Takashima, S.; Yamauchi, J. BIG1/Arfgef1 and Arf1 regulate the initiation of myelination by Schwann cells in mice. Sci. Adv. 2018, 4, eaar4471. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Torii, T.; Terao, M.; Takada, S.; Tanoue, A.; Katoh, H.; Yamauchi, J. Rnd2 differentially regulates oligodendrocyte myelination at different developmental periods. Mol. Biol. Cell 2021, 32, 769–787. [Google Scholar] [CrossRef]
- Zara, S.; De Colli, M.; Rapino, M.; Pacella, S.; Nasuti, C.; Sozio, P.; Di Stefano, A.; Cataldi, A. Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer’s disease rat model. Gerontology 2013, 59, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Vella, L.D.; Figueiredo, V.C.; Cameron-Smith, D. Ibuprofen treatment blunts early translational signaling responses in human skeletal muscle following resistance exercise. J. Appl. Physiol. 2014, 117, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.; Ma, Q.; Ding, X.-Q.; Qin, X.; Wang, C.; Zhang, J. Research on protective mechanism of ibuprofen in myocardial ischemia-reperfusion injury in rats through the PI3K/Akt/mTOR signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4465–4473. [Google Scholar] [PubMed]
- Singh, A.; Tripathi, P.; Singh, S. Neuroinflammatory responses in Parkinson’s disease: Relevance of ibuprofen in therapeutics. Inflammopharmacology 2021, 29, 5–14. [Google Scholar] [CrossRef]
- Zurita, M.P.; Muñoz, G.; Sepúlveda, F.J.; Gómez, P.; Castillo, C.; Burgos, C.F.; Fuentealba, J.; Opazo, C.; Aguayo, L.G. Ibuprofen inhibits the synaptic failure induced by the amyloid-beta peptide in hippocampal neurons. J. Alzheimer’s Dis. 2013, 35, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.K.; Alam, P.; Malik, S.; Majid, N.; Chaturvedi, S.K.; Rajan, S.; Ajmal, M.R.; Khan, M.V.; Uversky, V.N.; Khan, R.H. Stabilizing proteins to prevent conformational changes required for amyloid fibril formation. J. Cell. Biochem. 2019, 12, 2642–2656. [Google Scholar] [CrossRef]
- Hochstrasser, T.; Hohsfield, L.A.; Sperner-Unterweger, B.; Humpel, B. Beta-amyloid-induced effects on cholinergic, serotonergic, and dopaminergic neurons are differentially counteracted by anti-inflammatory drugs. J. Neurosci. Res. 2013, 91, 83–94. [Google Scholar]
- Vitola, P.L.; Balducci, C.; Cerovic, M.; Santamaria, G.; Brandi, E.; Grandi, F.; Caldinelli, L.; Colombo, L.; Morgese, M.G.; Trabace, L.; et al. Alpha-synuclein oligomers impair memory through glial cell activation and via Toll-like receptor 2. Brain Behav. Immun. 2018, 69, 591–602. [Google Scholar] [CrossRef]
- Wang, X.; Budel, S.; Baughman, K.; Gould, G.; Song, K.-H.; Strittmatter, S.M. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J. Neurotrauma 2009, 26, 81–95. [Google Scholar] [CrossRef]
- Xing, B.; Li, H.; Wang, H.; Mukhopadhyay, D.; Fisher, D.; Gilpin, C.J.; Li, S. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp. Neurol. 2011, 231, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Roloff, F.; Scheiblich, H.; Dewitz, C.; Dempewolf, S.; Stern, M.; Bicker, G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS ONE 2015, 10, e0118536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiblich, H.; Bicker, G. Regulation of microglial phagocytosis by RhoA/ROCK-inhibiting drugs. Cell. Mol. Neurobiol. 2017, 37, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, P.; Zepeda, A.; Arias, C. Nonsteroidal anti-inflammatory drugs attenuate amyloid-beta protein-induced actin cytoskeletal reorganization through Rho signaling modulation. Cell. Mol. Neurobiol. 2017, 37, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Kawase-Koga, Y.; Otaegi, G.; Sun, T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev. Dyn. 2009, 238, 2800–2812. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Shin, J.Y.; McManus, M.T.; Ptácek, L.J.; Fu, Y.H. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann. Neurol. 2009, 66, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Dugas, J.C.; Cuellar, T.L.; Scholze, A.; Ason, B.; Ibrahim, A.; Emery, B.; Zamanian, J.L.; Foo, L.C.; McManus, M.T.; Barres, B.A. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 2010, 65, 597–611. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; He, X.; Han, X.; Yu, Y.; Ye, F.; Chen, Y.; Hoang, T.; Xu, X.; Mi, Q.S.; Xin, M.; et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 2010, 65, 612–626. [Google Scholar] [CrossRef] [Green Version]
