Abstract
Introduction: Although the putamen has a significant role in reward-seeking and motivated behaviors, including eating and food-seeking, minorities’ diminished returns (MDRs) suggest that individual-level risk and protective factors have weaker effects for Non-Hispanic Black than Non-Hispanic White individuals. However, limited research is available on the relevance of MDRs in terms of the role of putamen functional connectivity on body mass index (BMI). Purpose: Building on the MDRs framework and conceptualizing race and socioeconomic status (SES) indicators as social constructs, we explored racial and SES differences in the associations between putamen functional connectivity to the salience network and children’s BMI. Methods: For this cross-sectional study, we used functional magnetic resonance imaging (fMRI) data of 6473 9–10-year-old Non-Hispanic Black and Non-Hispanic White children from the Adolescent Brain Cognitive Development (ABCD) study. The primary independent variable was putamen functional connectivity to the salience network, measured by fMRI. The primary outcome was the children’s BMI. Age, sex, neighborhood income, and family structure were the covariates. Race, family structure, parental education, and household income were potential moderators. For data analysis, we used mixed-effect models in the overall sample and by race. Results: Higher right putamen functional connectivity to the salience network was associated with higher BMI in Non-Hispanic White children. The same association was missing for Non-Hispanic Black children. While there was no overall association in the pooled sample, a significant interaction was found, suggesting that the association between right putamen functional connectivity to the salience network and children’s BMI was modified by race. Compared to Non-Hispanic White children, Non-Hispanic Black children showed a weaker association between right putamen functional connectivity to the salience network and BMI. While parental education and household income did not moderate our association of interest, marital status altered the associations between putamen functional connectivity to the salience network and children’s BMI. These patterns were observed for right but not left putamen. Other/Mixed Race children also showed a pattern similar to Non-Hispanic Black children. Conclusions: The association between right putamen functional connectivity to the salience network and children’s BMI may depend on race and marital status but not parental education and household income. While right putamen functional connectivity to the salience network is associated with Non-Hispanic White children’s BMI, Non-Hispanic Black children’ BMI remains high regardless of their putamen functional connectivity to the salience network. This finding is in line with MDRs, which attributes diminished effects of individual-risk and protective factors for Non-Hispanic Black children to racism, stratification, and segregation.
1. Introduction
The putamen, a round brain structure located at the basal ganglia, is a significant dorsal striatum [1]. The putamen is involved in a wide range of motivated behaviors, decision making, and addiction [1]. As part of the brain reward system, putamen is a part of the brain’s dopaminergic system that influences a wide range of motivated behaviors including eating [2,3,4].
As an element of the brain dopaminergic system, putamen regulates the brain reward system [5]. Altered putamen structure and function predict poor decision-making, impulsivity, and high-risk behaviors, including but not limited to substance use [6]. One of the mechanisms that increases individuals’ risk of tobacco [7,8,9], drug [10,11,12], and alcohol [13,14,15] use is altered putamen function, through its association with impulsivity and reward seeking [16].
According to minorities’ diminished returns (MDRs) [17], observed across study designs, cohorts, settings, age groups, socioeconomic status (SES) indicators, and health outcomes [18,19], individual-level risk and protective factors show weaker associations with outcomes of racial and ethnic minority people compared to Non-Hispanic Whites. For example, SES shows weaker associations with brain structure and function of Non-Hispanic Blacks than Non-Hispanic Whites [20,21,22,23,24,25,26,27]. The same pattern is shown for SES effects on trauma [28], attention deficit hyperactivity disorder (ADHD) [29], suicide [27], depression [30], aggression [31], tobacco use [31,32,33], impulsivity [34], school bonding [35], school performance [36], math performance [37], attention [38], and inhibitory control [39] in Non-Hispanic Black children compared with Non-Hispanic White children. Similar results are shown in the Adolescent Brain Cognitive Development (ABCD) study [26,27,39,40], Add Health [17], the Fragile Families and Child Wellbeing Study (FFCWS) [29,34,35,41,42,43,44], Monitoring the Future (MTF) [36], the National Survey of American Life (NSAL) [30], the Flint Adolescents Study (FAS) [45], the Population Assessment of Tobacco and Health (PATH) [31], the Early Childhood Longitudinal Study (ECLS) study [46], and the Family and Community Health Study (FACHS) [47,48].
The MDRs have been shown for outcomes such as obesity [44], tobacco use [31], suicide [27], and aggression [31], which are all linked to impulsivity. In the ABCD [26,27,39], FFCWS [29,34,44], and PATH [31] studies, high SES Non-Hispanic Black children have remained at a high level of impulsivity [21,39], risk-taking [31], and reward-dependency [26,49], to a level that is unexpected given their SES. For example, Non-Hispanic Black children from high SES backgrounds showed worse than expected impulsivity [34], inhibitory control [21], fun-seeking [49], and reward responsiveness [26]. As a result, high SES Non-Hispanic Black children remain at risk of obesity, regardless of their individual level risk and protective factors [44,50,51,52]. While this literature is mainly limited to the effects of SES, with the same line of reasoning, putamen function and functional connectivity may have less salience as determinants of children’s developmental and behavioral outcomes such as body mass index (BMI) for Non-Hispanic Black youth.
In this study, we conceptualized race and SES as social rather than biological constructs [53] and explored racial and SES differences in the association between putamen functional connectivity with salience network and children’s BMI. We focused on the putamen as it has major implications for risk-taking, impulsivity, and sensation seeking, all related to children’s BMI [54,55,56,57,58]. We hypothesized that putamen functional connectivity to the salience network would be related to BMI (Hypothesis 1) [55,59,60,61,62,63]. In line with the MDRs phenomenon, we expected a weaker association between putamen functional connectivity to the salience network and BMI for Non-Hispanic Black children in comparison with Non-Hispanic White children (Hypothesis 2). Again, this is not because Non-Hispanic Blacks and Non-Hispanic Whites are biologically different but because race, as a proxy for exposure to racism, racialization, stratification, discrimination, and adversities, interferes with the likelihood that individuals secure outcomes in the presence of resources and assets [53]. As some research has shown specific lateralization of brain reward system including putamen, and as past research has shown that reward-related to food is connected to right putamen activity, we expected MDRs for right but not left putamen. Finally, as race and SES closely overlap, we tested if similar MDRs can be seen across groups in terms of parental education, household income, and family structure.
