Abstract
Ventricular arrhythmias are a common disorder, and sometimes the etiology remains unclear. Present data support cardiac fatty tissue’s potential role as a substrate for ventricular arrhythmias. Diagnosing fatty tissue based on imaging markers and histopathological evidence is often challenging. Data about the influence of individual and multiple genetic variants on epicardial adipose tissue volume remain limited. In this review, we aimed to provide a comprehensive overview of the current understanding of the genetic basis of fatty tissue and its contribution to the pathogenesis of ventricular arrhythmias and to discuss the relationship between certain genetic variants and the development of ventricular arrhythmia.
1. Introduction
Strong data suggest that obesity-related comorbidities contribute to the arrhythmogenic substrates. Lately, there has been an increased interest in evaluating the impact of excessive adipose tissue on arrhythmia occurrence, in the absence of other risk factors [1]. Body mass index (BMI) is an established marker for quantifying obesity and the relationship between weight and cardiovascular diseases (CVDs). Also, there is significant interest in evaluating the impact of adipose deposits and CVD [2].
Epicardial adipose tissue (EAT), as presented in Figure 1, represents a type of visceral fat located between the myocardial surface and the visceral layer of the pericardium. Because of i common embryologic origin with visceral fat deposits [3], EAT is often confused with pericardial fat. Data obtained from genome-wide association studies show various single-nucleotide polymorphisms (SNPs) involved in biological processes such as oxidation, diabetes/obesity, the reninangiotensin system, and dyslipidemia, but associated with certain phenotypes, cardiovascular risk, and CVD [4]. Several polygenic risk scores have been created to evaluate the burden of these genetic variants as well as individual susceptibility [5].
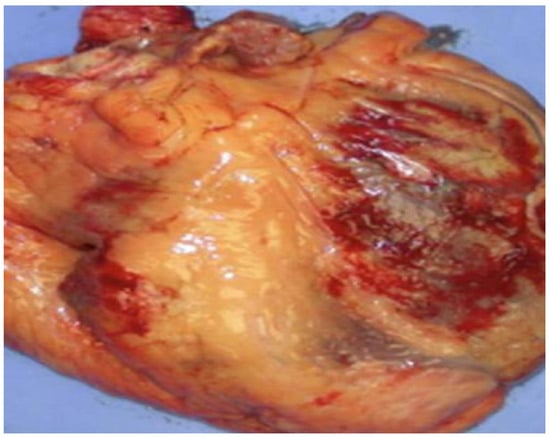
Figure 1.
Image of an explanted human heart (anterior view) with the epicardial adipose tissue colored in yellow. EAT is visceral fat localized between the myocardial surface and the visceral layer of the pericardium, most often observed on both atria, the free wall of the right ventricle and the left ventricle’s apex. EAT should be considered a biologically active structure connecting obesity and cardiovascular diseases, as well as a risk factor for the atherosclerotic process. EAT exhibits a heterogeneous distribution throughout the heart. Every EAT deposit presents a different transcriptome and proteome, leading to different effects on the adjacent heart structures.
Even though the fatty tissue disposed around the cardiac vessels seems to play a supportive action by reducing vascular tension and torsion [6], it is also responsible for releasing several cytokines and hormones into circulation, which play an important role in the myocardial tissue. EAT exhibits a wide range of thermogenic and mechanical functions through the released cytokines. Next, based on these cytokines’ pro- and anti-inflammatory effects, the ventricular myocardium and coronary arteries are affected, as the myocytes are closely situated near the zones with adipocytes and peri-cellular fibrosis. Even though the adipocytes express ion channels and gap junctional channels [7], there is scarce data on adipocyte–myocyte electrical coupling.
Cardiac diseases such as arrhythmogenic right ventricular cardiomyopathy (ARVC), myotonic dystrophy, and Anderson—Fabry disease frequently present extensive adipose tissue deposits and increased EAT [8]. Patients with ischemic cardiomyopathy and left ventricular (LV) hypertrophy present a constant fatty tissue/muscle ratio, compared to the pathologies mentioned above, where the fatty tissue/muscle ratio seems to increase [9].
Epicardial adipose tissue plays a unique role in the occurrence and development of CVD, as well as cardiac arrhythmia occurrence. This review summarizes the relationship between genetics and EAT and the impact on the occurrence of ventricular arrhythmias. From our knowledge, this is the first review concerning this issue. Arrhythmias, either atrial or ventricular, are multifactorial, so establishing the exact mechanism that leads to arrhythmia requires further work.
2. Fatty Tissue
2.1. General Data on Fatty Tissue Infiltration
Adipose tissue is represented by either white fatty tissue, the predominant type, responsible for fat storage, or brown fatty tissue, involved in thermogenesis. Intramyocardial adipose tissue (IAT) is a normal finding and consists of fatty tissue stored within the cardiomyocytes, representing less than 1% of the total organ mass. Human evidence shows that intramyocardial adipose deposits are related to triglyceride storage and seem to be increased in conditions such as type 2 diabetes, obesity, or impaired glucose tolerance. IAT is mostly located in the right ventricle (RV), starting from the epicardium and extending to the endocardium. Adipose cells are usually spread among the myocardial fibers without involving processes such as fibrosis, inflammation, or cardiomyocyte replacement.
EAT represents the adipose tissue deposits located between the myocardium and the visceral layer of the pericardium [10]. EAT is considered white adipose tissue and includes mainly adipocytes, smaller than those from visceral or subcutaneous adipose deposits. EAT exhibits different anatomical and physiological properties when compared to other fat deposits, so it may offer protection for the adjacent myocardium via brown fat-like thermogenic function, or it can be harmful through para or vasocrine secretion of various cytokines involved in inflammation and fibrosis.