- Shalak, V.; Kaminska, M.; Mitnacht-Kraus, R.; Vandenabeele, P.; Clauss, M.; Mirande, M. The EMAPII cytokine is released from the mammalian multisynthetase complex after cleavage of its p43/proEMAPII component. J. Biol. Chem. 2001, 276, 23769–23776. [Google Scholar] [CrossRef] [Green Version]
- Sawaguchi, S.; Tago, K.; Oizumi, H.; Ohbuchi, K.; Yamamoto, M.; Mizoguchi, K.; Miyamoto, Y.; Yamauchi, J. Hypomyelinating leukodystrophy 7 (HLD7)-associated mutation of POLR3A Is related to defective oligodendroglial cell differentiation, which Is ameliorated by ibuprofen. Neurol. Int. 2021, 22, 11–33. [Google Scholar] [CrossRef]



| Product Name | Target or Description | Company | Cat. No. | Lot. No. | Final Conc. |
|---|---|---|---|---|---|
| Anti-eIF4EBP1 (phospho T37) antibody | A phospho-peptide corresponding to residues surrounding threonine 37 of eIF4EBP1 | abcam | ab75767 | GR88680-14 | IF, 1/100; IB, 1/2500 |
| Anti-F-actin antibody | Filamentous actin (F-actin) | abcam | ab205 | GR319251-7 | IB, 1/80,000 |
| Anti-GFP mAb | Green fluorescent protein (GFP) | MBL | 598 | “078” | IB, 1/80,000 |
| Anti-KDEL mAb | KDEL-containing peptide of the endoplasmic reticulum (ER)-resident glucose-regulated protein (GRP78) | MBL | M181-3 | “004” | IF, 1/500 |
| Anti-PLP1 antibody | Myelin proteolipid protein (PLP) 1 | Atlas Antibodies | HPA004128 | B115828 | IB, 1/500 |
| Anti-RPS6 (phospho S240 + S244) antibody | Synthetic peptide within human S6 protein to the C-terminus (phospho S240 + S244) | abcam | ab215214 | GR3205097-3 | IF, 1/101; IB, 1/10,000 |
| CNPase (D83E10) XP Rabbit mAb | 2′, 3′-cyclic nucleotide 3′-phospho-diesterase (CNPase) | Cell Signaling TECHNOLOGY | #5664 | “001” | IB, 1/500 |
| Anti-LAMP-1 antibody (H4A3) | Lysosomal-associated membrane protein1 (LAMP1) | Santa Cruz Biotechnology | sc-20011 | J0919 | IF, 1/200 |
| Purified Mouse Anti-GM130 antibody | Golgi matrix protein of 130 kDa (GM130) | BD Biosciences | 610823 | 8352796 | IF, 1/501 |
| Anti-Sox-10 antibody (A-2) | SRY-related HMG-box 10 (Sox-10) | Santa Cruz Biotechnology | sc-365692 | J0720 | IB, 1/500 |
| Anti-elF4EBP1 (Y329) | elF4EBP1 | abcam | ab32024 | GR239794-12 | IB, 1/5000 |
| Ribosomal Protein S6 (C-8) | Ribosomal Protein S6 | Santa Cruz Biotechnology | sc-74459 | D2921 | IB, 1/500 |
| Alexa Fluor 488 goat anti-mouse IgG (H + L) | Mouse IgG (H + L) conjugated with Alexa Fluor 488 | Thermo Fisher Scientific | A-11001 | 774904 | IF, 1/500 |
| Alexa Fluor 488 goat anti-rabbit IgG (H + L) | Rabbit IgG (H + L) conjugated with Alexa Fluor 488 | Thermo Fisher Scientific | A-11008 | 751094 | IF, 1/500 |
| Alexa Fluor 594 goat anti-mouse IgG (H + L) | Mouse IgG (H + L) conjugated with Alexa Fluor 594 | Thermo Fisher Scientific | A-11005 | 2043369 | IF, 1/500 |
| Alexa Fluor 594 goat anti-rabbit IgG (H + L) | Rabbit IgG (H + L) conjugated with Alexa Fluor 594 | Thermo Fisher Scientific | A-11012 | 2018240 | IF, 1/500 |
| Anti-IgG (H + L chain) (Mouse) pAb-HRP | Mouse IgG F(ab’) conjugated with horseradish peroxidase | MBL | 330 | 366 | IB, 1/5000 |
| Anti-IgG (H + L chain) (Rabbit) pAb-HRP | Rabbit IgG F(ab’) conjugated with horseradish peroxidase | MBL | 458 | 352 | IB, 1/5000 |
| Molecular Target and/or Mechanism | Animal or Human Model | Disease | Effect | Reference |
|---|---|---|---|---|
| Alpha-synuclein oligomers (alpha-synOs) | Mouse model (in vivo) | Synucleinopathies | memory impairment induced by alpha-synOs (↓) | Pietro La Vitola, Claudia Balducci, Milica Cerovic, Giulia Santamaria, Edoardo Brandi, Federica Grandi, Laura Caldinelli, Laura Colombo, Maria Grazia Morgese, Luigia Trabace, Loredano Pollegioni, Diego Albani, Gianluigi Forloni: Alpha-synuclein oligomers impair memory through glial cell activation and via Toll-like receptor 2. Brain, Behavior, and Immunity 2018 69:591–602 |