2. Methods
2.1. Design and Settings
This is a secondary analysis of existing data. Data are from the Adolescent Brain Cognitive Development (ABCD) study [64,65,66,67]. The ABCD is a landmark brain development study in the United States. Although detailed information regarding ABCD study methods, sampling, sample, measures, and imaging techniques are available [64,65,66,67,68,69], we briefly review some key aspects of the study.
2.2. Participants and Sampling
Participants of the ABCD study were recruited as children between ages 9 and 11 from multiple cities across the USA. Overall, participants were enrolled from 21 sites. The primary source of recruitment for the ABCD sample was U.S. school systems. The sampling protocol of the ABCD study is described in detail elsewhere [64]. This analysis was based on 6473 participants; in this analysis, participants needed to have valid data on race, ethnicity, demographics, right putamen functional connectivity to the salience network, parental marital status, and children’s BMI. Participants were included if their Monetary Incentive Delay (MID) task was available, and T1 MRI was recommended to be used in the Data Exploration and Analysis Portal (DEAP).
2.3. Study Variables
The study variables included right putamen functional connectivity to the salience network (independent variables); child race (moderators); child age, ethnicity, sex, and parental marital status (confounders); and child BMI (dependent variables).
2.3.1. Main Outcomes
Body Mass Index (BMI). The main outcomes were the children’s BMI, calculated on the basis of the measured height and weight of the children.
2.3.2. Independent Variable
Right putamen functional connectivity to the salience network. Participants’ right putamen functional connectivity to the salience network education were a continuous variable. This variable is pre-calculated and available in the ABCD dataset. This was measured using resting functional magnetic resonance imaging (fMRI). For more information on ABCD fMRI techniques and details, please see Casey and others [69]. The ABCD imaging protocol was harmonized for three 3T scanner platforms (Siemens Prisma, General Electric (GE) 750, and Philips) and use of multi-channel coils capable of multiband echo planar imaging (EPI) acquisitions, using a standard adult-size coil. The scan session consisted of a fixed order of scan types that begin with a localizer, acquisition of 3D T1-weighted images, 2 runs of resting state fMRI, diffusion-weighted images, 3D T2-weighted images, 1–2 more runs of resting state fMRI (see motion detection below for when to acquire 1 versus 2 additional runs), and the task-based fMRI. Participants completed an MRI screening questionnaire for any contraindication for an MRI (e.g., braces, pacemakers, and other metal in the body including piercings, medical screw, or pins). This MR screening occurred 3 times: during initial recruitment, at scheduling, and just prior to the scan. Before the scan, participants were desensitized to the scanner environment with a simulator. The simulation occurred in dedicated mock scanners with a prerecorded scanner. A child-friendly movie was turned on as the child entered the scanner and remained on during acquisition of the localizer and 3D T1 scans, being also played during the 3D T2 and diffusion-weighted imaging acquisitions. The functional scans included 20 min of resting-state data acquired with eyes open and passive viewing of a cross-hair. One set of two 5 min runs was acquired immediately after the 3D T1 and another set was acquired after the 3D T2 scans. Real-time motion detection and correction for the structural scans were implemented by the ABCD DAIC hardware and software. A real-time head motion monitoring system called FIRMM (fMRI integrated real-time motion monitor (www.firmm.us (accessed on 1 March 2021)) [70], collaboratively developed at Washington University, St. Louis, and Oregon Health Sciences University was implemented for motion detection in resting state fMRI scans at the Siemens sites. Scan qualities were acceptable given the young age of the participants (9–10 years), length of the scan protocol (100–120 min), and that approximately 42% of the sample consisted of children who showed early signs of externalizing and internalizing symptoms and were considered at risk for substance abuse and other mental health problems [69].
2.3.3. Moderators
Race. Children’s race was self-identified by the parents. Race was a dichotomous variable: Non-Hispanic Black vs. Non-Hispanic White (reference category).
2.3.4. Confounders
Age, sex, parental marital status, and neighborhood income were included as covariates. Parents reported the child’s age, which was calculated as months between the date of birth and the study’s date. Sex of the child was a dichotomous variable that was coded 0 for males and 1 for females. Parental marital status was also a dichotomous variable, self-reported by the parent interviewed, and coded 1 vs. 0 for married and unmarried. Neighborhood median income was derived from ABCD residential history files. These data were collected from Zip codes. This variable was derived from residential history data of the ABCD and was calculated for current place of residence, regardless of duration of residence in the area. Neighborhood income was treated as a continuous measure, with higher neighborhood income being an indicator of higher area-level SES [71,72,73,74,75,76].