Visceral adipose tissue (VAT) can be found around the main coronary arteries, in the atrioventricular or interventricular grooves, and around the RV [11]. Paracardial fatty tissue is located between the pericardium layers and the external area of the parietal pericardium (Figure 2).
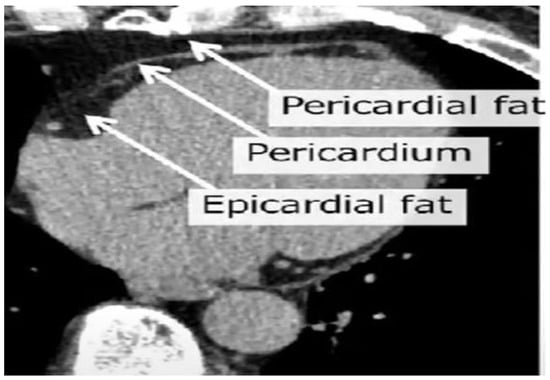
Figure 2.
Cardiac CT view highlighting the epicardial adipose tissue, localized between the myocardium and the visceral layer of the pericardium, and the pericardial adipose tissue, localized outside the parietal pericardium.
Even though pericardial and the epicardial fatty tissue evolve from brown fatty tissue as well as intra-abdominal visceral adipose tissue, they exhibit different embryological origins [12]. Paracardial fatty tissue originates in the primitive thoracic mesenchyme, while epicardial tissue originates from the splanchnopleuric mesoderm.
While white adipose tissue is involved in accumulating triglycerides in large lipid droplets and then in the hydrolyzation process when energy is needed, brown adipose tissue participates in the lipid oxidation process responsible for thermogenesis. White adipose tissue undergoes various changes in obese individuals as it increases, becomes dysfunctional, and is more prone to developing an inflammatory status. At the same time, brown adipose tissue volume and activity decline because of the conversion from brown- to white-like adipocytes, a process named “whitening” [13].
Obesity is associated with an increased arrhythmic risk. Obesity coexists with hypertension, obstructive sleep apnea, and diseases that are also pro—arrhythmogenic. At the same time, there is strong evidence linking adipose tissue volume to arrhythmias and also weight-loss to arrhythmia-free survival [14].
2.2. Pathogenesis
The distribution of cardiac adipose tissue is highly heterogeneous. Although adipose infiltration is frequently observed and it is considered a normal finding, data also show a pro-arrhythmogenic effect, as several cardiac pathologies are associated with an increase in adipose infiltration, especially at the epicardium level.
Studies have shown that 80% of the myocardial area is covered by adipose tissue and up to 20% of the cardiac weight is represented by fatty tissue [3,15]. At the level of the ventricular myocardium, the fatty deposits are located on the free wall of the RV and at the left ventricle’s (LV) apex. Data show that the amount of adipose tissue is similar for both LV and RV, although the total LV mass is superior to the RV mass [16]. The proposed cut-off for EAT thickness is 5 mm and EAT volume is 125 mL [17].
With aging, the EAT adipocytes become more prone to hemodynamic, metabolic and environmental changes, switching the EAT role of thermogenesis to energy storage [18]. Also, with aging, the proportion of EAT brown adipocytes tends to decrease in favor of white adipocytes.
Heart failure (HF) is a known cause of mortality and morbidity. Nowadays, HF with preserved ejection fraction (HFpEF) presents a significant increase in incidence and prevalence, especially in obese patients. Various studies have shown an association between HFpEF and increased EAT [19].
It is established that EAT is involved in arrhythmia mechanisms through automaticity or triggered or reentrant activity [20]. Adipocytes and other intra-myocardial cell types represent almost 70% of the cardiac cellular population. Myocardial infiltration mediated by proliferating cells leads to electrophysiological changes responsible for micro fibrosis. Myocyte remodeling may occur due to abnormal activity or crosstalk between myocytes and adjacent cells [21]. The subsequent imbalance created by the fatty tissue deposits, related to obesity and or inflammation, leads to significant remodeling of the electrical and structural properties of the myocytes. Next, changes in electrical propagation occur, either anisotropy or slowing of the electrical impulse conduction. Adipose deposits may also be responsible for an anatomical block of electrical impulse propagation, leading further to reentry-related arrhythmias.
The increase in EAT is also responsible for abnormal gene expression, myocyte activity, and nervous system activity through processes that involve cytokines, adipose infiltration, and oxidative stress (Table 1).
It is paramount to precisely locate the cardiac adipose deposits to perform further investigations into the link between adiposity andarrhythmic risk. Several receptor types, such as inflammation Toll-like receptors, play a significant role in signaling other factors, but further studies are needed.
First, EAT was presented as a marker of coronary atherosclerosis in the early 2000s. The EAT-atherosclerosis mechanisms are complex and based on inflammation, adipocyte stress, oxidative stress, endothelial damage, immunity response, lipid accumulation, and glucotoxicity [22]. The EAT surrounding the coronary arteries presents an enhanced expression of genes encoding factors regulating glucose and lipid metabolism, as well as pro-inflammatory adipokines. The coronary epicardial adipose tissue seems to be involved in the atherosclerosis process through the pro-inflammatory M1 macrophage infiltration from the EAT to the surrounding myocardium, through the release of inflammation factors such as CCL2, IL-6, and TNF, several adipokines as chemerin, resistin, intelectin 1, and serglycin, and the activation of immune response factors such as JUN N-terminal kinase (JNK), nuclear factor-κB (NF-κB) and Toll-like receptors (TLRs) [23].
The EAT from the left atrial level presents an enhanced expression of genes encoding pro-arrhythmogenic factors and participates in atrial fibrillation development by secreting profibrotic factors such as transforming growth factors like TGF -β1 and TGFβ2, pro-inflammatory cytokines such as IL-6 and tumor necrosis factor (TNF), connective tissue growth factor (cTGF), profibrotic factors such as matrix metalloproteinases (MMPs), free fatty acid infiltrations, and enhanced autonomic activity mediated through the ganglionated plexi.