| Amyloid-beta peptide (Abeta) | Rat model (in vitro) | Alzheimer’s disease (AD) | alter the ultrastructure of Abeta, its association to neuronal membranes and its synaptotoxic effect (↓) | Maria Paz Zurita, Gonzalo Muñoz, Fernando J Sepúlveda, Paulina Gómez, Carolina Castillo, Carlos F Burgos, Jorge Fuentealba, Carlos Opazo, Luis G Aguayo: Ibuprofen inhibits the synaptic failure induced by the amyloid-β peptide in hippocampal neurons. Journal of Alzheimer’s Disease 2013 35:463–473 |
| Abeta | Human model (in vitro) | may alter the ultrastructure of Abeta | Mohammad Khursheed Siddiqi, Parvez Alam, Sadia Malik, Nabeela Majid, Sumit Kumar Chaturvedi, Sudeepa Rajan, Mohd Rehan Ajmal, Mohsin Vahid Khan, Vladimir N Uversky, Rizwan Hasan Khan: Stabilizing proteins to prevent conformational changes required for amyloid fibril formation. Journal of Cellular Biochemistry 2019 12:2642–2656 | |
| Abeta | Rat model (in vitro) | may alter the ultrastructure of Abeta | Tanja Hochstrasser, Lindsay A Hohsfield, Barbara Sperner-Unterweger, Christian Humpel: Beta-Amyloid-induced effects on cholinergic, serotonergic, and dopaminergic neurons are differentially counteracted by anti-inflammatory drugs. Journal of Neuroscience Research 2013 91:83–94 | |
| Apolipoprotein (APO) E4 | Mouse model (in vivo) | AD | alter the expression pattern of APOE in APOE4 mice to that of APOE3 mice, dendritic spine density (↑) | Amanda M DiBattista, Sonya B Dumanis, Joshua Newman, G William Rebeck: Identification and modification of amyloid-independent phenotypes of APOE4 mice. Experimental Neurology 2016 280:97–105 |
| beta amyloid precursor protein (betaAPP) | Human model (in vitro) | AD | the secretion of total Abeta (↓), the accumulation of Abeta-42 and Abeta-40 (↓) | I Blasko, A Apochal, G Boeck, T Hartmann, B Grubeck-Loebenstein, G Ransmayr: Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiology of Disease 2001 8:1094–1101 |
| Beta-site amyloid precursor protein cleaving enzyme1 (BACE1), advanced glycation end product (AGE) and receptor for AGE (RAGE) | Rat model (in vivo) | Diabetic encephalopathy | the decline in learning and memory ability and loss of neurons in the CA1 and CA3 areas of the hippocampus (↓) | Yao-Wu Liu, Xia Zhu, Liang Zhang, Qian Lu, Fan Zhang, Hao Guo, Xiao-Xing Yin: Cerebroprotective effects of ibuprofen on diabetic encephalopathy in rats. Pharmacology Biochemistry and Behavior 2014 117:128–136 |
| Cyclooxygenase (Cox) family | Rat model (in vivo) | Forebrain-ischemia | post-ischemic thromboxane B2 and prostaglandin F2alpha in both caudate nucleus and dorsal hippocampus and neuronal injury (↓) | P M Patel, J C Drummond, T Sano, D J Cole, C J Kalkman, T L Yaksh: Effect of ibuprofen on regional eicosanoid production and neuronal injury after forebrain ischemia in rats. Brain Research 1993 614:315–324 |
| Cox1/2 | Rat model (in vitro) | Parkinson’s disease (PD) | 1-methyl-4-phenylpyridinium (MPP(+))-induced VM cell toxicity and VM dopaminergic cell apoptosis (↓) | Ya-Ching Hsieh, Ross B Mounsey, Peter Teismann: MPP(+)-induced toxicity in the presence of dopamine is mediated by COX-2 through oxidative stress. Naunyn-Schmiedeberg’s Archives of Pharmacolog 2011 384:157–167 |
| Cox1/2 | Mouse model (in vivo) | PD | protect neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced injury in the striatum | Maciej Swiątkiewicz, Małgorzata Zaremba, Ilona Joniec, Andrzej Członkowski, Iwona Kurkowska-Jastrzębska: Potential neuroprotective effect of ibuprofen, insights from the mice model of Parkinson’s disease. Pharmacological Reports 2013 65:1227–1236 |
