The Role of Apparent Diffusion Coefficient in the Differentiation between Cerebellar Medulloblastoma and Brainstem Glioma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anesthesia Procedure
2.3. MRI Procedure
2.4. Variables
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jallo, G.I.; Biser-Rohrbaugh, A.; Freed, D. Brainstem gliomas. Childs Nerv. Syst. 2004, 20, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2015, 16 (Suppl. S10), x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.S.; Jackler, R.K. Exophytic brain tumors mimicking primary lesions of the cerebellopontine angle. Laryngoscope 1997, 107, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, J.; Nishiyama, K.; Fukuda, M.; Watanabe, M.; Igarashi, H.; Fujii, Y. Adult cerebellopontine angle medulloblastoma originating in the pons mimicking focal brainstem tumor. J. Neuroimaging 2009, 19, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg. Rev. 2018, 41, 719–731. [Google Scholar] [CrossRef]
- Gits, H.C.; Anderson, M.; Stallard, S.; Pratt, D.; Zon, B.; Howell, C.; Kumar-Sinha, C.; Vats, P.; Kasaian, K.; Polan, D.; et al. Medulloblastoma therapy generates risk of a poorly-prognostic H3 wild-type subgroup of diffuse intrinsic pontine glioma: A report from the International DIPG Registry. Acta Neuropathol. Commun. 2018, 6, 67. [Google Scholar] [CrossRef]