Table 1.
EAT activity [24].
Table 1.
EAT activity [24].
| Properties | Actions | Diseases |
|---|---|---|
| 1. Metabolic activity [25,26] - increased lipolysis - decreased glycolysis - heat production | excess of free fatty acids myocardial protection against hypothermia | Coronary artery disease valvular diseases, diabetes metabolic syndrome |
| 2. Angiogenic factors [27] - angiogenin - endostatin - VEGF - thrombospondin-2 - angiopoietin | cell adhesion proliferation migration angiogenesis | CAD |
| 3. Growth and remodeling factors [28] - Activin A - follistatin - TGF 1, 2, 3 - MMP 1, 2, 3, 8, 9, 13 | fibrosis myocyte calcium signaling extracellular matrix remodeling | HF diabetes |
| 4. Adipocytokines [28,29,30,31,32,33] - adiponectin - leptin - resistin - visfatin - omentin - FABP4 | increased insulin sensitivity anti-inflammatory properties inflammation atherosclerosis negative inotropic effect | Obesity Metabolic syndrome |
| 5. Inflammatory cytokines and chemokines [33,34,35,36] - IL-6-1β - IL-6 and IL-7 receptor - PAI-1 - TNF-α - monocyte chemotactic protein-1 - chemokine ligands - adrenomedullin - phospholipase A2 | atherosclerosis vasodilator anti-inflammatory effects | Obesity CAD |
Monocyte chemotactic protein-1 (MCP 1), fatty acid-binding proteins (FABP4), coronary artery disease (CAD), tumor growth factor (TGF).
2.3. Adipose Tissue Diagnosis
Cardiac imaging such as echocardiography (TTE), computer tomography (CT), and magnetic resonance (MRI) are frequently used for describing cardiac fatty tissue, each one presenting advantages and disadvantages [37] (Table 2). Echocardiography allows the evaluation of adipose tissue based on thickness, is not expensive, and is widely spread. EAT is described as an echo-free and sometimes as an echo-dense space in patients with inflammation, between the myocardium and the visceral layer of the pericardium (Figure 3a). EAT is usually measured at end-systole, either in parasternal long- or short-axis view.

Table 2.
Relationship between imaging measures of cardiac adipose tissue and VA.
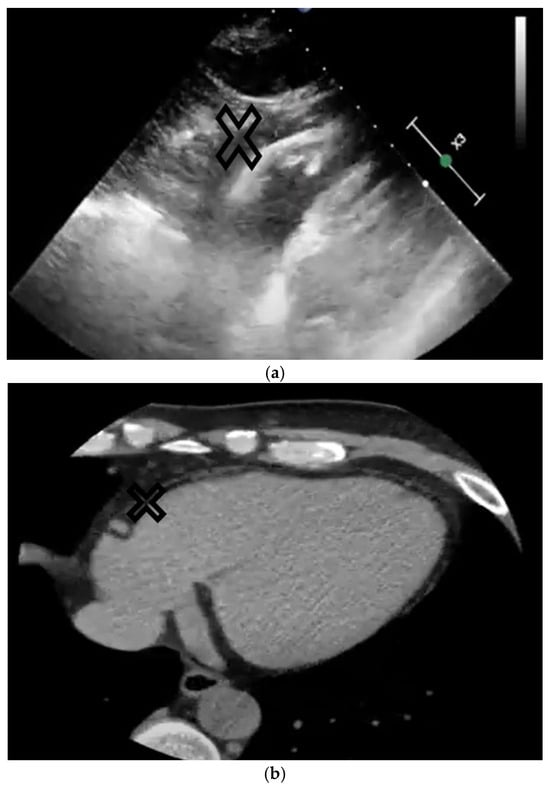
Figure 3.
The upper image (a) represents a subxiphoid echo view of the heart, and the lower image (b) represents a CT view of the heart. The epicardial adipose tissue is marked on both images with X.
Conversely, CT and MRI allow volume, area, and thickness measurements, but are more expensive, and have radiation complications and obesity-related limitations. Cardiac CT allows high spatial and temporal resolution and 3-D cardiac views (Figure 3b). Several software programs allow a semi-automated quantification of the EAT dependent on the attenuation thresholds. Based on 3-D reconstruction, the EAT volume is calculated automatically. Cardiac MRI allows EAT measurements as well as focal EAT deposits and may provide data concerning intramyocardial adipose infiltration. When compared to cardiac CT, MRI has a decreased spatial resolution, but a higher soft tissue contrast, allowing a precise delineation of the EAT from the other adipose layers. Overall, these imaging techniques improve the ability to localize fatty tissue deposits and infiltrations.
3. Ventricular Arrhythmias
Adipose tissue includes adipocytes, as well as pre-adipocytes, endothelial and stromal cells, macrophages, and lymphocytes. This type of population is responsible for releasing a variety of chemokines, cytokines, and other pro-inflammatory factors. These inflammatory settings create a substrate for arrhythmia development. Studies performed on coronary artery disease patients revealed increased levels of pro-inflammatory markers such as angiotensinogen, monocyte chemotactic protein (MCP1), IL1β, IL6, sIL6R, NFκB, and IKKβ when compared to the subcutaneous adipose tissue from the same individual [35,52]. EAT also exhibits a pro-fibrotic effect, as secretome analysis suggests through the detection of adipocytokines such as activin A, a member of the TGF-β family [28].
Studies also revealed contractile dysfunction, decreased insulin-mediated Akt Ser 473 phosphorylation, and enhanced SMAD 2 phosphorylation, suggesting that EAT can interfere with cardiomyocyte function and cardiac remodeling [29]. Also, there is an enhanced production of mitochondrial reactive oxygen species in the fatty tissue, which are promotors of inflammation. Meanwhile, oxidative stress plays a role in arrhythmia pathogenesis [52]. SERCA protein dysfunction leading to decreased or increased cytoplasmic calcium levels has also been related to arrhythmias [53].