2.4. Data Analysis
We used the Data Exploration and Analysis Portal (DEAP) for data analysis. Provided by the Data Analysis and Informatics Core of the ABCD study. The DEAP uses R and provides a user-friendly online platform for multivariable analysis of the ABCD data. The DEAP platform was obtained from https://deap.nimhda.org (accessed on 1 March 2021), and ABCD data were downloaded from https://nda.nih.gov/abcd (accessed on 1 March 2021). For our univariate analysis, we reported the mean (standard deviation (SD)) and frequency (%) of our variables depending on the variable type. We also reported the results of the chi-squared test to compare Non-Hispanic White and Non-Hispanic Black children. Our regression in DEAP was based on mixed-effect models, given the fact that participants are nested to families, and families are nested to sites. The primary outcome was the children’s BMI. The independent variable was the right putamen functional connectivity to the salience network. The moderator was race. Age, sex, family marital status, and neighborhood income were covariates. Overall, four models were fitted. Model 1 and Model 2 were run in Non-Hispanic White and Non-Hispanic Black children, respectively. Model 3 tested the additive effects, while covariates were in the model. This model did not include an interaction term. Model 4 tested the interaction term between right putamen functional connectivity to the salience network and race. Before running models, we checked a wide range of assumptions, including lack of collinearity between predictors, normal distribution of our outcome, distribution of errors for our model, as well as the association between observed and theoretical quantiles of our model (Appendix A). Other than the main analysis explained above, we performed additional analysis as below. First, we tested if the same patterns held for right and left putamen. Second, we ran models with parental education, household income, and family structure as moderators. Finally, as MDRs are not specific to Non-Hispanic Blacks, we also tested the same patterns for other non-White groups. From regression coefficients, we reported beta coefficients, Standard Errors (SE), and p-values. p-values smaller than 0.05 were significant.
2.5. Ethical Aspect
Our secondary analysis was found by the Charles R Drew University of Medicine and Science (CDU) Institutional Review Board (IRB) to be exempt from a full IRB review (IRB number 1665000-1; Date: 10/02/2020). However, the original ABCD study underwent an Institutional Review Board (IRB) in several institutions, including but not limited to the University of California, San Diego (UCSD). The IRB in multiple institutions approved the study protocol, and all children provided assent and parents signed consent.
3. Results
3.1. Sample Descriptive Data
Table 1 shows the descriptive data, overall and by race. Our sample included 6473 children who were either 9 or 10 years old. Among the sample, 5180 (80.0%) were Non-Hispanic White, and 1293 (20.0%) were Non-Hispanic Black. As Table 1 shows, right putamen functional connectivity to the salience network was higher in Non-Hispanic White than Non-Hispanic Black children. Non-Hispanic White and Non-Hispanic Black children also differed in terms of BMI. Non-Hispanic White children had lower average BMI than Non-Hispanic Black children.

Table 1.
Descriptive statistics in the sample overall and by race.
3.2. Model Fit
As Table 2 shows, the best fitting model was Model 4, which was performed in the full sample, and had the interaction term.

Table 2.
Model fit.
3.3. Effects in Non-Hispanic White and Non-Hispanic Black Participants
As shown in Table 3, the right putamen functional connectivity to the salience network positively associated children’s BMI in Non-Hispanic White children but not in Non-Hispanic Black children. For Non-Hispanic White children, the association was positive and significant. Although not significant, for Non-Hispanic Black children, the association was negative.

Table 3.
Parameter estimates for the effects of right putamen functional connectivity to the salience network on children’s body mass index (BMI).
Table 3 shows the results of Model 1 and Model 2. Higher right putamen functional connectivity to the salience network was associated with higher BMI in Non-Hispanic White children. The same association was missing for Non-Hispanic Black children.
3.4. Main Effects in the Pooled Sample
Table 4 summarizes the results of Model 3 and Model 4. As shown in Table 4, right putamen functional connectivity to the salience network had an inverse association with children’s BMI. As Table 4 shows, we also found a statistically significant interaction between the effects of right putamen functional connectivity to the salience network and race on children’s BMI. This interaction suggested that the gain in terms of low BMI from higher levels of right putamen functional connectivity to the salience network is diminished for Non-Hispanic Black compared to Non-Hispanic White children.

Table 4.
Parameter estimates for the effects of right putamen functional connectivity to the salience network on children’s BMI.
As shown in Figure 1, the right putamen functional connectivity to the salience network had a positive and significant association with the BMI for Non-Hispanic White children. For Non-Hispanic Black children, this positive association was reversed, although non-significant. As Figure 1 shows, we also found statistical interactions between right putamen functional connectivity to the salience network and race on the children’s BMI. These suggest that the inverse association between right putamen functional connectivity to the salience network and BMI is weaker for Non-Hispanic Black vs. Non-Hispanic White children.
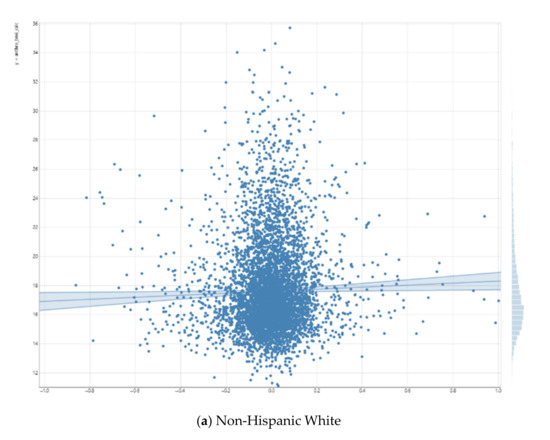
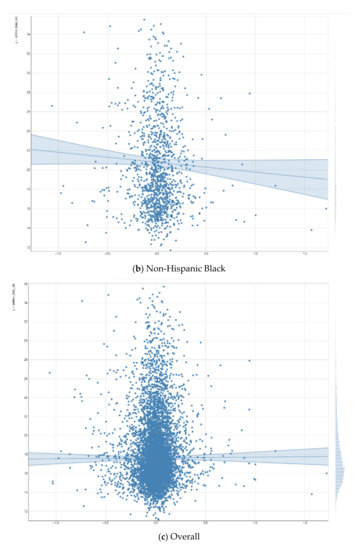
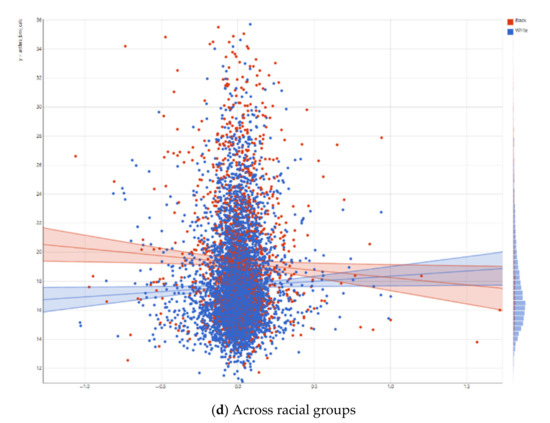
Figure 1.
