The QOSMOS Study: Pharmacist-Led Multicentered Observational Study on Quality of Life in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Design
2.3. Patient Characteristics
2.4. Data Collection
2.5. Statistical Analysis
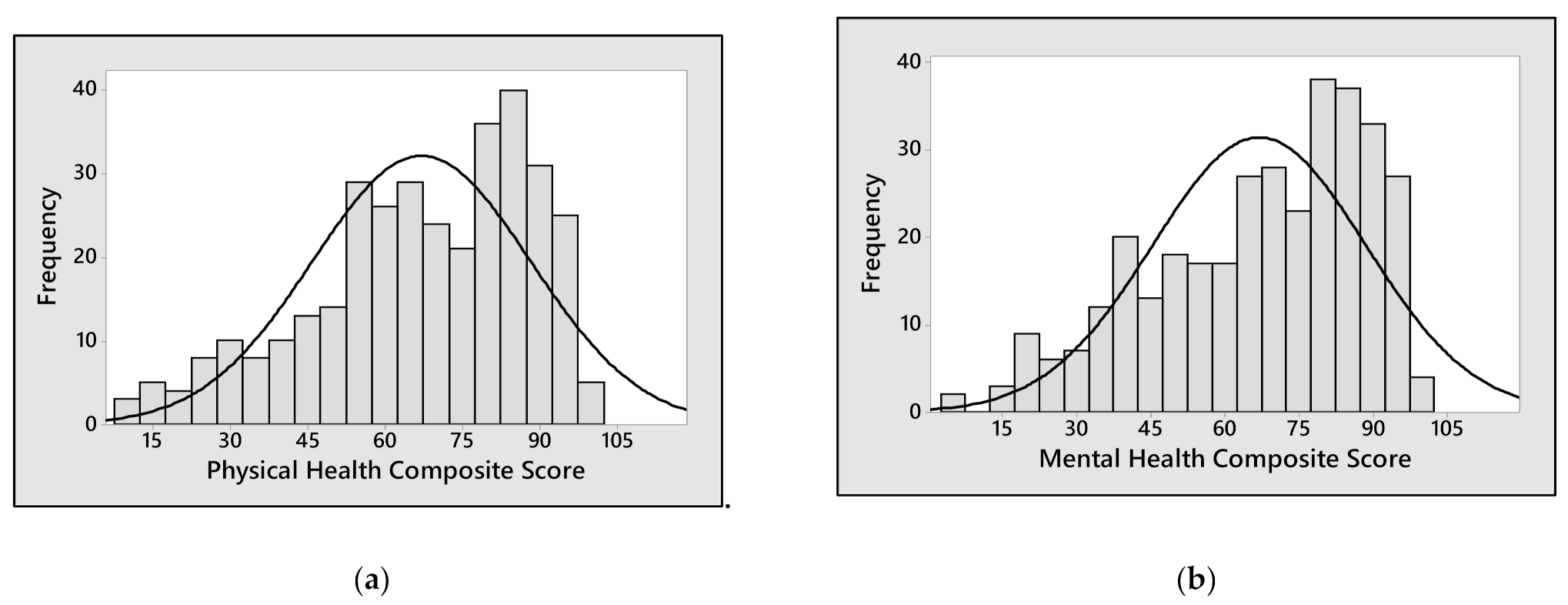
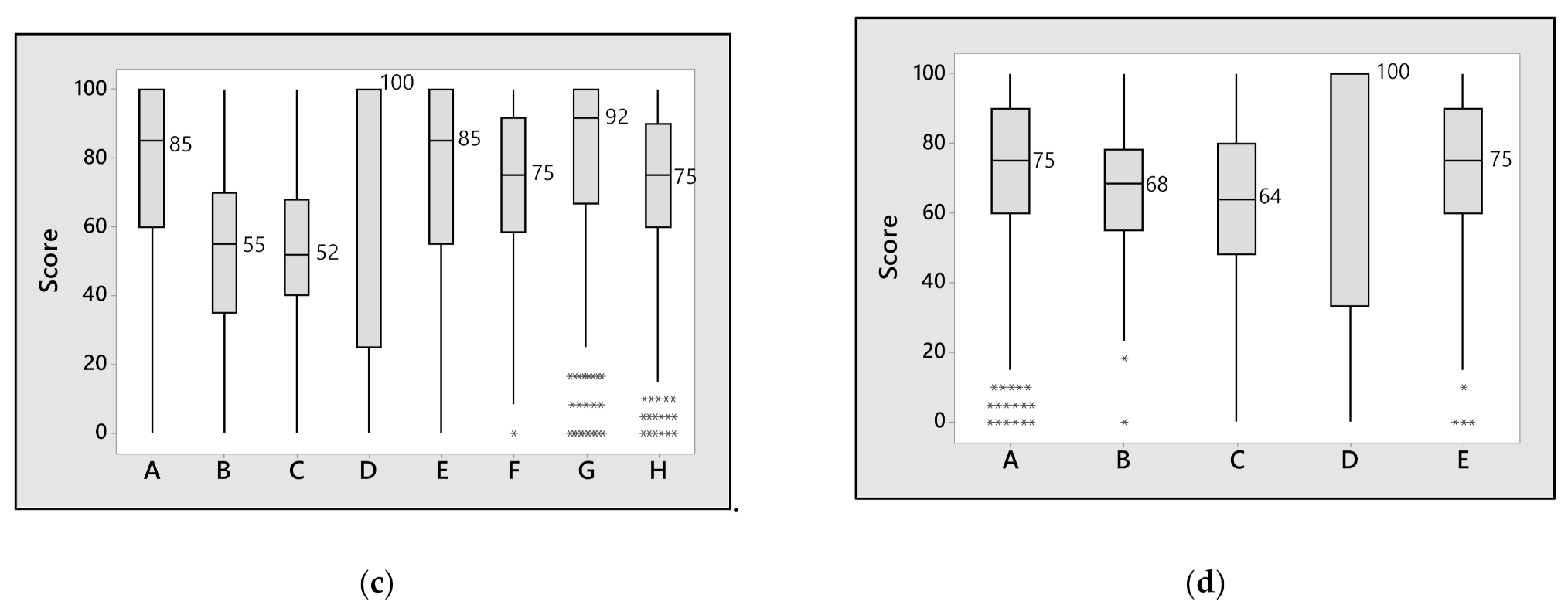
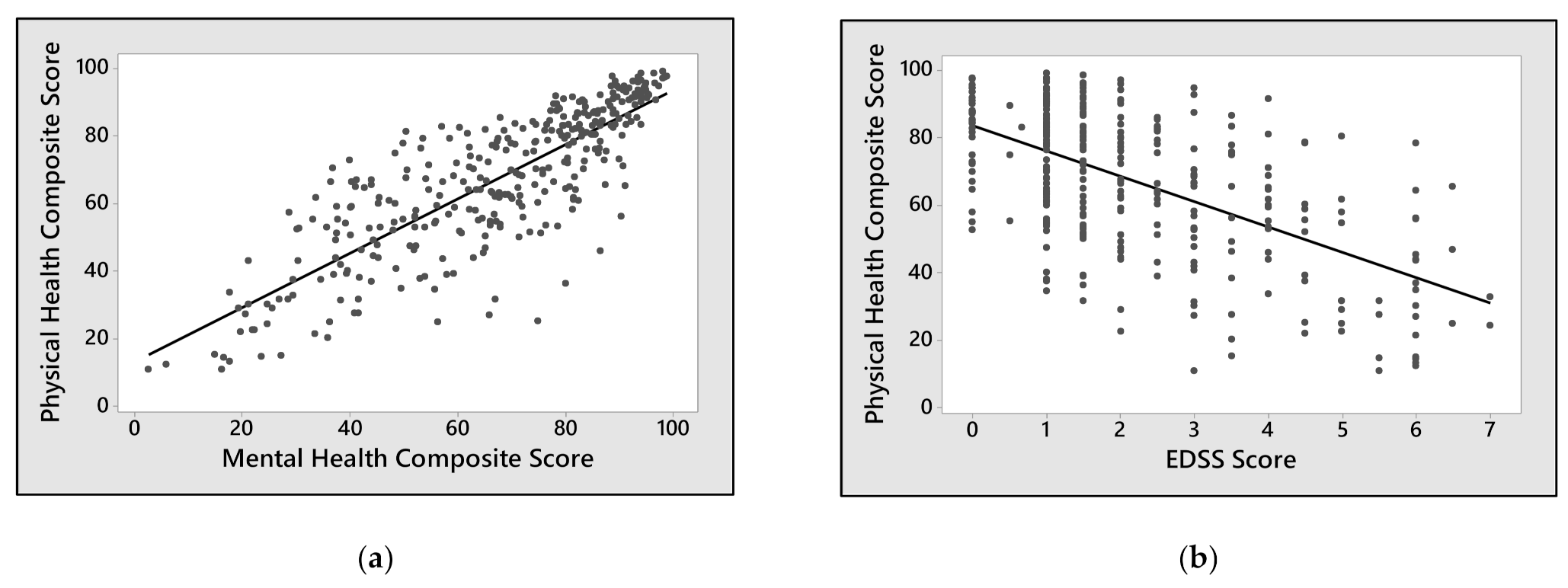
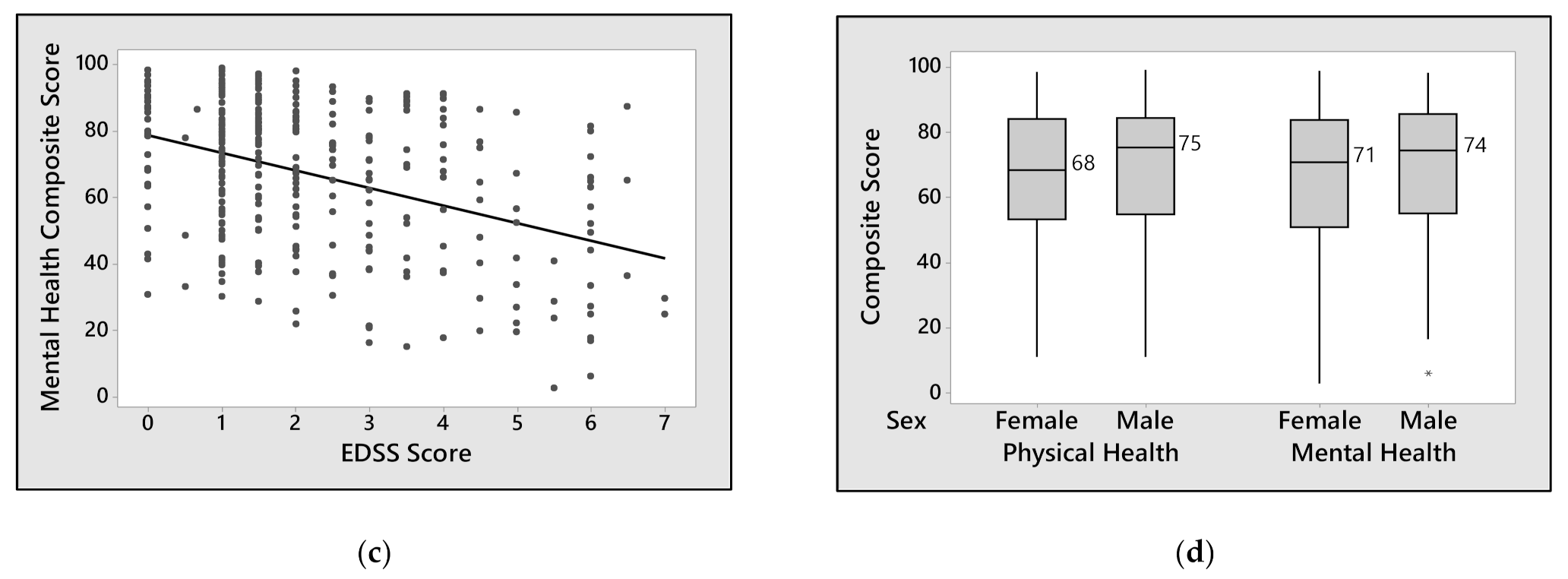
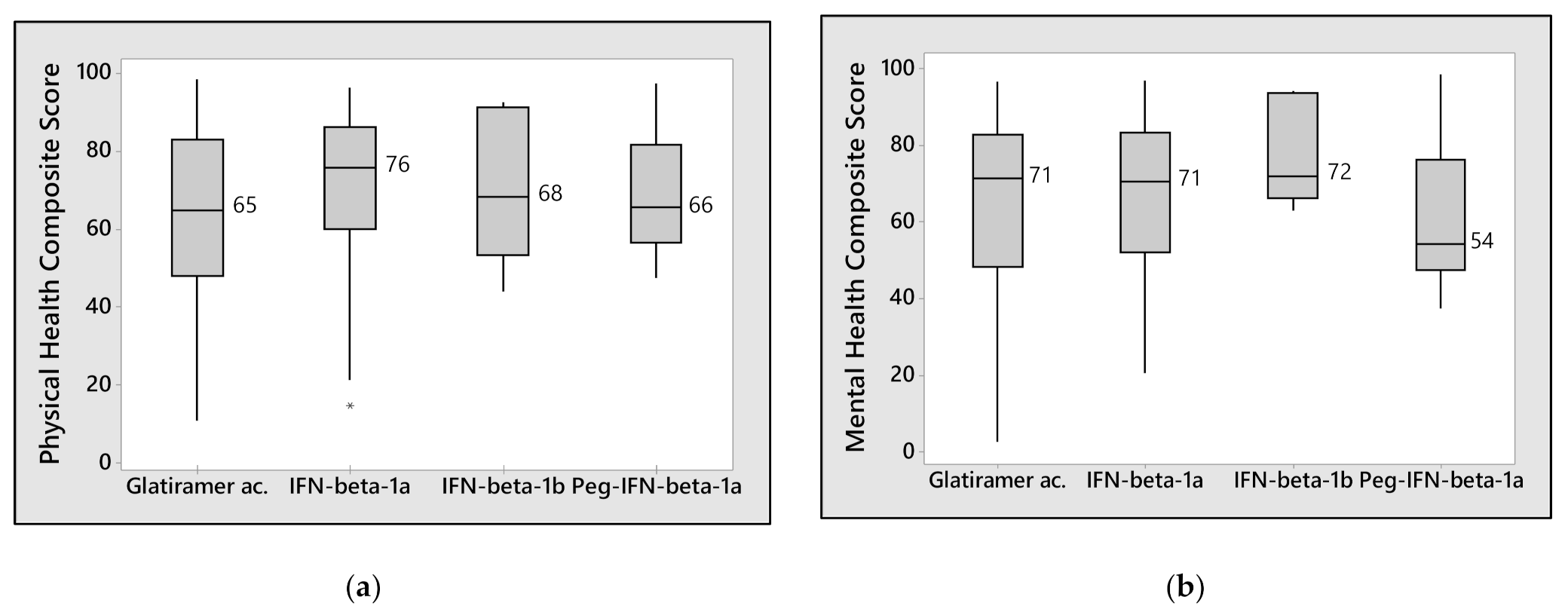
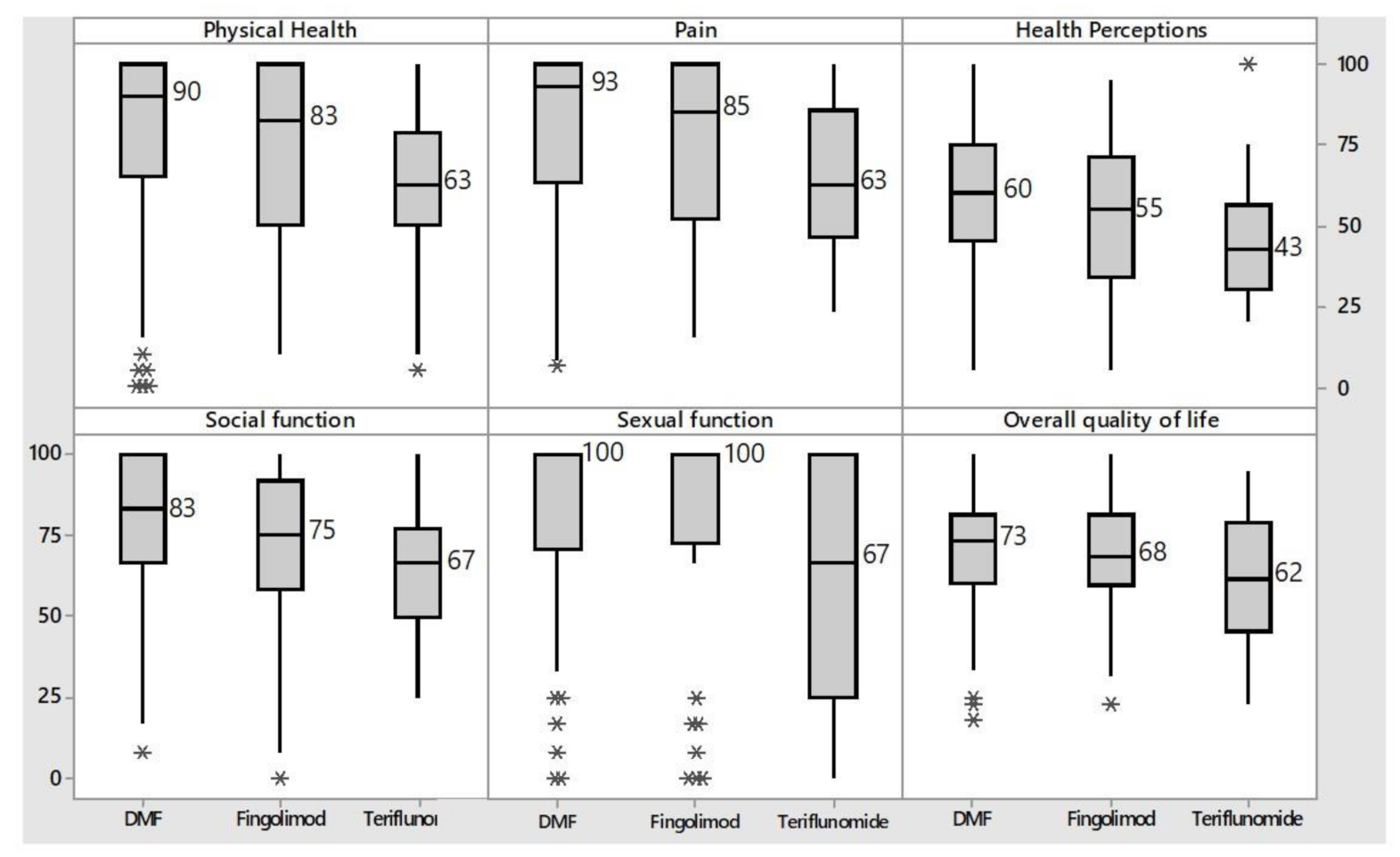
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pons, V.; Rivest, S. Beneficial Roles of Microglia and Growth Factors in MS, a Brief Review. Front. Cell Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Fiol, M.P.; Gaitán, M.I.; Correale, J. Quality of Life Assessment in Multiple Sclerosis: Different Perception between Patients and Neurologists. Front. Neurol. 2018, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Atlas of Multiple Sclerosis. Available online: www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms (accessed on 7 November 2021).
- Battaglia, M.A.; Bezzini, D. Estimated prevalence of multiple sclerosis in Italy in 2015. Neurol. Sci. 2017, 38, 473–479. [Google Scholar] [CrossRef]