The evidence concerning the link between EAT and ventricular arrhythmias is weak, but there is a strong correlation between adipose myocardial infiltration and cardiomyopathies. This group of patients presents localized or diffuse fibrosis, a pro-inflammatory status, and other comorbidities linked to arrhythmias. Studies have shown that intramyocardial fat is related to ventricular arrhythmias (VAs) in obese subjects or to some genetic disorders such as ARVC, myotonic dystrophy (MD), Fabry’s disease, myocardial infarction, and heart failure [54].
For ARVC patients, the intramyocardial adipose burden is correlated with the level of RV dysfunction and the VT substrate [39]. Studies have shown that most of the local abnormal ventricular electrograms are located around the border of the RV adipose tissue, suggesting that superimposing the cardiac CT on the 3-D electroanatomic mapping may help to localize ablation targets. The evaluation of ARVC patients for LV infiltrations is useful for predicting the risk of ventricular tachycardia or ventricular fibrillation, sudden cardiac death, or cardiac arrest, allowing a 5-year risk of events estimation [45].
Based on clinical and mapping/ablation studies, advances in understanding the arrhythmogenic process in ischemic heart disease have been noticed. Most ventricular arrhythmias in ischemic heart disease are initiated by a triggering premature ventricular beat (PVB), due to automaticity, particularly in acute ischemia episodes, micro reentry within the border zone, or triggered activity. Spontaneously occurring PVBs, couplets, and VT in patients with ischemic cardiomyopathy might be initiated and maintained by focal mechanisms without macro reentry evidence. The border zone plays a central role in arrhythmias perpetuation through the structural obstacles that induce conduction slowing and zig-zag conduction in the border zone, allowing the development of reentry circuits.
Acute myocardial ischemia and reperfusion involve metabolic, ionic, and neurohumoral mechanisms, causing mechanical and electrical complications, including cardiac death. Endocardial macro-reentry represents the main mechanism involved in the initiation and maintenance of VT, leading to VF during myocardial ischemia, usually involving multiple activation sites from or around the border region of the ischemic zone, although non-reentrant mechanisms originating from the subendocardium or subepicardium may also contribute.
Acute ischemia is responsible for opening the K(ATP) channels, leading to acidosis and hypoxia of myocardial cells and dispersion in repolarization across the border zone. Abnormalities of intracellular Ca2+ handling represent another possible cause of arrhythmias in patients with coronary artery disease. The substrate transforms triggers into VF and serves to perpetuate this through the fragmentation of waves in the ischemic zone.
The VF occurrence in myocardial ischemia is mainly caused by intramural reentry, mostly in the subendocardium and rarely in the subepicardium, with subsequent acceleration and then rapid recovery of excitability, causing conduction delay and an enhanced functional block. The ischemic substrate transforms triggers such as stretch, catecholamine, genetic predisposition, thrombin, etc. into VF and allows the maintenance of the fragmented waves in the ischemic area [55].
Data regarding patients with myocardial infarction show that intramyocardial adipose deposits are mostly located in the post-infarcted myocardium during lipomatous metaplasia [54]. Several studies performed in animals and humans have confirmed the association between this metaplasia and the electrophysiological changes in the myocardium [56], and with scar age and size, with low-amplitude intracardiac electrograms (both unipolar and bipolar) in ischemic cardiomyopathy patients, revealing this way the significant role of scarrelated VA in this setting [57]. Fragmented intracardiac electrograms seem to be more frequent in the areas with adipose tissue, especially in the subendocardial layer of the scar [47]. Data show that EAT is an independent predictor of VA recurrence and mortality post-ablation, suggesting the importance of this marker in risk stratification post-ventricular ablation [49].
Studies performed in patients with heart failure (HF) have revealed that LV hypertrophy, diastolic dysfunction, and mid or preserved ejection fraction HF are related to increased EAT [58]. The presence of EAT is associated with VA in patients with reduced ejection fraction HF. For patients with dilated cardiomyopathy, intramyocardial adipose tissue is related to LV global function and fibrosis volume, thus making intramyocardial adipose tissue a marker of the disease prognosis [50]. Data show that EAT is correlated with a prolonged QTc and an increased burden of ventricular ectopies, suggesting the arrhythmogenic potential of cardiac fatty tissue. Also, EAT thickness measured by echography was increased in patients with failed ventricular ectopy ablation [59]. Several studies did not report a relationship between EAT and QTc interval [60], but rather withPR prolongation [61], P wave [62], and QT dispersion [63].
Some studies reveal a positive relationship between elevated EAT and VA burden, as well as the efficacy of VA ablation [64] and the recurrence after ablation [65], fragmented QRS, or increased QRS duration through the reentry mechanism [66]. The most frequent VA mechanism is represented by reentry, while triggered activity due to early or delayed afterdepolarizations might also be involved [67]. Structural, mechanical, or neurohormonal factors and ischemia may cause imbalances in the electrophysiological status, with enhanced automaticity through issues with conduction and refractoriness [68].
4. Genetics and Adipose Tissue
In a study performed by Ramo et al. (2023) [69], with more than 44 000 Biobank UK participants included, pericardial adipose tissue was positively correlated with male sex, age, and BMI. 5 novel genetic loci for pericardial adipose tissue were identified near CDCA2, C5orf67/ANKRD55, WARS2, IP6K1, and PEPD, as well as two previously reported foci near TRIB2 and EBF1. This study detected a link between pericardial adipose tissue and coronary artery disease, as well as atrial fibrillation. To detect the causal genes in the seven associated loci, the Polygenic Priority Score (PoPs) was used. Transcriptional regulators of adipocyte morphology and brown adipogenesis, such as EBF1, EBF2, and CEBPA, as well as regulators of visceral adiposity (WARS2 and TRIB2) were prioritized by the PoPS, in this way sharing determinants with abdominal adiposity.