- Schumacher, M.; Schulte-Mönting, J.; Stoeter, P.; Warmuth-Metz, M.; Solymosi, L. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: A multicenter review. J. Neurosurg. 2007, 106, 111–119. [Google Scholar] [CrossRef]
- Albright, A.L. Diffuse brainstem tumors: When is a biopsy necessary? Pediatr. Neurosurg. 1996, 24, 252–255. [Google Scholar] [CrossRef]
- Rumboldt, Z.; Camacho, D.L.; Lake, D.; Welsh, C.T.; Castillo, M. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am. J. Neuroradiol. 2006, 27, 1362–1369. [Google Scholar]
- Jaremko, J.L.; Jans, L.B.; Coleman, L.T.; Ditchfield, M.R. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am. J. Neuroradiol. 2010, 31, 1613–1616. [Google Scholar] [CrossRef]
- D’Arco, F.; Khan, F.; Mankad, K.; Ganau, M.; Caro-Dominguez, P.; Bisdas, S. Differential diagnosis of posterior fossa tumours in children: New insights. Pediatr. Radiol. 2018, 48, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.F.; Azeem Ismail, A.A.; Hasan, D.I.; Essa, W.E. The role of apparent diffusion coefficient (ADC) value in the differentiation between the most common pediatric posterior fossa tumors. Egypt. J. Radiol. Nucl. Med. 2013, 44, 349–355. [Google Scholar] [CrossRef][Green Version]
- Poretti, A.; Meoded, A.; Huisman, T.A. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J. Magn. Reson. Imaging 2012, 35, 32–47. [Google Scholar] [CrossRef]