- Hobart, J.C.; Lamping, D.L.; Freeman, J.A.; Thompson, A.J. Reliability, validity and responsiveness of the Kurtzke expanded disability status scale (EDSS) in multiple sclerosis (MS) patients. Eur. J. Neurol. Name 1996, 3, 13. [Google Scholar]
- Ochoa, A.; Hernández-Mojica, T.; Paz, F.; Jara-Prado, A.; Santos, Z.T.-D.L.; Sánchez-Guzmán, M.; Guerrero-Camacho, J.; Corona-Vázquez, T.; Flores, J.; Camacho-Molina, A.; et al. Quality of life in patients with multiple sclerosis and its association with depressive symptoms and physical disability. Mult. Scler. Relat. Disord. 2019, 36, 101386. [Google Scholar] [CrossRef]
- Nortvedt, M.W.; Riise, T. The use of quality of life measures in multiple sclerosis research. Mult. Scler. J. 2003, 9, 63–72. [Google Scholar] [CrossRef]
- Vickrey, B.G.; Hays, R.D.; Harooni, R.; Myers, L.W.; Ellison, G.W. A health-related quality of life measure for multiple sclerosis. Qual. Life Res. 1995, 4, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Solari, A.; Filippini, G.; Mendozzi, L.; Ghezzi, A.; Cifani, S.; Barbieri, E.; Baldini, S.; Salmaggi, A.; La Mantia, L.; Farinotti, M.; et al. Validation of Italian multiple sclerosis quality of life 54 questionnaire. J. Neurol. Neurosurg. Psychiatry 1999, 67, 158–162. [Google Scholar] [CrossRef]
