Clinical Onset and Multiple Sclerosis Relapse after SARS-CoV-2 Infection
Abstract
:1. Introduction
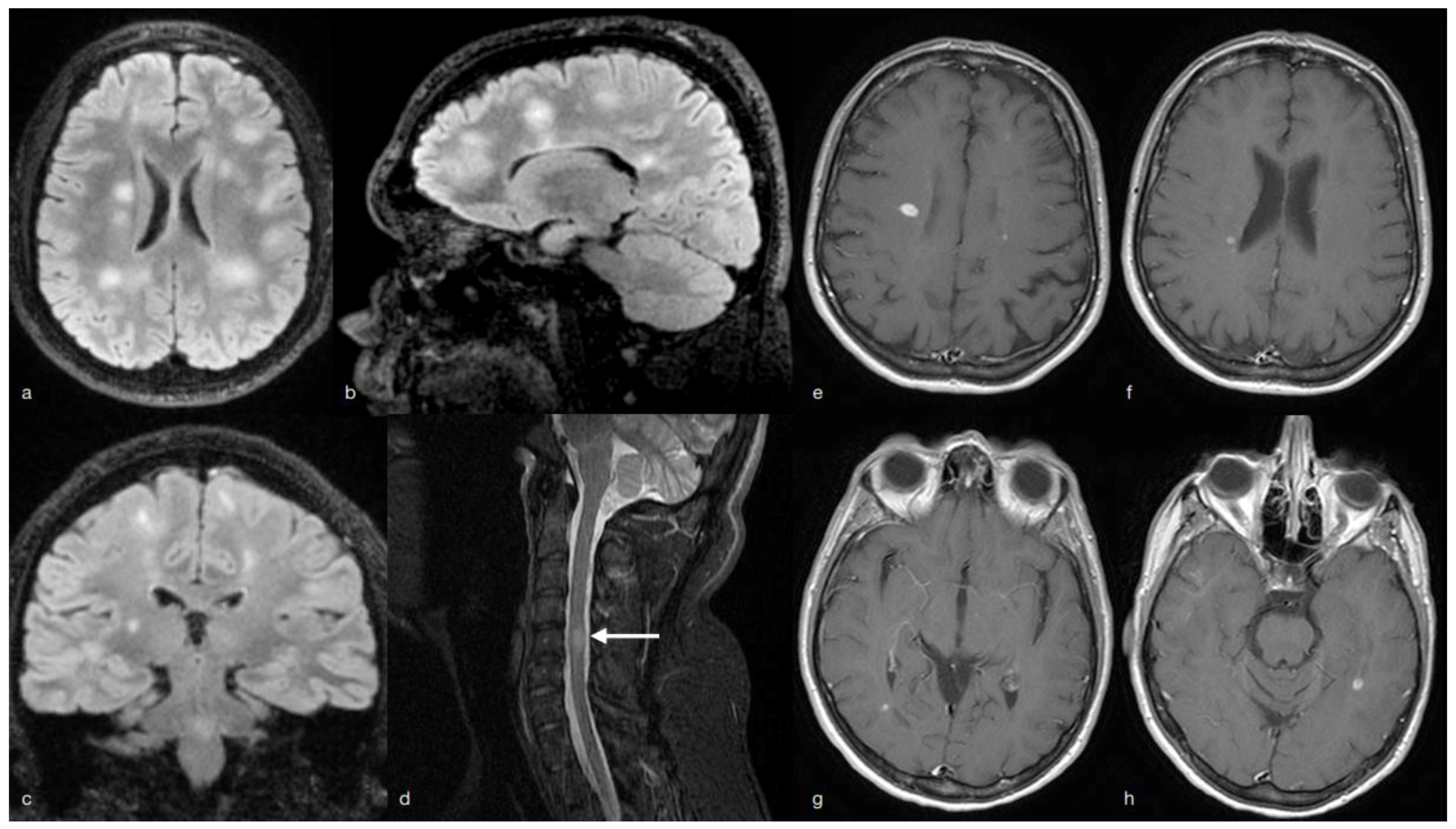
2. MS Onset
3. Multiple Sclerosis (MS) Relapse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilden, D.H. Multiple sclerosis exacerbations and infection. Lancet Neurol. 2002, 1, 145. [Google Scholar] [CrossRef]
- Munger, K.L.; Hongell, K.; Cortese, M.; Åivo, J.; Soilu-Hänninen, M.; Surcel, H.M.; Ascherio, A. Epstein–barr virus multiple sclerosis risk in the finnish maternity, cohort. Ann. Neurol. 2019, 86, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Buljevac, D.; Flach, H.Z.; Hop, W.C.J.; Hijdra, D.; Laman, J.D.; Savelkoul, H.F.J.; Van Der Meché, F.G.A.; Van Doorn, P.A.; Hintzen, R.Q. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 2002, 125, 952–960. [Google Scholar] [CrossRef]
- Blackmore, S.; Hernandez, J.; Juda, M.; Ryder, E.; Freund, G.G.; Johnson, R.W.; Steelman, A.J. Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyeli-tis. Proc. Natl. Acad. Sci. USA 2017, 114, E6107–E6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020 (No Date). Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 7 February 2021).