Association between right putamen functional connectivity to the salience network and children’s body mass index (BMI).
3.5. Further Results
When we ran similar models with family structure as the moderator, the interaction between family structure and putamen functional connectivity with salience network on children’s BMI was statistically significant. Similar to race, we found positive association in married and negative association in non-married families. However, we did not find any moderating effects of parental education or household income on our association of interest. In other terms, the association between putamen functional connectivity with salience network and children’s BMI showed similar patterns in households with low and high income or across various levels of parental education. Our additional analysis showed that similar to Non-Hispanic Black children, Other/Mixed Race children also showed more negative association between putamen functional connectivity with salience network and BMI compared to Non-Hispanic White children. Finally, we found that these MDRs can be only seen for right but not left putamen resting state functional connectivity with salience network.
4. Discussion
There were racial variations in the association between right but not left putamen functional connectivity to the salience network and BMI of 9–10-year-old American children. While we did not find any association between right putamen functional connectivity to the salience network and BMI in the total sample of American children, interaction terms were found between race and right putamen functional connectivity to the salience network on childhood BMI, indicating weaker effects for Non-Hispanic Black (and Other/Mixed Race) children than Non-Hispanic White children. In Non-Hispanic White children, there was a positive and significant association, and in Non-Hispanic Black and in Other/Mixed Race children, there was an inverse association between right putamen functional connectivity to the salience networks and BMI. In addition, marital status also showed a similar moderating effect, however, our association was similar across subgroups based on parental education and household income. This observation suggests that while marital status may be a reason race moderates the role of putamen functional connectivity as a neural determinant of childhood BMI, parental education and household income are not the reason we see racial differences in the salience of putamen functional connectivity as a predictor of childhood BMI.
Our result on the association between right putamen functional connectivity to the salience network and BMI in Non-Hispanic White children is in line with the other research on putamen function. Putamen closely operates with the neurotransmitter dopamine and influences motivated behaviors such as food-seeking [2,3,4]. Putamen can be seen as one of the central elements of the brain’s dopaminergic system that regulates the reward seeking, including but not limited to food consumption [5].
Our second finding that putamen functional connectivity to the salience network showing weaker association with BMI for Non-Hispanic Black children than Non-Hispanic White children is an extension of the MDRs literature. Our past research using the ABCD data [26,27,39,40], Add Health [17], FFCWS [29,34,35,41,42,43,44], MTF [36], NSAL [30], FAS [45], and FACHS [47,48] have all shown significantly weaker effects of individual-level risk and protective factors (e.g., SES, age, coping, and affect) on various health outcomes for Non-Hispanic Black children in comparison with Non-Hispanic White children. For example, family income and parental education showed stronger association with aggression [31], tobacco use [33], school bonding [35], school performance [36], ADHD [29], impulsivity [34], inhibitory control [21], stress [28,41], obesity [44], physical health [31], and depression [30] for Non-Hispanic White children when compared with Non-Hispanic Black children.
The current results can be seen as Non-Hispanic Black (and Other/Mixed Race) children’s diminished salience [19] of putamen functional connectivity on BMI. MDRs are defined as systematically smaller effects of individual-level risk and protective factors such as economic resources and psychological assets for Non-Hispanic Black families in comparison with Non-Hispanic White families. As the same pattern can be seen for Latino [31], Asian American [37], and Native American [77] families, this phenomenon is believed to be due to mineralization of all marginalized social groups. These MDRs are nothing specific to Black people as they are hold for LGBT [78,79], immigrant [80,81,82], and even marginalized Non-Hispanic White families [17]. As a result, they are believed to be due to marginalization, segregation, and discrimination [17]. In our study also, we could see similar MDRs for two non-White groups of children.
A number of recent studies have documented MDRs of SES effect on several brain morphometric and functional features in the ABCD data. In one study, SES showed a weaker effect on amygdala volume of Non-Hispanic Black than Non-Hispanic White children [83]. In another study, the effect of age on amygdala and cortical function to threat was significantly different in Non-Hispanic Black and Non-Hispanic White children [84]. In several other studies, effects of SES on neurocognitive outcomes such as attention, memory, executive function, and inhibitory control were all weaker for Non-Hispanic Black than Non-Hispanic White children.
As a result of MDRs, regardless of risk or protective factors such as age, SES, coping, health, or affect, Non-Hispanic Black children remain at risk of tobacco use [33], anxiety [45], ADHD [29], depression [85], suicide [27], obesity [44], poor diet [86], high screen time [87], and low physical activity [88]. While for Non-Hispanic White children, individual level risk factors have high salience on aggression, obesity, tobacco use, and chronic disease, for Non-Hispanic Black children, individual-level risk and protective factors show diminished effects [31]. These patterns are attributed to racism, segregation, and discrimination, all reducing the relevance of individual level assets and resources on securing outcomes for the marginalized group. Our finding also showed that race moderates the salience of putamen connectivity, but not because race is a proxy of SES. This was observed as parental education and household income did not alter the salience of putamen functional connectivity as a predictor of childhood BMI.
This paper documented MDRs in Non-Hispanic Blacks as well as Other/Mixed Race children. This finding is another piece of evidence suggesting that MDRs are not specific to Blacks but can also happen in other racial and ethnic groups that are marginalized. Although most MDRs are shown for Non-Hispanic Black [19] families, similar results have been shown for Latino [31], Asian American [37], Native American [77], LGBT [78,79], immigrant [80,81], and marginalized Non-Hispanic White [17] families. This systemic nature of MDRs suggests that it is the society and not a specific social group that is to blame. In other term, it is white privilege that strengthens the salience of individual-level risk and protective factors. Potential processes that penalize the marginalized groups include social stratification, societal injustice, and contextual factors such as neighborhood poverty and school segregation, all contributing to MDRs.