- Drulovic, J.; Pekmezovic, T.; Matejic, B.; Mesaros, S.; Manigoda, M.; Dujmović, I.; Stojsavljevic, N.; Kocev, N.; Gavric-Kezic, M.; Nikic, P.; et al. Quality of life in patients with multiple sclerosis in Serbia. Acta Neurol. Scand. 2007, 115, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Rodriguez-Mena, D.; Herrero, R.; Almarcegui, C.; Dolz, I.; Martin, J.; Ara, J.R.; Larrosa, J.M.; Polo, V.; Fernández, J.; et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology 2013, 81, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Schurch, B.; Denys, P.; Kozma, C.M.; Reese, P.R.; Slaton, T.; Barron, R. Reliability and validity of the Incontinence Quality of Life questionnaire in patients with neurogenic urinary incontinence. Arch. Phys. Med. Rehabil. 2007, 88, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.; Vickrey, B.; Fortin, K.; Lee, L. Two Multiple Sclerosis Quality-of-Life Measures: Comparison in a National Sample. Can. J. Neurol. Sci. 2015, 42, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Ponziani, G.; Rossi, F.; Liedl, C.L.; Stefanile, C.; Rossi, L. Quality of life in multiple sclerosis: The impact of depression, fatigue and disability. Mult. Scler. J. 2001, 7, 340–344. [Google Scholar] [CrossRef]
- Lily, O.; McFadden, E.; Hensor, E.; Johnson, M.; Ford, H. Disease-specific quality of life in multiple sclerosis: The effect of disease modifying treatment. Mult. Scler. J. 2006, 12, 808–813. [Google Scholar] [CrossRef]