| Cox1/2 | Mouse model (in vivo) | AD | modulate hippocampal gene expression in pathways involved in neuronal plasticity and norepinephrine and dopamine (↑) | Nathaniel S Woodling, Damien Colas, Qian Wang, Paras Minhas, Maharshi Panchal, Xibin Liang, Siddhita D Mhatre, Holden Brown, Novie Ko, Irene Zagol-Ikapitte, Marieke van der Hart, Taline V Khroyan, Bayarsaikhan Chuluun, Prachi G Priyam, Ginger L Milne, Arash Rassoulpour, Olivier Boutaud, Amy B Manning-Boğ, H Craig Heller, Katrin I Andreasson: Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer’s disease model mice. Brain 2016 139:2063–2081 |
| Cox2 | Rat model (in vivo) | Stroke | impairment of experience-dependent plasticity (↓), the Cox2 after stroke (↓) | Jan A Jablonka, Malgorzata Kossut, Otto W Witte, Monika Liguz-Lecznar: Experience-dependent brain plasticity after stroke: effect of ibuprofen and poststroke delay. European Journal of Neuroscience 2012 36:2632–2639 |
| Cox2 | Mouse model (in vivo) | Psychiatric disorders and neurodegenerative diseases | anti-inflammatory markers (↑), tryptophan 2,3-dioxygenase (TDO2) and PGE2 (↓) | Maria Teresa Golia, Silvia Poggini, Silvia Alboni, Stefano Garofalo, Naomi Ciano Albanese, Aurelia Viglione, Maria Antonietta Ajmone-Cat, Abygaël St-Pierre, Nicoletta Brunello, Cristina Limatola, Igor Branchi, Laura Maggi: Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain, Behavior, and Immunity 2019 81:484–494 |
| Cox2 and nucleotide-binding oligomerization domain (NOD) -like receptor 3 inflammasome | Rat model (in vivo) | Pentylenetetrazol-induced chronic epilepsy | seizure scores and the secretion of the inflammatory cytokine interleukin (IL)-18 (↓), loss of hippocampal neurons (↓) | Rui Liu, Shuhua Wu, Chong Guo, Zhongbo Hu, Jiangtao Peng, Ke Guo, Xinfan Zhang, Jianmin Li: Ibuprofen Exerts Antiepileptic and Neuroprotective Effects in the Rat Model of Pentylenetetrazol-Induced Epilepsy via the COX-2/NLRP3/IL-18 Pathway. Neurochemical Research 2020 45:2516–2526 |
| Cox2 and PGJ2 | Rat model (in vivo) | PD | dopaminergic neuronal loss (↓)and amoeboid microglia in the substantia nigra (↓), and the bias toward use of the ipsilateral over the contralateral forelimb (↓) | Chuhyon Corwin, Anastasia Nikolopoulou, Allen L Pan, Mariela Nunez-Santos, Shankar Vallabhajosula, Peter Serrano, John Babich, Maria E Figueiredo-Pereira: Prostaglandin D2/J2 signaling pathway in a rat model of neuroinflammation displaying progressive parkinsonian-like pathology: potential novel therapeutic targets. Journal of Neuroinflammation 2018 15:272 |
| Cox1/2 and arachidonic acid cascade | Mouse and rat models (in vitro, in vivo) | PD | the neuroinflammatory response (↓) | Ashish Singh, Pratibha Tripathi, Sarika Singh: Neuroinflammatory responses in Parkinson’s disease: relevance of Ibuprofen in therapeutics. Inflammopharmacology 2021 29:5–14 |
| Inducible nitric oxide synthase (iNOS) | Rat model (in vitro) | AD | iNOS mRNA and protein (↓) | N C Stratman, D B Carter, V H Sethy: Ibuprofen: effect on inducible nitric oxide synthase. Molecular Brain Research 1997 50:107–112 |
| IL-1 receptor antagonist proteins | Rat model (in vitro, in vivo) | Cerebral ischemia | protect CA1 hippocampal neurons for a long time, neurons (↑) | E-M Park, B-P Cho, B T Volpe, M O Cruz, T H Joh, S Cho: Ibuprofen protects ischemia-induced neuronal injury via upregulating interleukin-1 receptor antagonist expression. Neuroscience 2005 132:625–631 |
| Microglial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX2) | Human model (in vitro), mouse model (in vivo) | AD | oxidative damage (↓) and plaque clearance in the brain (↑) | Brandy L Wilkinson, Paige E Cramer, Nicholas H Varvel, Erin Reed-Geaghan, Qingguang Jiang, Alison Szabo, Karl Herrup, Bruce T Lamb, Gary E Landreth: Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s disease. Neurobiology of Aging 2012 33:197.e21–e32 |
| Mitogen-activated protein kinases (MAPKs) etc. | Rat model (in vivo) | PD and cypermethrin-induced Parkinsonism | cypermethrin-induced pathophysiological effects along with expression of pro-inflammatory and/or apoptosis-related proteins in the nigrostriatal tissue (↓) | Ashish Singh, Pratibha Tripathi, Om Prakash, Mahendra Pratap Singh: Ibuprofen abates cypermethrin-induced expression of pro-inflammatory mediators and mitogen-activated protein kinases and averts the nigrostriatal dopaminergic neurodegeneration. Molecular Neurobiology 2016 53:6849–6858 |