Finally, we found MDRs for right but not left putamen functional connectivity. Interestingly, past research has suggested that food reward is predominantly processed in right but not left putamen and other parts of the basal ganglia. Some research suggests that money, erotic, and food rewards have different lateralizations. Our finding is in support of relevance of right but not left putamen functional connectivity to children’s BMI.
5. Limitations
This study only described the MDRs without exploring the underlying mechanisms behind them. A wide range of potential societal processes may cause MDRs. It is still unknown if these MDRs can be seen across all neighborhoods or require specific social and physical contexts to emerge. Residential and school segregation, neighborhood SES and crime, and environmental toxins may contribute to the observed MDRs of SES for marginalized families. There is a need to study how parents can interfere with the emergence of these MDRs in Non-Hispanic Black children. Future research may quantify how segregation, discrimination, and school quality reduce the effects of known risk factors for Non-Hispanic Black children. Finally, there is a need to study variations across geographic places. Such research may suggest public policies that can mitigate such MDRs.
6. Conclusions
The association between right but not left putamen’s functional connectivity to the salience network and children’s BMI depends on race and marital status but not household income and parental education. We found that putamen’s functional connectivity to the salience network is a weaker predictor of BMI for Non-Hispanic Black and Other/Mixed Race children than Non-Hispanic White children. That is, while right putamen functional connectivity to the salience network is related to children’s BMI for Non-Hispanic White children, this association is diminished for some of the Non-White racial groups. Future research should investigate the role of obesogenic environment, stress, and other factors that may keep racial minority children at risk of high BMI, regardless of putamen functional connectivity to the salience network. Residential segregation, neighborhood disorder, fast food availability, family structure, and family’s food environment may explain the weaker association of putamen functional connectivity and BMI for Non-Hispanic Black and Other/Mixed Race children.
Author Contributions
Conceptualization, formal analysis, writing—original draft preparation, writing—review, and editing: S.A. Conceptualization, writing—review, and editing: S.B. All authors have read and agreed to the published version of the manuscript.
Funding
Shervin Assari is supported by the grants with the numbers DA035811-05, U54MD007598, U54MD008149, D084526-03, and U54CA229974 by the National Institutes of Health (NIH).
Institutional Review Board Statement
Our secondary analysis was found by the Charles R Drew University of Medicine and Science (CDU) Institutional Review Board (IRB) to be exempt from a full IRB review (IRB number 1665000-1; Date: 10 February 2020). However, the original ABCD study underwent an Institutional Review Board (IRB) in several institutions, including but not limited to the University of California, San Diego (UCSD).
Informed Consent Statement
Parents reported consent and youth provided assent.
Data Availability Statement
ABCD data are available at https://nda.nih.gov/abcd (accessed on 1 March 2021) and can be accessed after NIH approval. More information about how to access ABCD data please see https://abcdstudy.org/ (accessed on 1 March 2021) and https://nda.nih.gov/abcd (accessed on 1 March 2021).
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org (accessed on 1 March 2021)), held in the National Institute of Mental Health (NIMH) Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators (accessed on 1 March 2021). A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html (accessed on 1 March 2021). ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health (NIH) or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from [NIMH Data Archive Digital Object Identifier (http://dx.doi.org/10.15154/1504041) (accessed on 1 March 2021)]. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The author wishes to thank Gavin Wells for his edits to this paper.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A. Distribution of Variables and Model Assumptions
Figure A1.
Distribution of our predictor.
Figure A2.
Distribution of our outcome.
Figure A3.
Quantiles distribution.
Figure A4.
Model residuals (error terms).
References
- Wang, Z.; Yan, X.; Liu, Y.; Spray, G.J.; Deng, Y.; Cao, F. Structural and functional abnormality of the putamen in children with developmental dyslexia. Neuropsychologia 2019, 130, 26–37. [Google Scholar] [CrossRef]
- Aceves, J.; Cuello, A.C. Dopamine release induced by electrical stimulation of microdissected caudate-putamen and substantia nigra of the rat brain. Neuroscience 1981, 6, 2069–2075. [Google Scholar] [CrossRef]
- Hu, X.T.; Wachtel, S.R.; Galloway, M.P.; White, F.J. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J. Neurosci. 1990, 10, 2318–2329. [Google Scholar] [CrossRef]
- York, D.H. Electrophysiology of the nigro-putamen dopamine pathway. Pharmacol. Ther. B 1976, 2, 1–7. [Google Scholar] [CrossRef]
- Joyce, J.N.; Loeschen, S.K.; Sapp, D.W.; Marshall, J.F. Age-related regional loss of caudate-putamen dopamine receptors revealed by quantitative autoradiography. Brain Res. 1986, 378, 158–163. [Google Scholar] [CrossRef]