- Jongen, P.J. Health-Related Quality of Life in Patients with Multiple Sclerosis: Impact of Disease-Modifying Drugs. CNS Drugs 2017, 31, 585–602. [Google Scholar] [CrossRef]
- Coyle, P.K.; Khatri, B.; Edwards, K.R.; Meca-Lallana, J.E.; Cavalier, S.; Rufi, P.; Benamor, M.; Thangavelu, K.; Robinson, M.; Gold, R. Patient-reported outcomes in patients with relapsing forms of MS switching to teriflunomide from other disease-modifying therapies: Results from the global Phase 4 Teri-PRO study in routine clinical practice. Mult. Scler. Relat. Disord. 2018, 26, 211–218. [Google Scholar] [CrossRef]
- Kita, M.; Fox, R.J.; Gold, R.; Giovannoni, G.; Phillips, J.T.; Sarda, S.P.; Kong, J.; Viglietta, V.; Sheikh, S.I.; Okwuokenye, M.; et al. Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: An integrated analysis of the phase 3 DEFINE and CONFIRM studies. Clin. Ther. 2014, 36, 1958–1971. [Google Scholar] [CrossRef]
- Lee, A.; Pike, J.; Edwards, M.R.; Petrillo, J.; Waller, J.; Jones, E. Quantifying the Benefits of Dimethyl Fumarate Over β Interferon and Glatiramer Acetate Therapies on Work Productivity Outcomes in MS Patients. Neurol. Ther. 2017, 6, 79–90. [Google Scholar] [CrossRef]