| Mitogen-activated protein kinases (MAPKs) etc. | Rat model (in vivo) | cypermethrin-induced Parkinsonism | may resist cypermethrin-induced pathophysiological effects | Pratibha Tripathi, Ashish Singh, Lakshmi Bala, Devendra Kumar Patel, Mahendra Pratap Singh: Ibuprofen Protects from Cypermethrin-Induced Changes in the Striatal Dendritic Length and Spine Density. Molecular Neurobiology 2018 55:2333–2339 |
| Neuroglobin | Rat model (in vivo) | AD | neuroglobin (↑), protein kinase B (Akt) signaling (↑) | Susi Zara, Marianna De Colli, Monica Rapino, Stephanie Pacella, Cinzia Nasuti, Piera Sozio, Antonio Di Stefano, Amelia Cataldi: Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer’s disease rat model. Gerontology 2013 59:250–260 |
| Neuroinflammatory mediator molecules | Rat model (in vivo) | Hypoxia-ischemia | the P3 HI-induced reductions in brain serotonin levels, serotonin transporter expression, and numbers of serotonergic neurons in the dorsal raphé nuclei (↓) | Julie A Wixey, Hanna E Reinebrant, Kathryn M Buller: Post-insult ibuprofen treatment attenuates damage to the serotonergic system after hypoxia-ischemia in the immature rat brain. Journal of Neuropathology Experimental Neurology 2012 71:1137–1148 |
| Neuronal pentraxin | Mouse model (in vivo) | AD | cognitive function (↑), IL-1beta (↓) | Anum Jamil, Aamra Mahboob, Touqeer Ahmed: Ibuprofen targets neuronal pentraxins expression and improves cognitive function in mouse model of AlCl 3-induced neurotoxicity. Experimental and Therapeutic Medicine 2016 11:601–606 |
| Nuclear factor (NF) kappa B inhibitor alpha protein and dopamine- and cAMP-regulated phosphoprotein-32 (DARPP-32) neuronal marker | Human model (in vitro), mouse model (in vivo) | Machado–Joseph disease | synaptic function and neural progenitors proliferation markers (↑), neuropathology and motor coordination (↑) | Liliana S Mendonça, Clévio Nóbrega, Silvia Tavino, Maximilian Brinkhaus, Carlos Matos, Sandra Tomé, Ricardo Moreira, Daniel Henriques, Brian K Kaspar, Luís Pereira de Almeida: Ibuprofen enhances synaptic function and neural progenitors proliferation markers and improves neuropathology and motor coordination in Machado–Joseph disease models. Human Molecular Genetics 2019 28:3691–3703 |
| Protein kinase C epsilon-mediated matrix metalloproteinase-2/9 (PKC epsilon-mediated MMP-2/9) | Rat model (in vivo) | AD | PKC epsilon-mediated MMP-2 and MMP-9 (↓), control symptoms of AD | S Zara, M Rapino, P Sozio, A Di Stefano, C Nasuti, A Cataldi: Ibuprofen and lipoic acid codrug 1 control Alzheimer’s disease progression by downregulating protein kinase C ε-mediated metalloproteinase 2 and 9 levels in β-amyloid infused Alzheimer’s disease rat model. Brain Research 2011 1412:79–87 |
| RhoA | Human and chick models (in vitro), mouse and rat models (in vitro) | Spinal cord injury | ligand-induced Rho signaling and myelin-induced inhibition (↓), the recovery of rats from a clinically relevant spinal cord trauma (↑) | Xingxing Wang, Stephane Budel, Kenneth Baughman, Grahame Gould, Kang-Ho Song, Stephen M Strittmatter: Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. Journal of Neurotrauma 2009 26:81–95 |
| RhoA | Rat model (in vitro, in vivo) | Spinal cord injury | may protect spinal cord injury | Bin Xing, Hui Li, Hongyu Wang, Dhriti Mukhopadhyay, Daniel Fisher, Christopher J Gilpin, Shuxin Li: RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Experimental Neurology 2011 231:247–260 |
| RhoA | Human model (in vitro) | Axonal injury | may protect axonal injury | Frank Roloff, Hannah Scheiblich, Carola Dewitz, Silke Dempewolf, Michael Stern, Gerd Bicker: Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS One 2015 10:e0118536 |
| RhoA | Human and mouse models (in vitro) | Neurodegenerative diseases | RhoA activation and microglial phagocytosis of neuronal cell fragments (↓) | Hannah Scheiblich, Gerd Bicker: Regulation of Microglial Phagocytosis by RhoA/ROCK-Inhibiting Drugs. Cellular and Molecular Neurobiology 2017 37:461–473 |