Another study published by Sousa et al. [70] included 996 participants who were prospectively enrolled. The epicardial adipose tissue was evaluated by cardiac CT and, based on the genotyped and linked SNPs, a multiplicative genetic risk score (mGRS) was created. This study revealed the fact the subjects with above-median EAT were older and presented a higher BMI as well as other cardiac risk factors such as hypertension, diabetes, hypercholesterolemia, and metabolic syndrome. This higher EAT was also associated with a higher GRS, and this was considered an independent predictor of higher EAT volumes. Among the 33 variants, only 1 was strongly associated with EAT volume, but only in the univariate analysis, rs1333049 (CDKN2B-AS1) at the 9p21 locus. The variant rs1801133 of MTHFR677 was significantly and independently associated with an EAT volume above the median. This gene variant is involved in regulating plasma homocysteine levels.
The Sousa study revealed an increased number of different mutations linked to EAT volume compared to the studies previously published so far. Most of the previous studies evaluated the expression of the EAT genes on in vivo samples from patients undergoing CABG surgery, resulting in a limited number of participants [71]. Another study including coronary artery disease patients was published by Vacca et al. [72], and revealed the role of miRNA in the crosstalk between EAT and the coronary arteries. M-RNAs are small non–coding RNAs modulating gene expression. The study published by Tan et al. [73] showed that pro-inflammatory and immunological genes are upregulated in EAT coronary artery disease patients and play an important role in the coronary atherosclerosis process.
Data regarding the impact between sex and EAT volume are still under debate. Some studies have shown increased EAT volume in females [74] and some in men [75].
5. Correlation Between Genetics, Adipose Infiltration, and Cardiac Diseases
It seems that ARVC and myotonic dystrophy are correlated with fatty infiltration and VA [16]. This observation supports the involvement of fatty tissue in the pathogenesis of cardiac arrhythmias.
Up to 30% of patients diagnosed with myotonic dystrophy type 1 develop cardiac complications, related to the number of CTG repeats [76]. Usually, conduction disturbances are most frequent, but ventricular arrhythmias and ventricular systolic dysfunction are also reported. Three-dimensional mapping in patients with DM1 revealed low-voltage areas spread around both the right atria and ventricle [77], while myocardial biopsies showed extensive fibrosis, adipose deposits, inflammation, and myocardial hypertrophy [78].
ARVC represents an inherited cardiomyopathy with autosomal transmission and low penetrance, based on RV enlargement and dysfunction, fibro-fatty replacement of myocytes in the right ventricle, ECG abnormalities, and arrhythmias originating from the RV. Adipose infiltrations are correlated with advanced right ventricular structural disease in patients associated with the highest arrhythmic risk [41].
Almost 50% of the symptomatic patients present a mutation in one of the five major components of the cardiac desmosome [79], comprising PKP2 (encoding plakophilin-2), DSP (encoding desmoplakin), DSC2 (encoding desmocollin-2), DSG2 (encoding desmoglein-2), and JUP (encoding junctional plakoglobin). Genetic testing is advised for patients with ARVC or ARVD as well as their family members. In cases where the affected individual (proband) with ARVC or ARVD does not present a genetic defect, it will not be present in other family members [59].
Fabry disease represents a X-linked lysosomal storage disease caused by reduced activity of alpha-galactosidase A, leading to lysosomal accumulations of neutral glycosphingolipids and globotriaosylceramide GL-3. Studies have identified hundreds of mutations involved in Fabry disease. Milder types associated with missense mutation are linked to cardiac involvement [80,81]. Ventricular tachycardia and ventricular fibrillation are the most frequent VAs described in Fabry disease. The prevalence of these arrhythmias is between 13 and 18% [82]. α-GAL A deficiency together with the accumulation of glycosphingolipid interferes with the expression of sodium and calcium ion channels, modifying in this way the cellular electrophysiological properties, which are responsible for Fabry disease-related VAs [83].
In the study performed by Sahasrabuddhe [84], 43 patients undergoing cardiac surgery were included, 27 patients for coronary artery bypass graft (CABG) and 16 for valvular surgery. After analyzing the gene expression obtained from the EAT samples, they observed an upregulation and higher expression of pro-inflammatory chemokines such as monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion molecule-1 (VCAM-1), and tumor necrosis factor-alpha (TNF-α) for the CABG group compared to non-CABG group, as was observed in other studies [35,85,86]. Downregulation was noted for anti-inflammatory chemokines such as uncoupling protein-1, adiponectin, and the adenosine A1 receptor (ADORA-1) [87,88]. In this way, EAT might be involved in the development of coronary artery diseases.
MCP-1 is responsible for macrophage infiltration infatty tissue, as described in previous studies. A higher expression of MCP-1 from epicardial fatty tissue when compared to subcutaneous fatty tissue and omental fat was observed in several studies [89,90]. EBF1 is involved in the regulation of the adipose cell morphology and lipolysis process [91], and decreased levels of EBF1 are associated with white adipose tissue hypertrophy. Meanwhile, EBF2 is involved in brown-like/beige adipose cell differentiation [92]. CEBPA encodes the transcription factor CCAAT/enhancer binding protein alpha (C/EBPα). The last one shares binding sites with Peroxisome proliferator-activated receptor gamma (PPARγ) and acts as a co-stimulator of adipogenesis and adipocyte differentiation. TRIB2 is a promoter of CCAAT/enhancer binding protein beta, which transactivates the expression of both C/EBPα and PPARγ [93], whereas WARS2 is responsible for encoding mitochondrial tryptophan-tRNA synthetase [94].