- Koeller, K.K.; Rushing, E.J. From the archives of the AFIP: Medulloblastoma: A comprehensive review with radiologic-pathologic correlation. Radiographics 2003, 23, 1613–1637. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.P.; Kemp, S.S.; Tarr, R.W. MR imaging features of medulloblastomas. AJR Am. J. Roentgenol. 1992, 158, 859–865. [Google Scholar] [CrossRef]
- Pierce, T.T.; Provenzale, J.M. Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol. J. 2014, 27, 63–74. [Google Scholar] [CrossRef]
- Chen, H.J.; Panigrahy, A.; Dhall, G.; Finlay, J.L.; Nelson, M.D., Jr.; Blüml, S. Apparent diffusion and fractional anisotropy of diffuse intrinsic brain stem gliomas. AJNR Am. J. Neuroradiol. 2010, 31, 1879–1885. [Google Scholar] [CrossRef]
- Lober, R.M.; Cho, Y.J.; Tang, Y.; Barnes, P.D.; Edwards, M.S.; Vogel, H.; Fisher, P.G.; Monje, M.; Yeom, K.W. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J. Neurooncol. 2014, 117, 175–182. [Google Scholar] [CrossRef]
- Duc, N.M.; Huy, H.Q.; Bang, M.T.L.; Truong, L.M.; Tri, V.H.; Canh, B.N.; Hoa, P.N.; Thong, P.M. Clinical applications of diffusion-weighted magnetic resonance imaging. Imaging Med. 2018, 10, 79–84. [Google Scholar]
- Duc, N.M.; Huy, H.Q. Magnetic resonance imaging features of common posterior fossa brain tumors in children: A preliminary Vietnamese study. Open Access Maced. J. Med. Sci. 2019, 7, 2413–2418. [Google Scholar] [CrossRef]