- Jacobsen, L.K.; Giedd, J.N.; Gottschalk, C.; Kosten, T.R.; Krystal, J.H. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am. J. Psychiatry 2001, 158, 486–489. [Google Scholar] [CrossRef]
- Akkermans, S.E.A.; Luijten, M.; van Rooij, D.; Franken, I.H.A.; Buitelaar, J.K. Putamen functional connectivity during inhibitory control in smokers and non-smokers. Addict. Biol. 2018, 23, 359–368. [Google Scholar] [CrossRef]
- Bahk, J.Y.; Li, S.; Park, M.S.; Kim, M.O. Dopamine D1 and D2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 1095–1104. [Google Scholar] [CrossRef]
- Naha, N.; Li, S.P.; Yang, B.C.; Park, T.J.; Kim, M.O. Time-dependent exposure of nicotine and smoke modulate ultrasubcellular organelle localization of dopamine D1 and D2 receptors in the rat caudate-putamen. Synapse 2009, 63, 847–854. [Google Scholar] [CrossRef]
- Izenwasser, S.; Newman, A.H.; Katz, J.L. Cocaine and several sigma receptor ligands inhibit dopamine uptake in rat caudate-putamen. Eur. J. Pharmacol. 1993, 243, 201–205. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Chen, Z.; Xie, M.; Huang, L.; Xue, J.; Liu, Y.; Liu, N.; Guo, F.; Zheng, Y.; et al. Cocaine activates Rac1 to control structural and behavioral plasticity in caudate putamen. Neurobiol. Dis. 2015, 75, 159–176. [Google Scholar] [CrossRef]
- Werme, M.; Thoren, P.; Olson, L.; Brene, S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur. J. Neurosci. 2000, 12, 2967–2974. [Google Scholar] [CrossRef]
- Budygin, E.A.; Oleson, E.B.; Mathews, T.A.; Lack, A.K.; Diaz, M.R.; McCool, B.A.; Jones, S.R. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology 2007, 193, 495–501. [Google Scholar] [CrossRef]
- Cuzon Carlson, V.C.; Seabold, G.K.; Helms, C.M.; Garg, N.; Odagiri, M.; Rau, A.R.; Daunais, J.; Alvarez, V.A.; Lovinger, D.M.; Grant, K.A. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 2011, 36, 2513–2528. [Google Scholar] [CrossRef]
- Freund, G.; Ballinger, W.E., Jr. Neuroreceptor changes in the putamen of alcohol abusers. Alcohol. Clin. Exp. Res. 1989, 13, 213–218. [Google Scholar] [CrossRef]
- Wiers, C.E.; Shokri-Kojori, E.; Cabrera, E.; Cunningham, S.; Wong, C.; Tomasi, D.; Wang, G.-J.; Volkow, N.D. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci. Lett. 2016, 617, 27–31. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Caldwell, C.H.; Zimmerman, M.A. Place-Based Diminished Returns of Parental Educational Attainment on School Performance of Non-Hispanic White Youth. Front. Educ. 2020, 5. [Google Scholar] [CrossRef]
- Assari, S. Unequal Gain of Equal Resources across Racial Groups. Int. J. Health Policy Manag. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Assari, S. Health Disparities due to Diminished Return among Black Americans: Public Policy Solutions. Soc. Issues Policy Rev. 2018, 12, 112–145. [Google Scholar] [CrossRef]
- Akhlaghipour, G.; Assari, S. Parental Education, Household Income, Race, and Children’s Working Memory: Complexity of the Effects. Brain Sci. 2020, 10, 950. [Google Scholar] [CrossRef]
- Assari, S. Parental Education and Youth Inhibitory Control in the Adolescent Brain Cognitive Development (ABCD) Study: Blacks’ Diminished Returns. Brain Sci. 2020, 10, 312. [Google Scholar] [CrossRef]
- Assari, S. Youth Social, Emotional, and Behavioral Problems in the ABCD Study: Minorities’ Diminished Returns of Family Income. J. Econ. Public Financ. 2020, 6, 1–19. [Google Scholar] [CrossRef]
- Assari, S. Dimensional Change Card Sorting of American Children: Marginalization-Related Diminished Returns of Age. Child. Teenagers 2020, 3, 72–92. [Google Scholar] [CrossRef]
- Assari, S. Parental Education, Household Income, and Cortical Surface Area among 9-10 Years Old Children: Minorities’ Diminished Returns. Brain Sci. 2020, 10, 956. [Google Scholar] [CrossRef]
- Assari, S.; Akhlaghipour, G. Not Race or Age but Their Interaction Predicts Pre-Adolescents’ Inhibitory Control. Child. Teenagers 2020, 3, 50–71. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Akhlaghipour, G.; Bazargan, M.; Caldwell, C.H. Reward Responsiveness in the Adolescent Brain Cognitive Development (ABCD) Study: African Americans’ Diminished Returns of Parental Education. Brain Sci. 2020, 10, 391. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Caldwell, C.H. African Americans’ Diminished Returns of Parental Education on Adolescents’ Depression and Suicide in the Adolescent Brain Cognitive Development (ABCD) Study. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Family Socioeconomic Status and Exposure to Childhood Trauma: Racial Differences. Children 2020, 7, 57. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H. Family Income at Birth and Risk of Attention Deficit Hyperactivity Disorder at Age 15: Racial Differences. Children 2019, 6, 10. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H. High Risk of Depression in High-Income African American Boys. J. Racial Ethn. Health Disparities 2018, 5, 808–819. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Bazargan, M. Association Between Parental Educational Attainment and Youth Outcomes and Role of Race/Ethnicity. JAMA Netw. Open 2019, 2, e1916018. [Google Scholar] [CrossRef]
- Assari, S.; Mistry, R.; Bazargan, M. Race, Educational Attainment, and E-Cigarette Use. J. Med. Res. Innov. 2020, 4, e000185. [Google Scholar] [CrossRef]
- Assari, S.; Mistry, R.; Caldwell, C.H.; Bazargan, M. Protective Effects of Parental Education Against Youth Cigarette Smoking: Diminished Returns of Blacks and Hispanics. Adolesc. Health Med. Ther. 2020, 11, 63–71. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Mincy, R. Family Socioeconomic Status at Birth and Youth Impulsivity at Age 15; Blacks’ Diminished Return. Children 2018, 5, 58. [Google Scholar] [CrossRef]