A total of 41 studies were evaluated in the literature review, but only 23 were considered relevant and included in this review (Table 3).

Table 3.
Studies from the literature regarding patients with cardiac diseases and the histopathologic and genetic observations.
6. Future Directions
EAT volume is the result of a complex interaction between environmental, genetic, and epigenetic factors, which we started to uncover. Furthermore, EAT represents more than fat deposits: it represents a biologically active structure and an association between obesity and cardiovascular diseases.
EAT has attracted special interest lately as an important player in the pathophysiology of cardiovascular disease and is considered a risk factor for the atherosclerotic process. More important seems to be the earlier identification of the disease and its complications at a subclinical stage, preventing further progression. Many loci associated with adipose tissue are a targeted subset of drivers of unhealthy adiposity, unlike many loci linked with BMI which may exert their effects via neuronal pathways and hunger regulation [114]. Even though EAT can be a risk factor for the beginning of atherosclerotic development, its usefulness could lie in the detection of the disease at a subclinical stage, preventing future progression through preventive and even therapeutic measures.
It is well known that EAT is a source of free fatty acids, adipokines, and cytokines. In the presence of various inflammatory disorders, EAT participates in the atherosclerosis process, as well as myocardial fibrosis, and then contributes to arrhythmias and heart failure development. The relationship between adipose tissue and arrhythmia is not strictly related to obesity, but to the activation of multiple pathways, so for treating this matter, there is a need for a multidisciplinary approach including weight reduction, risk factors, and lifestyle modification. Investigating all the pro-arrhythmogenic mechanisms enhanced by obesity requires further research, as arrhythmias are multifactorial. In this regard, possible targets that need to be investigated to prevent or treat arrhythmias are EAT activity, mitochondrial reactive oxygen species, TGF- β 1, and SERCA proteins.
Beyond its utility as a flexible and modifiable risk marker, epicardial adipose tissue is also a therapeutic target for preventing atherosclerotic plaque growth and cardiovascular events. Despite the positive effects of lifestyle modification and bariatric surgery on EAT, there are no specific pharmacological drugs developed for EAT reduction. High-dose statin therapy seems to have a positive dose-dependent effect on EAT lowering. Studies performed with pro-protein convertase subtilisin/kexin 9 (PSCK9) inhibitors revealed reduction in EAT [115].
Glucose-lowering drugs, such as SGLT2 inhibitors and GLP-1 RA, exhibit cardioprotective effects and can target both atrial and coronary EAT for the treatment and prevention of AF and coronary artery disease by lowering EAT inflammation and increasing free fatty acid oxidation. GLP-1 receptor agonists are a type of glucose-lowering drug that lowers HbA1c, and modestly improves blood lipids and body weight while decreasing the risk of hypoglycemia by stimulating insulin secretion and lowering glucagon secretion, delaying gastric emptying, and reducing appetite [116]. Studies involving GLP1 RA showed the positive effect of this drug in reducing cardiovascular mortality, non-fatal stroke, and non-fatal myocardial infarction [117]. Preclinical studies revealed that GLP1 RA can reduce atrial fibrosis and atrial arrhythmias in rat models with myocardial infarction [118], as well as reduce the risk of development of ventricular arrhythmias after myocardial ischemia, the incidence of VF, and the number of VT and VF episodes [119]. The presence of GLP 1 receptors in the ventricular myocardium is still a matter of debate, as well as the effects of GLP-1RAs on arrhythmia development. The studies showed either no effects [120], or reduced risk for atrial arrhythmias [121], or efficient opposing of the β-adrenoceptor stimulation, thus reducing ventricular arrhythmic potential [122]. These results seem to be mediated through the release of acetylcholine and NO from the cardiac vagal neurons [123].
7. Conclusions
There is variability in the strength of the causative relationship between EAT and VA, mainly caused by the different populations studied, the different disease stages and adipose tissue locations (atrial, ventricular, and vascular), and the indexes used, such as volume and thickness. Research is needed to evaluate the connection between adipose tissue and arrhythmias, as various factors such as oxidative stress, autonomic tone, autophagy, variability of adipose tissue deposits, propagation of abnormal signals, development of reentry circuits, and myocyte death are involved. Next, possible therapeutic targets can be investigated.
