Improvement of Tardive Dyskinesia during Mindfulness Meditation
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cornett, E.; Novitch, M.; Kaye, A.; Kata, V.; Kaye, A. Medication-Induced Tardive Dyskinesia: A Review and Update. Ochsner J. 2017, 17, 162–174. [Google Scholar] [PubMed]
- Waln, O.; Jankovic, J. An update on tardive dyskinesia: From phenomenology to treatment. Tremor Other Hyperkinetic Mov. 2013, 3. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Yoshida, K.; Bies, R.R.; Suzuki, T.; Remington, G.; Pollock, B.G.; Mizuno, Y.; Mimura, M.; Uchida, H. Tardive dyskinesia in relation to estimated dopamine D2 receptor occupancy in patients with schizophrenia: Analysis of the CATIE data. Schizophr. Res. 2014, 153, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Anusa, A.; Thavarajah, R.; Nayak, D.; Joshua, E.; Rao, U.; Ranganathan, K. A Study on Drug-Induced Tardive Dyskinesia: Orofacial Musculature Involvement and Patient’s Awareness. J. Orofac. Sci. 2018, 10, 86–95. [Google Scholar] [PubMed]
- Soares-Weiser, K.; Rathbone, J.; Ogawa, Y.; Shinohara, K.; Bergman, H. Miscellaneous treatments for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst. Rev. 2018, 3, CD000208. [Google Scholar] [CrossRef] [PubMed]
- Bergman, H.; Soares-Weiser, K. Anticholinergic medication for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst. Rev. 2018, 1, CD000204. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.; Bernstein, C.J.; Davies, G.; Tang, N.K.Y.; Belhag, M.; Tingle, A.; Field, M.; Foss, J.; Lindahl, A.; Underwood, M.; et al. Combined cognitive-behavioural and mindfulness programme for people living with dystonia: A proof-of-concept study. BMJ Open 2016, 6, e011495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szekely, B.C.; Turner, S.M.; Jacob, R.G. Behavioral control of L-dopa induced dyskinesia in Parkinsonism. Biofeedback Self-Regul. 1982, 7, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Abnormal Involuntary Movement Scale (117-AIMS). ECDEU Assessment Manual for Psychopharmacology; Guy, W., Ed.; National Institute of Mental Health: Rockville, MD, USA, 1976; pp. 534–537. [Google Scholar]
- Tang, Y.-Y.; Ma, Y.; Wang, J.; Fan, Y.; Feng, S.; Lu, Q.; Yu, Q.; Sui, D.; Rothbart, M.K.; Fan, M.; et al. Short-term meditation training improves attention and self-regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 17152–17156. [Google Scholar] [CrossRef] [PubMed]
- MacCoon, D.G.; Imel, Z.E.; Rosenkranz, M.A.; Sheftel, J.G.; Weng, H.Y.; Sullivan, J.C.; Bonus, K.A.; Stoney, C.M.; Salomons, T.V.; Davidson, R.J.; et al. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR). Behav. Res. Ther. 2012, 50, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G.; Sawyer, A.T.; Witt, A.A.; Oh, D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J. Consult. Clin. Psychol. 