- Assari, S. Family Socioeconomic Position at Birth and School Bonding at Age 15; Blacks’ Diminished Returns. Behav. Sci. 2019, 9, 26. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Caldwell, C.H. Diminished Returns of Parental Education in Terms of Youth School Performance: Ruling out Regression toward the Mean. Children 2020, 7, 74. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Caldwell, C.H. Mathematical Performance of American Youth: Diminished Returns of Educational Attainment of Asian-American Parents. Educ. Sci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M. Subjective Family Socioeconomic Status and Adolescents’ Attention: Blacks’ Diminished Returns. Children 2020, 7, 80. [Google Scholar] [CrossRef]
- Assari, S.; Islam, S. Diminished Protective Effects of Household Income on Internalizing Symptoms among African American than European American Pre-Adolescents. J. Econ. Trade Mark. Manag. 2020, 2, 38–56. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Caldwell, C.H. Race, Socioeconomic Status, and Sex Hormones among Male and Female American Adolescents. Reprod. Med. 2020, 1, 108–121. [Google Scholar] [CrossRef]
- Assari, S. Parental Education and Spanking of American Children: Blacks’ Diminished Returns. World J. Educ. Res. 2020, 7, 19–44. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Mincy, R.B. Maternal Educational Attainment at Birth Promotes Future Self-Rated Health of White but Not Black Youth: A 15-Year Cohort of a National Sample. J. Clin. Med. 2018, 7, 93. [Google Scholar] [CrossRef]
- Assari, S.; Mardani, A.; Maleki, M.; Bazargan, M. Black-White Differences in the Association between Maternal Age at Childbirth and Income. Womens Health Bull. 2019, 6, 36–42. [Google Scholar]
- Assari, S.; Thomas, A.; Caldwell, C.H.; Mincy, R.B. Blacks’ Diminished Health Return of Family Structure and Socioeconomic Status; 15 Years of Follow-up of a National Urban Sample of Youth. J. Urban Health 2018, 95, 21–35. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Zimmerman, M.A. Family Structure and Subsequent Anxiety Symptoms; Minorities’ Diminished Return. Brain Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Boyce, S.; Bazargan, M.; Caldwell, C.H.; Zimmerman, M.A.; Assari, S. Parental Educational Attainment and Social Environment of Urban Public Schools in the U.S.: Blacks’ Diminished Returns. Children 2020, 7, 44. [Google Scholar] [CrossRef]
- Assari, S.; Gibbons, F.X.; Simons, R. Depression among Black Youth; Interaction of Class and Place. Brain Sci. 2018, 8, 108. [Google Scholar] [CrossRef]
- Assari, S.; Gibbons, F.X.; Simons, R.L. Perceived Discrimination among Black Youth: An 18-Year Longitudinal Study. Behav. Sci. 2018, 8, 44. [Google Scholar] [CrossRef]
- Assari, S.; Akhlaghipour, G.; Boyce, S.; Bazargan, M.; Caldwell, C.H. African American Children’s Diminished Returns of Subjective Family Socioeconomic Status on Fun Seeking. Children 2020, 7, 75. [Google Scholar] [CrossRef]
- Assari, S.; Bazargan, M.; Chalian, M. The Unequal Effect of Income on Risk of Overweight/Obesity of Whites and Blacks with Knee Osteoarthritis: The Osteoarthritis Initiative. J. Racial Ethn. Health Disparities 2020, 7, 776–784. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M.; Mincy, R.; Caldwell, C.H. Unequal Protective Effects of Parental Educational Attainment on the Body Mass Index of Black and White Youth. Int. J. Environ. Res. Public Health 2019, 16, 3641. [Google Scholar] [CrossRef]
- Assari, S. Family Income Reduces Risk of Obesity for White but Not Black Children. Children 2018, 5, 73. [Google Scholar] [CrossRef]
- Williams, D.R. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann. N. Y. Acad. Sci. 1999, 896, 173–188. [Google Scholar] [CrossRef]
- Dorton, H.M.; Luo, S.; Monterosso, J.R.; Page, K.A. Influences of Dietary Added Sugar Consumption on Striatal Food-Cue Reactivity and Postprandial GLP-1 Response. Front. Psychiatry 2017, 8, 297. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Veit, R.; Ketterer, C.; Schick, F.; Haring, H.U.; Fritsche, A.; Preissl, H. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 2012, 33, 1052–1061. [Google Scholar] [CrossRef]
- Murdaugh, D.L.; Cox, J.E.; Cook, E.W., 3rd; Weller, R.E. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 2012, 59, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Li, W.; Wen, Q.; Liu, F.; Xu, J.; Xu, Q.; Zhu, D.; Ye, Z.; Yu, C. Right Posterior Insula and Putamen Volume Mediate the Effect of Oxytocin Receptor Polygenic Risk for Autism Spectrum Disorders on Reward Dependence in Healthy Adults. Cereb. Cortex 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dong, H.; Zheng, H.; Du, X.; Dong, G.H. Inhibitory neuromodulation of the putamen to the prefrontal cortex in Internet gaming disorder: How addiction impairs executive control. J. Behav. Addict. 2020, 9, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Aotani, D.; Ebihara, K.; Sawamoto, N.; Kusakabe, T.; Aizawa-Abe, M.; Kataoka, S.; Sakai, T.; Iogawa, H.; Ebihara, C.; Fujikura, J.; et al. Functional magnetic resonance imaging analysis of food-related brain activity in patients with lipodystrophy undergoing leptin replacement therapy. J. Clin. Endocrinol. Metab. 2012, 97, 3663–3671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borowitz, M.A.; Yokum, S.; Duval, E.R.; Gearhardt, A.N. Weight-Related Differences in Salience, Default Mode, and Executive Function Network Connectivity in Adolescents. Obesity 2020, 28, 1438–1446. [Google Scholar] [CrossRef]