- Simone, I.L.; Ceccarelli, A.; Tortorella, C.; Bellacosa, A.; Pellegrini, F.; Plasmati, I.; De Caro, M.F.; Lopez, M.; Girolamo, F.; Livrea, P. Influence of Interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual. Life Outcomes 2006, 4, 96. [Google Scholar] [CrossRef]
- Patti, F.; Pappalardo, A.; Montanari, E.; Pesci, I.; Barletta, V.; Pozzilli, C. Interferon-beta-1a treatment has a positive effect on quality of life of relapsing-remitting multiple sclerosis: Results from a longitudinal study. J. Neurol. Sci. 2014, 337, 180–185. [Google Scholar] [CrossRef]
- Lanzillo, R.; Sparaco, M.; Lavorgna, L.; Carmisciano, L.; Signoriello, E.; Signori, A.; Costabile, T.; Maniscalco, G.T.; Saccà, F.; Cepparulo, S.; et al. A snapshot on patient-reported outcome measures of people with multiple sclerosis on first-line therapies in a real world setting. Neurol. Sci. 2020, 41, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Padureanu, R.; Albu, C.V.; Pirici, I.; Mititelu, R.R.; Subtirelu, M.S.; Turcu-Stiolica, R.A.; Sintonen, H.; Padureanu, V.; Turcu-Stiolica, A. Personal Autonomy as Quality of Life Predictor for Multiple Sclerosis Patients. J. Clin. Med. 2020, 9, 1349. [Google Scholar] [CrossRef]