- Duc, N.M.; Huy, H.Q.; Nadarajan, C.; Keserci, B. The Role of Predictive Model Based on Quantitative Basic Magnetic Resonance Imaging in Differentiating Medulloblastoma from Ependymoma. Anticancer Res. 2020, 40, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.M. The role of diffusion tensor imaging metrics in the discrimination between cerebellar medulloblastoma and brainstem glioma. Pediatr. Blood Cancer 2020, 67, e28468. [Google Scholar] [CrossRef]
- Duc, N.M. The effect of semi-quantitative T1-perfusion parameters for the differentiation between pediatric medulloblastoma and ependymoma. Egypt. J. Radiol. Nucl. Med. 2020, 51, 1–6. [Google Scholar] [CrossRef]
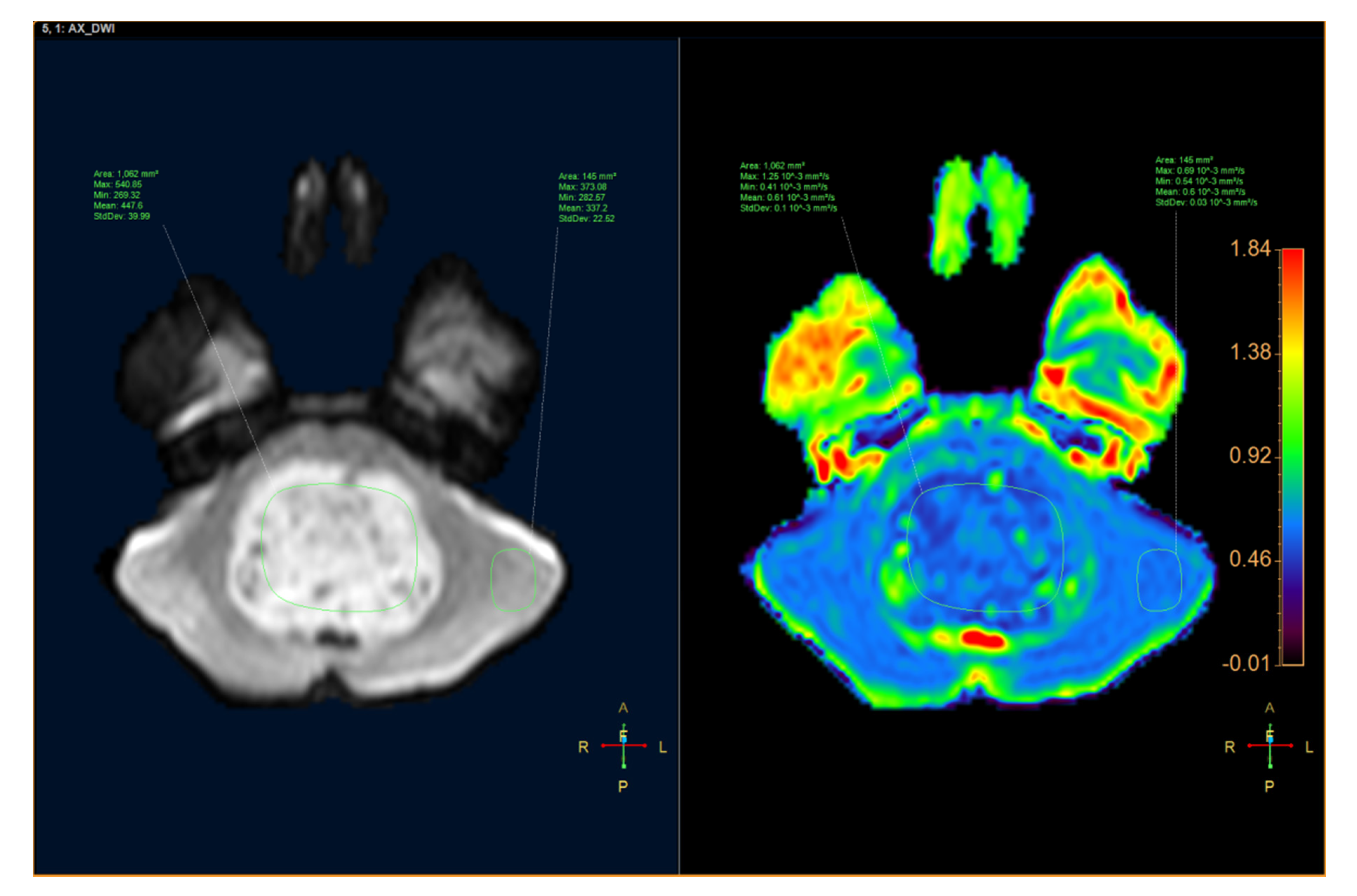
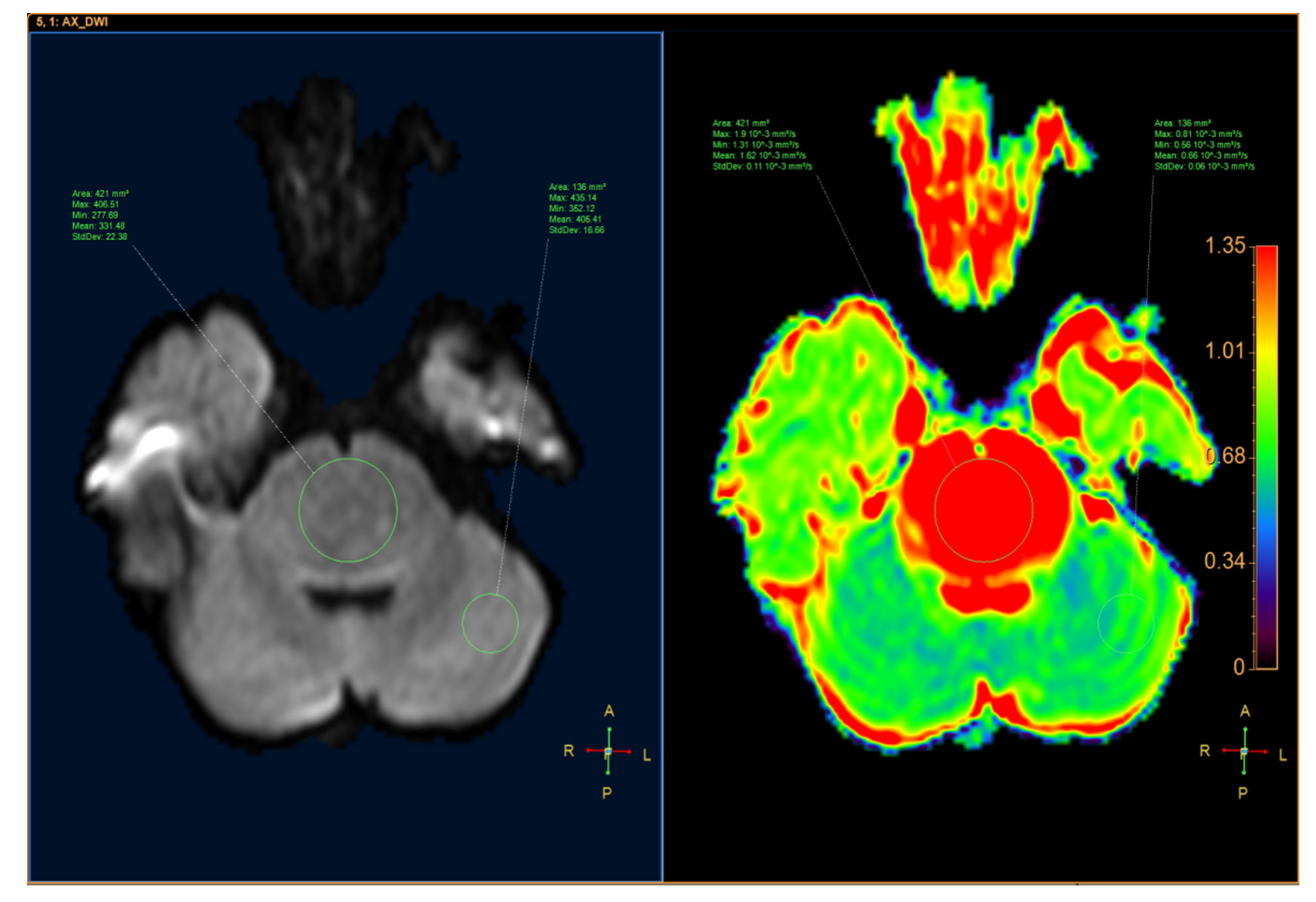
| Medulloblastoma n = 22 | Brainstem Glioma n = 10 | p | |
|---|---|---|---|
| ADC | |||
| ADCmax (10−3 mm2/s) | 1.06 (0.58) | 1.64 (0.45) | 0.003 § |
| ADCmin (10−3 mm2/s) | 0.43 (0.11) | 1.11 (0.34) | <0.001 § |
| ADCmean (10−3 mm2/s) | 0.62 (0.19) | 1.39 (0.33) | <0.001 § |
| ADCsd | 0.11 (0.06) | 0.10 (0.05) | 0.967 |
| ADC Ratio | |||
| rADCmax | 1.61 (0.87) | 1.96 (0.46) | 0.067 |
| rADCmin | 0.80 (0.17) | 1.87 (0.61) | <0.001 § |
| rADCmean | 1.01 (0.23) | 2.05 (0.51) | <0.001 § |
| rADCsd | 3.87 (2.88) | 2.25 (2.50) | 0.185 |
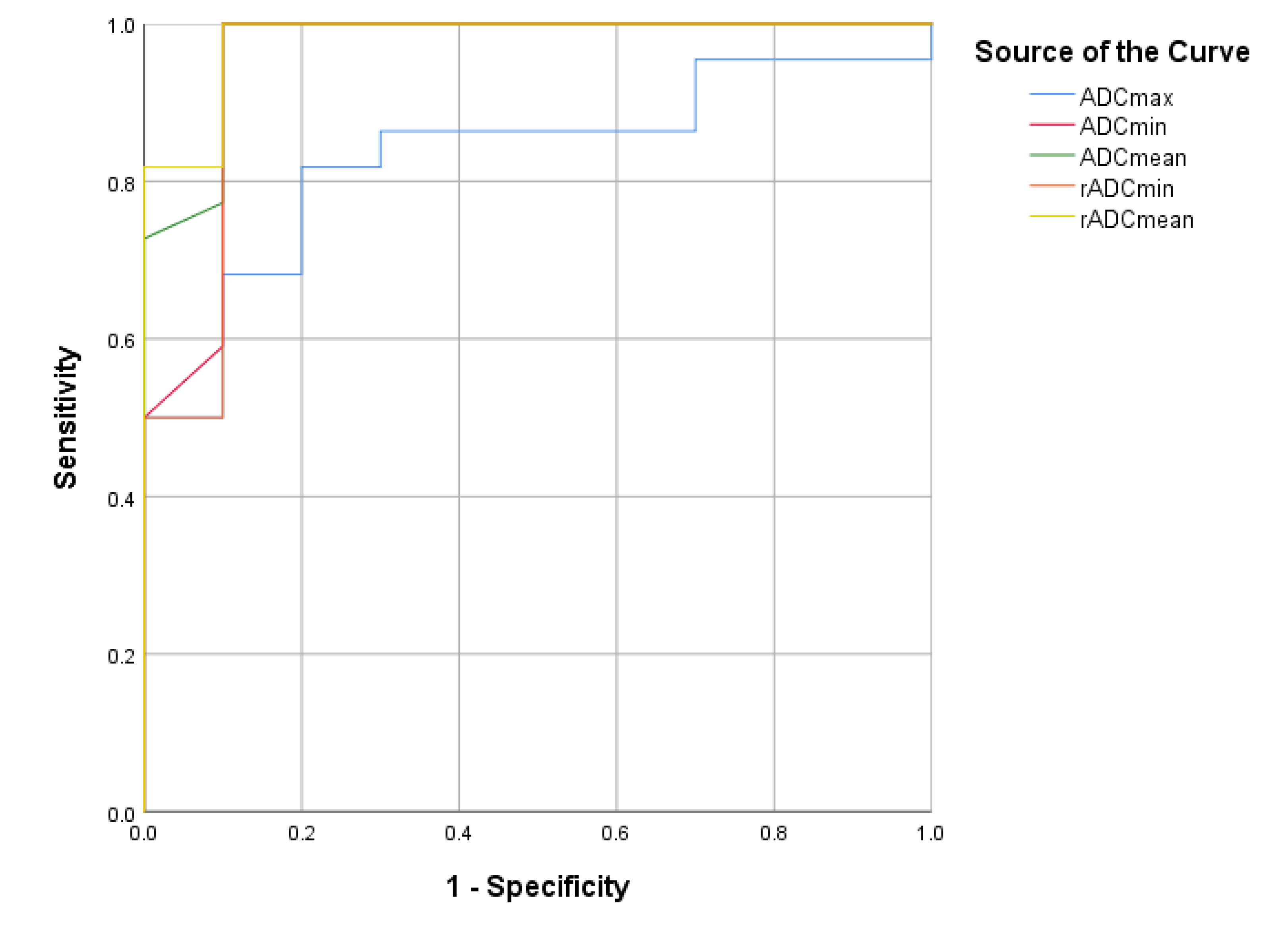
| Cut-Off Point | AUC | Sensitivity | Specificity | 95% CI | |