2010, 78, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.R.; Hartigan, J.A.; Mikulas, W.L. Concentration and Mindfulness Meditations: Unique Forms of Consciousness? Appl. Psychophysiol. Biofeedback 1999, 24, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Stegmayer, K.; Walther, S.; van Harten, P. Tardive Dyskinesia Associated with Atypical Antipsychotics: Prevalence, Mechanisms and Management Strategies. CNS Drugs 2018, 32, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C. From State-to-Trait Meditation: Reconfiguration of Central Executive and Default Mode Networks. Eneuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Wielgosz, J.; Goldberg, S.B.; Kral, T.R.A.; Dunne, J.D.; Davidson, R.J. Mindfulness Meditation and Psychopathology. Annu. Rev. Clin. Psychol. 2019, 15, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Supekar, K.; Menon, V.; Dougherty, R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 2009, 19, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.W.; Kerr, C.E.; Wasserman, R.H.; Gray, J.R.; Greve, D.N.; Treadway, M.T.; McGarvey, M.; Quinn, B.T.; Dusek, J.A.; Benson, H.; et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005, 16, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; Greischar, L.L.; Rawlings, N.B.; Ricard, M.; Davidson, R.J. Long-term meditators self-induce highamplitude gamma synchrony during mental practice. Proc. Natl. Acad. Sci. USA 2004, 101, 16369–16373. [Google Scholar] [CrossRef] [PubMed]
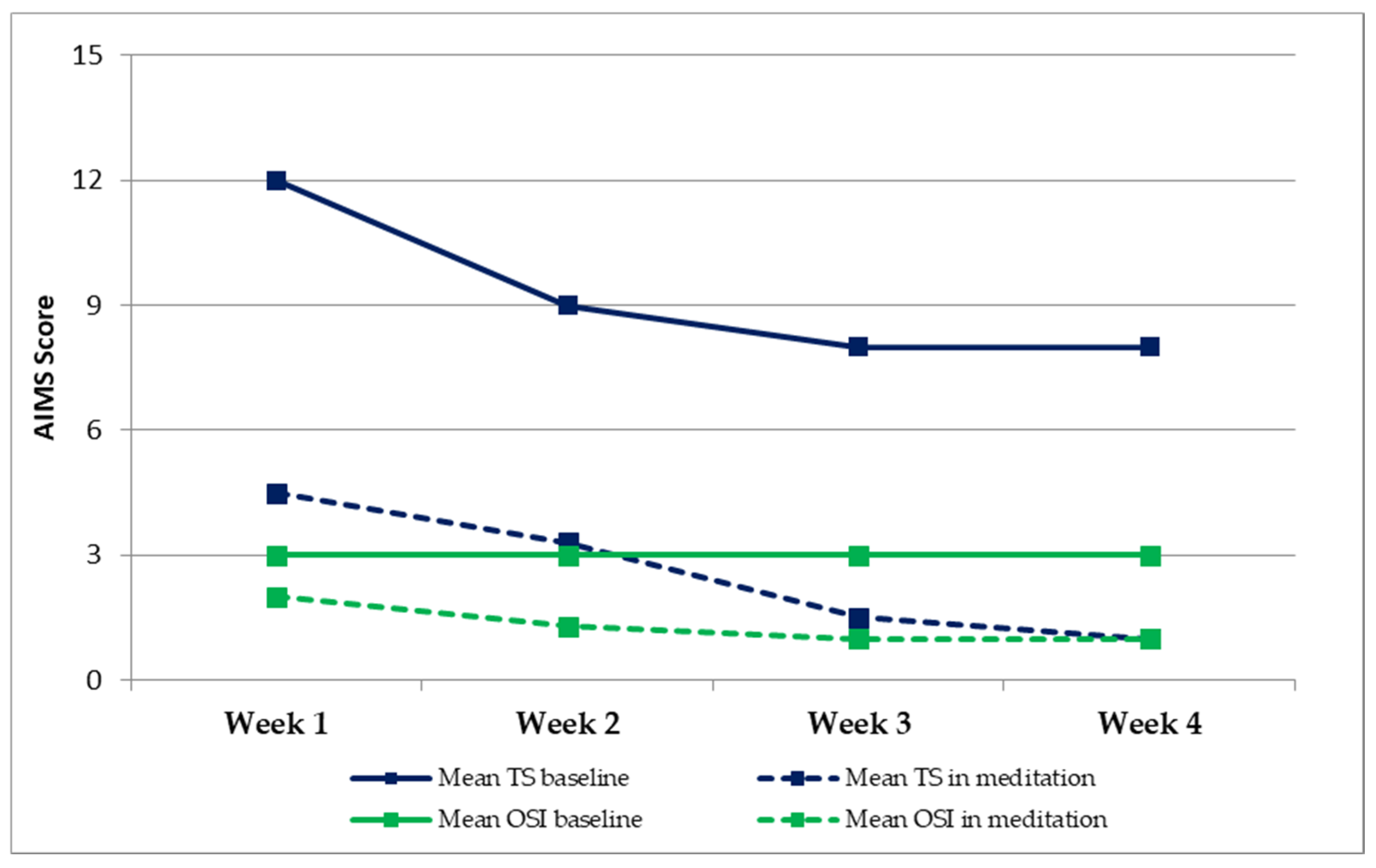
| Mean TS Baseline | Mean TS in Meditation | Δ TS | Mean OSI Baseline | Mean OSI in Meditation | Δ OSI | |
|---|---|---|---|---|---|---|
| Week 1 | 12 | 4.5 | 7.5 | 3 | 2 | 1 |
| Week 2 | 9 | 3.3 | 5.7 | 3 | 1.3 | 1.6 |
| Week 3 | 8 | 1.5 | 6.5 | 3 | 1 | 1.5 |
| Week 4 | 8 | 1 | 7 | 3 | 1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, M.A.; English, I.; Sezer, I.; Amagat, M.; Ly, F.; Chaneac, E.; Cailliez, P.; Bottemanne, H. Improvement of Tardive Dyskinesia during Mindfulness Meditation. Neurol. Int. 2021, 13, 439-444. https://doi.org/10.3390/neurolint13030043
Santoro MA, English I, Sezer I, Amagat M, Ly F, Chaneac E, Cailliez P, Bottemanne H. Improvement of Tardive Dyskinesia during Mindfulness Meditation. Neurology International. 2021; 13(3):439-444. https://doi.org/10.3390/neurolint13030043
Chicago/Turabian StyleSantoro, Maria Angela, Isolde English, Idil Sezer, Mickael Amagat, Frank Ly, Edouard Chaneac, Patricia Cailliez, and Hugo Bottemanne. 2021. "Improvement of Tardive Dyskinesia during Mindfulness Meditation" Neurology International 13, no. 3: 439-444. https://doi.org/10.3390/neurolint13030043
APA StyleSantoro, M. A., English, I., Sezer, I., Amagat, M., Ly, F., Chaneac, E., Cailliez, P., & Bottemanne, H. (2021). Improvement of Tardive Dyskinesia during Mindfulness Meditation. Neurology International, 13(3), 439-444. https://doi.org/10.3390/neurolint13030043