| RhoA | Human model (in vitro) | AD | neurite collapse and formation of stress fibers induced by Abeta (↓) | Patricia Ferrera, Angélica Zepeda, Clorinda Arias: Nonsteroidal anti-inflammatory drugs attenuate amyloid-β protein-induced actin cytoskeletal reorganization through Rho signaling modulation. Cellular and Molecular Neurobiology 2017 37:1311–1318 |
| Transcription factor peroxisome proliferator-activated receptor gamma (PPARgamma) | Rat model (in vitro) | AD and spinal cord injury | amyloid-beta42 peptide (↓) via inactivation of RhoA signaling | John Dill, Ankur R Patel, Xiao-Li Yang, Robert Bachoo, Craig M Powell, Shuxin Li: A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. The Journal of Neuroscience 2010 30:963–972 |
| Unknown | Mouse model (in vitro, in vivo) | AD | plaque burden (↓) | Ji-Kyung Choi, Bruce G Jenkins, Isabel Carreras, Sukru Kaymakcalan, Kerry Cormier, Neil W Kowall, Alpaslan Dedeoglu: Anti-inflammatory treatment in AD mice protects against neuronal pathology. Experimental Neurology 2010 223:377–384 |
| Unknown | Mouse model (in vivo) | AD | neuritic plaque pathology and inflammation (↓) | Dikmen Dokmeci: Ibuprofen and Alzheimer’s disease. Folia Med 2004 46:5–10 |
| Unknown | Mouse model (in vitro, in vivo) | Ataxia telangiectasia | cytodegeneration, cytological damage, young mutant pups’ LPS-induced behavioral deficits (↓) | Chin Wai Hui, Xuan Song, Fulin Ma, Xuting Shen, Karl Herrup: Ibuprofen prevents progression of ataxia telangiectasia symptoms in ATM-deficient mice. Journal of Neuroinflammation 2018 15:308 |
| Unknown | Piglet model (in vivo) | Intrauterine growth restriction | the inflammatory response and neuronal and matter impairment (↓) | Julie A. Wixey, Kishen R. Sukumar, Rinaldi Pretorius, Kah Meng Lee, Paul B. Colditz, S. Tracey Bjorkman, Kirat K. Chand: Ibuprofen Treatment Reduces the Neuroinflammatory Response and Associated Neuronal and White Matter Impairment in the Growth Restricted Newborn. Frontiers in Physiology 2019 10:541 |
| Unknown | Mouse model (in vitro) | Cerebral ischemia | neuronal cell death induced by kainate excitotoxicity or N-methyl-D-aspartate (↓) | Yusuke Iwata, Olivier Nicole, David Zurakowski, Toru Okamura, Richard A Jonas: Ibuprofen for neuroprotection after cerebral ischemia. The Journal of Thoracic and Cardiovascular Surgery 2010 139:489–493 |
| Unknown | Rat model (in vivo) | PD | reverse rotenone-induced motor deficits and depressive-like behavior | Tiago Zaminelli, Raísa Wendhausen Gradowski, Taysa Bervian Bassani, Janaína Kohl Barbiero, Ronise M Santiago, Daniele Maria-Ferreira, Cristiane Hatsuko Baggio, Maria A B F Vital: Antidepressant and antioxidative effect of Ibuprofen in the rotenone model of Parkinson’s disease. Neurotoxicity Research 2014 26:351–362 |
| Unknown | Mouse model (in vivo) | Age-dependent impairment of cognitive function | astrocyte activation (↓), synaptic plasticity and memory function (↑) | Justin T Rogers, Chia-Chen Liu, Na Zhao, Jian Wang, Travis Putzke, Longyu Yang, Mitsuru Shinohara, John D Fryer, Takahisa Kanekiyo, Guojun Bu: Subacute ibuprofen treatment rescues the synaptic and cognitive deficits in advanced-aged mice. Neurobiology of Aging 2017 53:112–121 |
| Unknown | Rat model (in vivo) | Intervertebral foramen inflammation (IVFI) | severity and duration of IVFI-induced thermal hyperalgesia and mechanical allodynia (↓), hyperexcitability of the inflamed DRG neurons (↓) | Zhi-Jiang Huang, Erica Hsu, Hao-Chuan Li, Anthony L Rosner, Ronald L Rupert, Xue-Jun Song: Topical application of compound Ibuprofen suppresses pain by inhibiting sensory neuron hyperexcitability and neuroinflammation in a rat model of intervertebral foramen inflammation. The Journal of Pain 2011 12:141–152 |