Author Contributions
R.S.P., collecting data and writing, O.P., collecting data and writing, and I.M., reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hatem, S.N.; Redheuil, A.; Gandjbakhch, E. Cardiac adipose tissue and atrial fibrillation: The perils of adiposity. Cardiovasc. Res. 2016, 109, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Abed, H.S.; Molaee, P.; Nelson, A.J.; Brooks, A.G.; Sharma, G.; Leong, D.P.; Lau, D.H.; Middeldorp, M.E.; Roberts-Thomson, K.C.; et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J. Am. Coll. Cardiol. 2011, 57, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Jermendy, A.L.; Merkely, B.; Maurovich-Horvat, P. Clinical importance of epicardial adipose tissue. Arch. Med. Sci. 2017, 4, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Iozzo, P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011, 34 (Suppl. 2), S371–S379. [Google Scholar] [CrossRef]
- Burke, S.; Nagajyothi, F.; Thi, M.M.; Hanani, M.; Scherer, P.E.; Tanowitz, H.B.; Spray, D.C. Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: Implications for Chagas disease. Microbes Infect. 2014, 16, 893–901. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Brooks, A.G.; Shipp, N.J.; Manavis, J.; Wood, J.P.; Finnie, J.W.; Samuel, C.S.; Royce, S.G.; Twomey, D.J.; et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J. Am. Coll. Cardiol. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Gorter, P.M.; van Lindert, A.S.; de Vos, A.M.; Meijs, M.F.; van der Graaf, Y.; Doevendans, P.A.; Prokop, M.; Visseren, F.L. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 2008, 197, 896–903. [Google Scholar] [CrossRef]
- Koepp, K.E.; Obokata, M.; Reddy, Y.; Olson, T.P.; Borlaug, B.A. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2020, 8, 657–666. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Ding, J.; Carr, J.J.; Allison, M.A.; Budoff, M.J.; Tracy, R.P.; Burke, G.L.; McClelland, R.L.; Arai, A.E.; Bluemke, D.A. Pericardial fat and the risk of heart failure. J. Am. Coll. Cardiol. 2021, 77, 2638–2652. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef] [PubMed]
- E Middeldorp, M.; Pathak, R.K.; Meredith, M.; Mehta, A.B.; Elliott, A.D.; Mahajan, R.; Twomey, D.; Gallagher, C.; Hendriks, J.M.L.; Linz, D.; et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: The REVERSE-AF study. EP Eur. 2018, 20, 1929–1935. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef]
- Corradi, D.; Maestri, R.; Callegari, S.; Pastori, P.; Goldoni, M.; Luong, T.V.; Bordi, C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004, 13, 313–316. [Google Scholar] [CrossRef]
- Bertaso, A.G.; Bertol, D.; Duncan, B.B.; Foppa, M. Epicardial fat: Definition, measurements and systematic review of main outcomes. Arq. Bras. Cardiol. 2013, 101, e18–e28. [Google Scholar] [CrossRef]
- Fainberg, H.P.; Birtwistle, M.; Alagal, R.; Alhaddad, A.; Pope, M.; Davies, G.; Woods, R.; Castellanos, M.; May, S.T.; Ortori, C.A.; et al. Transcriptional analysis of adipose tissue during development reveals depot-specific responsiveness to maternal dietary supplementation. Sci. Rep. 2018, 8, 9628. [Google Scholar] [CrossRef]
- Timóteo, A.T.; Albuquerque, F.B.; Teixeira, B.L. Pericardium, epicardial adipose tissue, and heart failure with preserved ejection fraction: Pathophysiology, quantification and treatment target11All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. Int. J. Cardiol. 2024, 412, 132303. [Google Scholar]
- Pandit, S.V.; Anumonwo, J.; Jalife, J. Atrial fibrillation susceptibility in obesity: An excess adiposity and fibrosis complicity? Circ. Res. 2016, 118, 1468–1471. [Google Scholar] [CrossRef]
- Zhang, P.; Su, J.; Mende, U. Cross talk between cardiac myocytes and fibroblasts: From multiscale investigative approaches to mechanisms and functional consequences. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1385–H1396. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.N.; Sanders, P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc. Res. 2014, 102, 205–213. [Google Scholar] [CrossRef]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp. Biochem. Physiol. B 1989, 94, 225–232. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2013, 36, 795–805. [Google Scholar] [CrossRef]
- Greulich, S.; Maxhera, B.; Vandenplas, G.; de Wiza, D.H.; Smiris, K.; Mueller, H.; Heinrichs, J.; Blumensatt, M.; Cuvelier, C.; Akhyari, P.; et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012, 126, 2324–2334. [Google Scholar] [CrossRef]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef]
- Cherian, S.; Lopaschuk, G.D.; Carvalho, E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E937–E949. [Google Scholar] [CrossRef]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Carter, R.A.; Tichansky, D.S.; Madan, A.K. Identification of omentin mRNA in human epicardial adipose tissue: Comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int. J. Obes. 2008, 32, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Langheim, S.; Dreas, L.; Veschini, L.; Maisano, F.; Foglieni, C.; Ferrarello, S.; Sinagra, G.; Zingone, B.; Alfieri, O.; Ferrero, E.; et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H746–H753. [Google Scholar] [CrossRef] [PubMed]
- Lamounier-Zepter, V.; Look, C.; Alvarez, J.; Christ, T.; Ravens, U.; Schunck, W.H.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Morano, I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: A new link between obesity and heart disease. Circ. Res. 2009, 105, 326–334. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Dutour, A.; Achard, V.; Sell, H.; Naour, N.; Collart, F.; Gaborit, B.; Silaghi, A.; Eckel, J.; Alessi, M.-C.; Henegar, C.; et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J. Clin. Endocrinol. Metab. 2010, 95, 963–967. [Google Scholar] [CrossRef]