- Romero-Sánchez, C.M.; Díaz-Maroto, I.; Fernández-Díaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J.; Segura, T. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID, registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef]
- Meppiel, E.; Peiffer-Smadja, N.; Maury, A.; Bekri, I.; Delorme, C.; Desestret, V.; Zarrouk, V. Neurologic manifestations associated with COVID-19: A multicentre, registry. Clin. Microbiol. Infect. 2021, 27, 458. [Google Scholar] [CrossRef] [PubMed]
- Donati, D. Viral infections and multiple sclerosis. Drug Discov. Today Dis. Model. 2020, 32, 27–33. [Google Scholar] [CrossRef]
- Confavreux, C.; Suissa, S.; Saddier, P.; Bourdès, V.; Vukusic, S. Vaccinations and the Risk of Relapse in Multiple Sclerosis. N. Engl. J. Med. 2001, 344, 319–326. [Google Scholar] [CrossRef]
- Nguyen, J.; Hardigan, P.; Kesselman, M.M.; Beckler, M.D. Immunogenicity of The Influenza Vaccine in Multiple Sclerosis Patients: ASystematic Review, Meta-Analysis. Mult. Scler. Relat. Disord. 2021, 48, 102698. [Google Scholar] [CrossRef] [PubMed]
- Wouk, J.; Rechenchoski, D.Z.; Rodrigues, B.C.D.; Ribelato, E.V.; Faccin-Galhardi, L.C. Viral infections and their relationship to neurological disorders. Arch. Virol. 2021, 166, 733–753. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A. Perspectives of Neuro-COVID: Myasthenia. Front. Neurol. 2021, 12, 635747. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.O.; Jacobson, S. Viruses multiple sclerosis. CNS Neurol. Disord. Drug Targets 2012, 11, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J. Neurol. 2021, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.-N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. J. 2021, 27, 864–870. [Google Scholar] [CrossRef]
- Di Filippo, M.; Cordioli, C.; Malucchi, S.; Annovazzi, P.; Cavalla, P.; Clerici, V.T.; Ragonese, P.; Nociti, V.; Radaelli, M.; Laroni, A.; et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Palao, M.; Fernández-Díaz, E.; Gracia-Gil, J.; Romero-Sánchez, C.M.; Díaz-Maroto, I.; Segura, T. Multiple sclerosis following SARS-CoV-2, infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Kataria, S.; Tandon, M.; Melnic, V.; Sriwastava, S. A case series and literature review of multiple sclerosis and COVID-19: Clinical characteristics, outcomes and a brief review of immunotherapies. eNeurologicalSci 2020, 21, 100287. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.H.; Tsang, O.T.; Hui, D.S.; Kwan, M.Y.; Chan, W.H.; Chiu, S.S.; Peiris, M. Neutralizing antibody titres in SARS-CoV-2, infections. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Kevadiya, B.D. A Role for Extracellular Vesicles in SARS-CoV-2 Therapeutics, Prevention. J. Neuroimmune Pharmacol. 2021, 16, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, K.D.; Collins, L.E.; McLoughlin, A.; Cummins, P.M. Tumour necrosis factor-α-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory, interleukin-6. J. Neurochem. 2016, 136, 564–572. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignolo, A.; Aprile, M.; Gagliardo, C.; Giammanco, G.M.; D’Amelio, M.; Aridon, P.; La Tona, G.; Salemi, G.; Ragonese, P. Clinical Onset and Multiple Sclerosis Relapse after SARS-CoV-2 Infection. Neurol. Int. 2021, 13, 695-700. https://doi.org/10.3390/neurolint13040066
Pignolo A, Aprile M, Gagliardo C, Giammanco GM, D’Amelio M, Aridon P, La Tona G, Salemi G, Ragonese P. Clinical Onset and Multiple Sclerosis Relapse after SARS-CoV-2 Infection. Neurology International. 2021; 13(4):695-700. https://doi.org/10.3390/neurolint13040066
Chicago/Turabian StylePignolo, Antonia, Maria Aprile, Cesare Gagliardo, Giovanni Maurizio Giammanco, Marco D’Amelio, Paolo Aridon, Giuseppe La Tona, Giuseppe Salemi, and Paolo Ragonese. 2021. "Clinical Onset and Multiple Sclerosis Relapse after SARS-CoV-2 Infection" Neurology International 13, no. 4: 695-700. https://doi.org/10.3390/neurolint13040066
APA StylePignolo, A., Aprile, M., Gagliardo, C., Giammanco, G. M., D’Amelio, M., Aridon, P., La Tona, G., Salemi, G., & Ragonese, P. (2021). Clinical Onset and Multiple Sclerosis Relapse after SARS-CoV-2 Infection. Neurology International, 13(4), 695-700. https://doi.org/10.3390/neurolint13040066