| Unknown | Mouse model (in vivo) | Batten disease and juvenile neuronal ceroid lipofuscinosis | the performance on the vertical pole test (concomitant use with lamotrigine) (↑), slightly ameliorate microgliosis | Marta A Tarczyluk-Wells, Christoph Salzlechner, Allison R Najafi, Ming J Lim, David Smith, Frances M Platt, Brenda P Williams, Jonathan D Cooper: Combined Anti-inflammatory and Neuroprotective Treatments Have the Potential to Impact Disease Phenotypes in Cln3−/− Mice. Frontiers in Neurology 2019 10:963 |
| Unknown | Rat model (in vitro) | Glutamate excitotoxicity and PD | dopamine uptake caused by glutamate (↓), protect both dopaminergic neurons and neurons overall against glutamate toxicity | D Casper, U Yaparpalvi, N Rempel, P Werner: Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. Neuroscience Letters 2000 289:201–204 |
| Unknown | Mouse model (in vivo) | PD | loss of mesencephalic dopaminergic neurons (↓), the number of CD68+/ Iba-1+ cells, the microglia/neurons interactions, and the pro-inflammatory cytokines (concomitant use with 1-deoxynojirimycin) (↓) | Tcs Costa, E Fernandez-Villalba, V Izura, A M Lucas-Ochoa, N J Menezes-Filho, R C Santana, M D de Oliveira, F M Araújo, C Estrada, Vda Silva, S L Costa, M T Herrero: Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. Journal of Neuroimmune Pharmacology 2021 16:390–402 |
| Unknown | Rat model (in vivo) | Exercise-induced fatigue | the acetylcholinesterase (AChE) activity (↓), neuronal tumor necrosis factor-alpha (TNF-alpha) and IL-1beta (↓) | F D Lima, D N Stamm, I D Della Pace, L R Ribeiro, L M Rambo, G Bresciani, J Ferreira, M F Rossato, M A Silva, M E Pereira, R P Ineu, A R Santos, F Bobinski, M R Fighera, L F Royes: Ibuprofen intake increases exercise time to exhaustion: A possible role for preventing exercise-induced fatigue. Scandinavian Journal of Medicine & Science in Sports 2016 26:1160–1170 |
| Unknown | Rat model (in vivo) | PD | delay the development of dyskinesia, Cox2 and vascular endothelial growth factor (VEGF) in striatal (↓) | Asmaa M Teema, Sawsan A Zaitone, Yasser M Moustafa: Ibuprofen or piroxicam protects nigral neurons and delays the development of l-dopa induced dyskinesia in rats with experimental Parkinsonism: Influence on angiogenesis. Neuropharmacology 2016 107:432–450 |
| Unknown | Human model (in vitro) | Hypomyelinating leukodystrophy 3 | reverse mutant-mediated inhibitory differentiation and the localization in the lysosome. | Yu Takeuchi, Marina Tanaka, Nanako Okura, Yasuyuki Fukui, Ko Noguchi, Yoshihiro Hayashi, Tomohiro Torii, Hiroaki Ooizumi, Katsuya Ohbuchi, Kazushige Mizoguchi, Yuki Miyamoto, Junji Yamauchi: Rare Neurologic Disease-Associated Mutations of AIMP1 are Related with Inhibitory Neuronal Differentiation Which is Reversed by Ibuprofen. Medicines (Basel) 2020 7:25 |
| Unknown | Rodent model (in vivo) | Hypoxia-ischemia | Cox2, IL-1β and TNF-alpha (↓), the loss O4- and O1-positive oligodendrocyte progenitor cells and myelin basic protein (MBP)-positive myelin content (↓) | M L Carty, J A Wixey, H E Reinebrant, G Gobe, P B Colditz, K M Buller: Ibuprofen inhibits neuroinflammation and attenuates white matter damage following hypoxia-ischemia in the immature rodent brain. Brain Research 2011 1402:9–19 |
| Unknown | Mouse model (in vitro, in vivo) | Manganism | the manganese-induced increase in cerebral F(2)-isoprostanes (↓) and protect the MSNs from dendritic atrophy and dendritic spine loss | Dejan Milatovic, Ramesh C Gupta, Yingchun Yu, Snjezana Zaja-Milatovic, Michael Aschner: Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicology and Applied Pharmacology 2011 256:219–226 |
| Unknown | Rat model (in vivo) | Intermittent hypoxia | oxidative stress, gp91 (phox) expression and macrophage infiltration in the CB (↓) | Siu-Yin Lam, Yu Liu, Kwong-Man Ng, Chi-Fai Lau, Emily C Liong, George L Tipoe, Man-Lung Fung: Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochemistry and Cell Biology 2012 137:303–317 |