- Vickrey, B.G.; Lee, L.; Moore, F.; Moriarty, P. EDSS Change Relates to Physical HRQoL While Relapse Occurrence Relates to Overall HRQoL in Patients with Multiple Sclerosis Receiving Subcutaneous Interferon β-1a. Mult. Scler. Int. 2015, 2015, 631989. [Google Scholar] [CrossRef] [PubMed]
- Gyllensten, H.; Kavaliunas, A.; Alexanderson, K.; Hillert, J.; Tinghög, P.; Friberg, E. Costs and quality of life by disability among people with multiple sclerosis: A register-based study in Sweden. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318783352. [Google Scholar] [CrossRef] [PubMed]
- Calkwood, J.; Cree, B.; Crayton, H.; Kantor, D.; Steingo, B.; Barbato, L.; Hashmonay, R.; Agashivala, N.; McCague, K.; Tenenbaum, N.; et al. Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: Post hoc analyses of the EPOC trial. BMC Neurol. 2014, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Li, D.K.; Freedman, M.S.; Truffinet, P.; Benzerdjeb, H.; Wang, D.; Bar-Or, A.; Traboulsee, A.; Reiman, L.E.; O’Connor, P.W.; et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: Safety and efficacy results up to 8.5 years. Mult. Scler. 2012, 18, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- L’uso dei Farmaci in Italia—Rapporto OsMed. 2017. Available online: https://www.aifa.gov.it/sites/default/files/Rapporto_OsMed_2017_AIFA.pdf (accessed on 11 November 2021).