- Dekkers, I.A.; Jansen, P.R.; Lamb, H.J. Obesity, Brain Volume, and White Matter Microstructure at MRI: A Cross-sectional UK Biobank Study. Radiology 2019, 291, 763–771. [Google Scholar] [CrossRef]
- Gupta, A.; Mayer, E.A.; Labus, J.S.; Bhatt, R.R.; Ju, T.; Love, A.; Bal, A.; Tillisch, K.; Naliboff, B.; Sanmiguel, C.P.; et al. Sex Commonalities and Differences in Obesity-Related Alterations in Intrinsic Brain Activity and Connectivity. Obesity 2018, 26, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Woods, C.; Zhen, J.; Antonio, T.; Carr, K.D.; Reith, M.E. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J. Neurochem. 2017, 140, 728–740. [Google Scholar] [CrossRef]
- Garavan, H.; Bartsch, H.; Conway, K.; Decastro, A.; Goldstein, R.Z.; Heeringa, S.; Jernigan, T.; Potter, A.; Thompson, W.; Zahs, D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Auchter, A.M.; Hernandez Mejia, M.; Heyser, C.J.; Shilling, P.D.; Jernigan, T.L.; Brown, S.A.; Tapert, S.F.; Dowling, G.J. A description of the ABCD organizational structure and communication framework. Dev. Cogn. Neurosci. 2018, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Feldstein Ewing, S.W.; Bjork, J.M.; Luciana, M. Implications of the ABCD study for developmental neuroscience. Dev. Cogn. Neurosci. 2018, 32, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Karcher, N.R.; Barch, D.M. The ABCD study: Understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology 2020. [Google Scholar] [CrossRef]
- Bjork, J.M.; Straub, L.K.; Provost, R.G.; Neale, M.C. The ABCD study of neurodevelopment: Identifying neurocircuit targets for prevention and treatment of adolescent substance abuse. Curr. Treat. Options Psychiatry 2017, 4, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H.; et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Koller, J.M.; Earl, E.A.; Miranda-Dominguez, O.; Klein, R.L.; Van, A.N.; Snyder, A.Z.; Nagel, B.J.; Nigg, J.T.; Nguyen, A.L. Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage 2017, 161, 80–93. [Google Scholar] [CrossRef]
- Kind, A.J.; Jencks, S.; Brock, J.; Yu, M.; Bartels, C.; Ehlenbach, W.; Greenberg, C.; Smith, M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann. Intern. Med. 2014, 161, 765–774. [Google Scholar] [CrossRef]
- Chan, P.S.; McNally, B.; Vellano, K.; Tang, Y.; Spertus, J.A. Association of Neighborhood Race and Income With Survival After Out-of-Hospital Cardiac Arrest. J. Am. Heart Assoc. 2020, 9, e014178. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Killian, J.M.; Therneau, T.M.; Jacobsen, S.J.; Roger, V.L. Neighborhood income and individual education: Effect on survival after myocardial infarction. Mayo Clin. Proc. 2008, 83, 663–669. [Google Scholar] [CrossRef]
- Galea, S.; Ahern, J.; Tracy, M.; Vlahov, D. Neighborhood income and income distribution and the use of cigarettes, alcohol, and marijuana. Am. J. Prev. Med. 2007, 32, S195–S202. [Google Scholar] [CrossRef]
- Kobetz, E.; Daniel, M.; Earp, J.A. Neighborhood poverty and self-reported health among low-income, rural women, 50 years and older. Health Place 2003, 9, 263–271. [Google Scholar] [CrossRef]
- Pickett, K.E.; Ahern, J.E.; Selvin, S.; Abrams, B. Neighborhood socioeconomic status, maternal race and preterm delivery: A case-control study. Ann. Epidemiol. 2002, 12, 410–418. [Google Scholar] [CrossRef]
- Assari, S.; Bazargan, M. Protective Effects of Educational Attainment Against Cigarette Smoking; Diminished Returns of American Indians and Alaska Natives in the National Health Interview Survey. Int. J. Travel Med. Glob. Health 2019, 7, 105. [Google Scholar] [CrossRef]
- Assari, S.; Bazargan, M. Education Level and Cigarette Smoking: Diminished Returns of Lesbian, Gay and Bisexual Individuals. Behav. Sci. 2019, 9, 103. [Google Scholar] [CrossRef]
- Assari, S. Education Attainment and ObesityDifferential Returns Based on Sexual Orientation. Behav. Sci. 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Income and Mental Well-Being of Middle-Aged and Older Americans: Immigrants’ Diminished Returns. Int. J. Travel Med. Glob. Health 2020, 8, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Socioeconomic Status and Current Cigarette Smoking Status: Immigrants’ Diminished Returns. Int. J. Travel Med. Glob. Health 2020, 8, 66–72. [Google Scholar] [CrossRef]
- Assari, S.; Akhlaghipour, G.; Boyce, S.; Bazargan, M.; Caldwell, C.H. Parental Human Capital and Adolescents’ Executive Function: Immigrants’ Diminished Returns. Med. Res. Arch. 2020, 8. [Google Scholar] [CrossRef]
- Assari, S.; Boyce, S.; Bazargan, M. Subjective Socioeconomic Status and Children’s Amygdala Volume: Minorities’ Diminish Returns. NeuroSci 2020, 1, 59–74. [Google Scholar] [CrossRef]
- Assari, S.; Akhlaghipour, G.; Saqib, M.; Boyce, S.; Bazargan, M. Prefrontal Cortex Response to Threat: Race by Age Variation in 9–10 Year Old Children. J. Ment. Health Clin. Psychol. 2020, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Social Determinants of Depression: The Intersections of Race, Gender, and Socioeconomic Status. Brain Sci. 2017, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Lankarani, M. Educational Attainment Promotes Fruit and Vegetable Intake for Whites but Not Blacks. J. Multidiscip. Sci. J. 2018, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. American Children’s Screen Time: Diminished Returns of Household Income in Black Families. Information 2020, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Educational Attainment and Exercise Frequency in American Women; Blacks’ Diminished Returns. Womens Health Bull. 2019, 6, e87413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).