|---|---|---|---|---|---|
| ADC | |||||
| ADCmax | 1.45 | 0.832 | 0.818 | 0.8 | 0.688–0.975 |
| ADCmin | 0.68 | 0.955 | 1 | 0.9 | 0.865–1.000 |
| ADCmean | 0.95 | 0.975 | 1 | 0.9 | 0.922–1.000 |
| ADC Ratio | |||||
| rADCmin | 1.25 | 0.950 | 1 | 0.9 | 0.853–1.000 |
| rADCmean | 1.47 | 0.982 | 1 | 0.9 | 0.941–1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minh Thong, P.; Minh Duc, N. The Role of Apparent Diffusion Coefficient in the Differentiation between Cerebellar Medulloblastoma and Brainstem Glioma. Neurol. Int. 2020, 12, 34-40. https://doi.org/10.3390/neurolint12030009
Minh Thong P, Minh Duc N. The Role of Apparent Diffusion Coefficient in the Differentiation between Cerebellar Medulloblastoma and Brainstem Glioma. Neurology International. 2020; 12(3):34-40. https://doi.org/10.3390/neurolint12030009
Chicago/Turabian StyleMinh Thong, Pham, and Nguyen Minh Duc. 2020. "The Role of Apparent Diffusion Coefficient in the Differentiation between Cerebellar Medulloblastoma and Brainstem Glioma" Neurology International 12, no. 3: 34-40. https://doi.org/10.3390/neurolint12030009
APA StyleMinh Thong, P., & Minh Duc, N. (2020). The Role of Apparent Diffusion Coefficient in the Differentiation between Cerebellar Medulloblastoma and Brainstem Glioma. Neurology International, 12(3), 34-40. https://doi.org/10.3390/neurolint12030009