- L’uso dei Farmaci in Italia—Rapporto OsMed. 2018. Available online: https://www.aifa.gov.it/documents/20142/o/Rapporto_OsMed_2018.pdf (accessed on 11 November 2021).
- Kappos, L.; Gold, R.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Sarda, S.P.; Agarwal, S.; Zhang, A.; Sheikh, S.I.; et al. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing–remitting multiple sclerosis: The DEFINE study. Mult. Scler. J. 2013, 20, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Fox, R.J.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.K.; Sarda, S.P.; Agarwal, S.; Kong, J.; Zhang, A.; Viglietta, V.; et al. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: Findings from the CONFIRM study. Mult. Scler. 2014, 20, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Hendin, B.; Naismith, R.T.; Wray, S.E.; Huang, D.; Dong, Q.; Livingston, T.; Jones, D.L.; Watson, C.; Jhaveri, M. Treatment satisfaction significantly improves in patients with multiple sclerosis switching from interferon beta therapy to peginterferon beta-1a every 2 weeks. Patient Prefer. Adherence 2018, 12, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Damuzzo, V.; Agnoletto, L.; Rampazzo, R.; Cammalleri, F.; Cancanelli, L.; Chiumente, M.; Costantino, S.; Michielan, S.; Milani, F.; Sartori, A.; et al. Experience in managing independent clinical research, the best training strategy for future clinical pharmacists: The QOSMOS project. Eur. J. Hosp. Pharm. 2021. published online ahead of print. [Google Scholar] [CrossRef] [PubMed]

| Number of Enrolled Patients | 349 | |
|---|---|---|
| Sex | Female [n (%)] | 241 (69) |
| Male [n (%)] | 108 (31) | |
| Age [median (min, max)] | 44 (0, 75) | |
| Disease [n (%)] | Relapsing–Remitting MS (RRMS) | 325 (93.1) |
| Secondary-Progressive MS (SPMS) | 12 (3.4) | |
| Progressive-Relapsing MS (PRMS) | 3 (0.9) | |
| Primary-Progressive MS (PPMS) | 1 (0.3) | |
| Other (Clinically isolated syndrome, partial transverse myelitis) | 8 (2.3) | |
| EDSS Score at enrolment [median (min, max)] | 1.5 (0.0, 7.0) | |
| Number of relapses in previous two years [median (min, max)] | 0.0 (0.0, 6.0) | |
| Time since diagnosis-years [median (min, max)] | 6.8 (0, 34.4) | |
| Current treatment [n (%)] | Azathioprine | 2 (0.6) |
| Dimethyl fumarate | 121 (34.7) | |
| Fingolimod | 42 (12.0) | |
| Glatiramer acetate | 59 (16.9) | |
| Interferon β-1a | 61 (17.5) | |
| Interferon β-1b | 5 (1.43) | |
| Peg-Interferon β-1a | 19 (5.44) | |
| Teriflunomide | 34 (9.7) | |
| Combined regimens | 6 (1.7) | |
| Number of drugs used previously [median (min, max)] | 1 (0.0, 4.0) | |
| Treatment | n | Missing | Minimum | Q1 | Median | Q3 | Maximum |
|---|---|---|---|---|---|---|---|
| Dimethyl fumarate | 114 | 7 | 0 | 1 | 1.5 | 3 | 7 |
| Interferon β-1a | 58 | 3 | 0 | 1 | 1.5 | 2.125 | 6 |
| Glatiramer acetate | 53 | 6 | 0 | 1 | 2.5 | 4 | 6.5 |
| Fingolimod | 40 | 2 | 0 | 1.125 | 2 | 3.875 | 6.5 |
| Teriflunomide | 32 | 2 | 0 | 1 | 2 | 3.875 | 6 |
| Peg-Interferon β-1a | 16 | 3 | 0 | 1 | 1.5 | 2 | 3 |