| Unknown | Human model (in vitro), mouse model (in vivo) | oxaliplatin(OXA)-induced painful neuropathy | the neurotoxic OXA effects (↓) (a significant dose-dependent decrease in viability, a large increase in reactive oxygen species (ROS) and NO production, lipid peroxidation and mitochondrial impairment) | France Massicot, Guillaume Hache, Ludivine David, Dominique Chen, Charlotte Leuxe, Laure Garnier-Legrand, Patrice Rat, Olivier Laprévote, François Coudoré: P2X7 Cell Death Receptor Activation and Mitochondrial Impairment in Oxaliplatin-Induced Apoptosis and Neuronal Injury: Cellular Mechanisms and In Vivo Approach. PLoS One 2013 8:e66830 |
| Unknown | Human model (in vitro), rat model (in vivo) | Pentylenetetrazol-induced epilepsy | the proliferation of astrocytes (↓) by increasing autophagy | Jiangtao Peng, Shuhua Wu, Chong Guo, Ke Guo, Weiguo Zhang, Rui Liu, Jianmin Li, Zhongbo Hu: Effect of Ibuprofen on Autophagy of Astrocytes During Pentylenetetrazol-Induced Epilepsy and its Significance: An Experimental Study. Neurochemical Research 2019 44:2566–2576 |
| Unknown | Mouse model (in vivo) | Dementia with Lewy bodies | protein aggregation and astrogliosis (↓) | Kazunari Sekiyama, Masayo Fujita, Akio Sekigawa, Yoshiki Takamatsu, Masaaki Waragai, Takato Takenouchi, Shuei Sugama, Makoto Hashimoto: Ibuprofen ameliorates protein aggregation and astrocytic gliosis, but not cognitive dysfunction, in a transgenic mouse expressing dementia with Lewy bodies-linked P123H β-synuclein. Neuroscience Letters 2012 515:97–101 |
| Unknown | Rat model (in vivo) | Traumatic brain injury | CD45 and TGF-beta1 (↓) | T Cao, T C Thomas, J M Ziebell, J R Pauly, J Lifshitz: Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience 2012 225:65–75 |
| Unknown | Rat model (in vivo) | Hyperammonemia and hepatic encephalopathy | microglial activation (↓) and cognitive and motor functions (↑) | Regina Rodrigo, Omar Cauli, Ulises Gomez-Pinedo, Ana Agusti, Vicente Hernandez-Rabaza, Jose-Manuel Garcia-Verdugo, Vicente Felipo: Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 2010 139:675–684 |
| Unknown | Rat model (in vitro) | Hyperprolinemias | the glutamate and glutamine (↓), glutamine synthetase and AChE activities (↓), and acetylcholine (Ach) (↑) | Samanta Oliveira Loureiro, Daniele Susana Volkart Sidegum, Helena Biasibetti, Mery Stefani Leivas Pereira, Diogo Losch de Oliveira, Regina Pessoa-Pureur, Angela T S Wyse: Crosstalk Among Disrupted Glutamatergic and Cholinergic Homeostasis and Inflammatory Response in Mechanisms Elicited by Proline in Astrocytes. Molecular Neurobiology 2016 53:1065–1079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawaguchi, S.; Suzuki, R.; Oizumi, H.; Ohbuchi, K.; Mizoguchi, K.; Yamamoto, M.; Miyamoto, Y.; Yamauchi, J. Hypomyelinating Leukodystrophy 8 (HLD8)-Associated Mutation of POLR3B Leads to Defective Oligodendroglial Morphological Differentiation Whose Effect Is Reversed by Ibuprofen. Neurol. Int. 2022, 14, 212-244. https://doi.org/10.3390/neurolint14010018
Sawaguchi S, Suzuki R, Oizumi H, Ohbuchi K, Mizoguchi K, Yamamoto M, Miyamoto Y, Yamauchi J. Hypomyelinating Leukodystrophy 8 (HLD8)-Associated Mutation of POLR3B Leads to Defective Oligodendroglial Morphological Differentiation Whose Effect Is Reversed by Ibuprofen. Neurology International. 2022; 14(1):212-244. https://doi.org/10.3390/neurolint14010018
Chicago/Turabian StyleSawaguchi, Sui, Rimi Suzuki, Hiroaki Oizumi, Katsuya Ohbuchi, Kazushige Mizoguchi, Masahiro Yamamoto, Yuki Miyamoto, and Junji Yamauchi. 2022. "Hypomyelinating Leukodystrophy 8 (HLD8)-Associated Mutation of POLR3B Leads to Defective Oligodendroglial Morphological Differentiation Whose Effect Is Reversed by Ibuprofen" Neurology International 14, no. 1: 212-244. https://doi.org/10.3390/neurolint14010018
APA StyleSawaguchi, S., Suzuki, R., Oizumi, H., Ohbuchi, K., Mizoguchi, K., Yamamoto, M., Miyamoto, Y., & Yamauchi, J. (2022). Hypomyelinating Leukodystrophy 8 (HLD8)-Associated Mutation of POLR3B Leads to Defective Oligodendroglial Morphological Differentiation Whose Effect Is Reversed by Ibuprofen. Neurology International, 14(1), 212-244. https://doi.org/10.3390/neurolint14010018