| Combined regimens | 5 | 0 | 1 | 1 | 1 | 1.5 | 2 |
| Interferon β-1b | 4 | 1 | 1 | 1 | 1.25 | 4.88 | 6 |
| Azathioprine | 2 | 1 | 5 | * | 5.5 | * | 6 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Standardized β | p-Value | Standardized β | p-Value | |
| Age | −0.4632 | <0.001 | 0.0038 | 0.968 |
| n° of relapses in the last two years | −4.94 | <0.001 | −2.26 | 0.039 |
| n° of prior treatments | −3.02 | 0.011 | −0.75 | 0.468 |
| EDSS score | −7.509 | <0.001 | −7.207 | <0.001 |
| Dimethyl fumarate | 5.72 | 0.016 | 2.17 | 0.294 |
| Interferon β-1a | 4.21 | 0.156 | ||
| Glatiramer acetate | −5.01 | 0.096 | ||
| Fingolimod | −0.94 | 0.787 | ||
| Teriflunomide | −11.62 | 0.002 | −9.7 | 0.003 |
| Peg-Interferon β-1a | 2.17 | 0.664 | ||
| Combined regimens | 2.26 | 0.812 | ||
| Interferon β-1b | 4.33 | 0.649 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Standardized β | p-Value | Standardized β | p-Value | |
| Age | −0.21 | 0.032 | 0.142 | 0.198 |
| n° of relapses in the last two years | −4.4 | 0.001 | −2.48 | 0.055 |
| n° of prior treatments | −1.11 | 0.361 | ||
| EDSS score | −5.339 | <0.0001 | −5.309 | <0.001 |
| Dimethyl fumarate | 4.96 | 0.041 | 3.28 | 0.165 |
| Interferon β-1a | 0.85 | 0.780 | ||
| Glatiramer acetate | −2.52 | 0.413 | ||
| Fingolimod | 1.06 | 0.766 | ||
| Teriflunomide | −5.94 | 0.127 | ||
| Peg-Interferon β-1a | −5.43 | 0.286 | ||
| Combined regimens | −3.84 | 0.693 | ||
| Interferon β-1b | 11.19 | 0.249 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damuzzo, V.; Agnoletto, L.; Rampazzo, R.; Cammalleri, F.; Cancanelli, L.; Chiumente, M.; Costantino, S.; Michielan, S.; Milani, F.; Sartori, A.; et al. The QOSMOS Study: Pharmacist-Led Multicentered Observational Study on Quality of Life in Multiple Sclerosis. Neurol. Int. 2021, 13, 682-694. https://doi.org/10.3390/neurolint13040065
Damuzzo V, Agnoletto L, Rampazzo R, Cammalleri F, Cancanelli L, Chiumente M, Costantino S, Michielan S, Milani F, Sartori A, et al. The QOSMOS Study: Pharmacist-Led Multicentered Observational Study on Quality of Life in Multiple Sclerosis. Neurology International. 2021; 13(4):682-694. https://doi.org/10.3390/neurolint13040065
Chicago/Turabian StyleDamuzzo, Vera, Laura Agnoletto, Roberta Rampazzo, Francesca Cammalleri, Luca Cancanelli, Marco Chiumente, Stefano Costantino, Silvia Michielan, Federica Milani, Alessia Sartori, and et al. 2021. "The QOSMOS Study: Pharmacist-Led Multicentered Observational Study on Quality of Life in Multiple Sclerosis" Neurology International 13, no. 4: 682-694. https://doi.org/10.3390/neurolint13040065
APA StyleDamuzzo, V., Agnoletto, L., Rampazzo, R., Cammalleri, F., Cancanelli, L., Chiumente, M., Costantino, S., Michielan, S., Milani, F., Sartori, A., Rivano, M., & Mengato, D. (2021). The QOSMOS Study: Pharmacist-Led Multicentered Observational Study on Quality of Life in Multiple Sclerosis. Neurology International, 13(4), 682-694. https://doi.org/10.3390/neurolint13040065