- Silaghi, A.; Achard, V.; Paulmyer-Lacroix, O.; Scridon, T.; Tassistro, V.; Duncea, I.; Clément, K.; Dutour, A.; Grino, M. Expression of adrenomedullin in human epicardial adipose tissue: Role of coronary status. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1443–E1450. [Google Scholar] [CrossRef]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2016, 38, 1294–1302. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Cochet, H.; Denis, A.; Komatsu, Y.; Jadidi, A.S.; Aït Ali, T.; Sacher, F.; Derval, N.; Relan, J.; Sermesant, M.; Corneloup, O.; et al. Automated Quantification of Right Ventricular Fat at Contrast-enhanced Cardiac Multidetector CT in Arrhythmogenic Right Ventricular Cardiomyopathy. Radiology 2015, 275, 683–691. [Google Scholar] [CrossRef]
- Ghasabeh, M.; Te Riele, A.; James, C.; Chen, V.; Tichnell, C.; Murray, B.; Eng, J.; Kral, B.G.; Tandri, H.; Calkins, H.; et al. Epicardial Fat Distribution Assessed with Cardiac CT in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Radiology 2018, 289, 641–648. [Google Scholar] [CrossRef]
- Rastegar, N.; Te Riele, A.S.; James, C.A.; Bhonsale, A.; Murray, B.; Tichnell, C.; Calkins, H.; Tandri, H.; Bluemke, D.A.; Kamel, I.R.; et al. Fibrofatty Changes: Incidence at Cardiac MR Imaging in Patients with Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Radiology 2016, 280, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Bauce, B.; De Lazzari, M.; Rigato, I.; Bariani, R.; Meneghin, S.; Pilichou, K.; Motta, R.; Aliberti, C.; Thiene, G.; et al. Arrhythmogenic Right Ventricular Cardiomyopathy: Characterization of Left Ventricular Phenotype and Differential Diagnosis With Dilated Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e014628. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Pingitore, A.; Di Bella, G.; Piaggi, P.; Gaeta, R.; Grigoratos, C.; Altinier, A.; Pantano, A.; Strata, E.; De Caterina, R.; et al. Prognostic Role of Cardiac Magnetic Resonance in Arrhythmogenic Right Ventricular Cardiomyopathy. Am. J. Cardiol. 2018, 122, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, J.; Li, G.; Zhou, D.; Li, S.; Zhuang, B.; Chen, X.; Duan, X.; Li, L.; Fan, X.; et al. Arrhythmogenic Left Ventricular Cardiomyopathy: A Clinical and CMR Study. Sci. Rep. 2020, 10, 533. [Google Scholar] [CrossRef]
- Aquaro, G.D.; De Luca, A.; Cappelletto, C.; Raimondi, F.; Bianco, F.; Botto, N.; Lesizza, P.; Grigoratos, C.; Minati, M.; Dell’Omodarme, M.; et al. Prognostic Value of Magnetic Resonance Phenotype in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 2753–2765. [Google Scholar] [CrossRef]
- Sasaki, T.; Calkins, H.; Miller, C.F.; Zviman, M.M.; Zipunnikov, V.; Arai, T.; Sawabe, M.; Terashima, M.; Marine, J.E.; Berger, R.D.; et al. New insight into scar-related ventricular tachycardia circuits in ischemic cardiomyopathy: Fat deposition after myocardial infarction on computed tomography—A pilot study. Heart Rhythm. 2015, 12, 1508–1518. [Google Scholar] [CrossRef]
- Cheniti, G.; Sridi, S.; Sacher, F.; Chaumeil, A.; Pillois, X.; Takigawa, M.; Frontera, A.; Vlachos, K.; Martin, C.A.; Teijeira, E.; et al. Post-Myocardial Infarction Scar With Fat Deposition Shows Specific Electrophysiological Properties and Worse Outcome After Ventricular Tachycardia Ablation. J. Am. Heart Assoc. 2019, 8, e012482. [Google Scholar] [CrossRef]
- Mordi, I.; Radjenovic, A.; Stanton, T.; Gardner, R.S.; McPhaden, A.; Carrick, D.; Berry, C.; Tzemos, N. Prevalence and Prognostic Significance of Lipomatous Metaplasia in Patients With Prior Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1111–1112. [Google Scholar] [CrossRef]
- Parisi, V.; Conte, M.; Petraglia, L.; Grieco, F.V.; Bruzzese, D.; Caruso, A.; Grimaldi, M.G.; Campana, P.; Gargiulo, P.; Paolillo, S.; et al. Echocardiographic Epicardial Adipose Tissue Thickness for Risk Stratification of Patients With Heart Failure. Front. Physiol. 2020, 11, 43. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, S.; Jiang, S.; Yin, G.; Wang, C.; Zhang, Y.; Liu, Q.; Cheng, H.; Ma, N.; Zhao, T.; et al. Fat deposition in dilated cardiomyopathy assessed by CMR. JACC Cardiovasc. Imaging 2013, 6, 889–898. [Google Scholar] [CrossRef]
- Kırış, A.; Turan, O.E.; Kırış, G.; İlter, A.; Öztürk, M.; Aydın, M. The relationship between epicardial fat tissue thickness and frequent ventricular premature beats. Kardiol. Pol. 2015, 73, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Fernández, A.L.; González-Juanatey, J.R.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef] [PubMed]
- Erkasap, N. SERCA in genesis of arrhythmias: What we already know and what is new? Anadolu Kardiyol. Derg. 2007, 7 (Suppl. 1), 43–46. [Google Scholar] [PubMed]
- Homan, E.A.; Reyes, M.V.; Hickey, K.T.; Morrow, J.P. Clinical overview of obesity and diabetes mellitus as risk factors for atrial fibrillation and sudden cardiac death. Front. Physiol. 2019, 9, 1847. [Google Scholar] [CrossRef]
- Pogwizd, S.M.; Corr, P.B. Mechanisms underlying the development of ventricular fibrillation during early myocardial ischemia. Circ. Res. 1990, 66, 672–695. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Kitagawa, K.; Chino, S.; Ishida, M.; Matsuoka, K.; Tanigawa, T.; Nakamura, T.; Hirano, T.; Takeda, K.; Sakuma, H. Adipose tissue detected by multislice computed tomography in patients after myocardial infarction. JACC Cardiovasc. Imaging 2009, 2, 548–555. [Google Scholar] [CrossRef]
- Samanta, R.; Kumar, S.; Chik, W.; Qian, P.; Barry, M.A.; Al Raisi, S.; Bhaskaran, A.; Farraha, M.; Nadri, F.; Kizana, E.; et al. Influence of intramyocardial adipose tissue on the accuracy of endocardial contact mapping of the chronic myocardial infarction substrate. Circ. Arrhythmia Electrophysiol. 2017, 10, e004998. [Google Scholar] [CrossRef]
- Fontes-Carvalho, R.; Fontes-Oliveira, M.; Sampaio, F.; Mancio, J.; Bettencourt, N.; Teixeira, M.; Gonçalves, F.R.; Gama, V.; Leite-Moreira, A. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am. J. Cardiol. 2014, 114, 1663–1669. [Google Scholar] [CrossRef]
- Tam, W.-C.; Lin, Y.-K.; Chan, W.-P.; Huang, J.-H.; Hsieh, M.-H.; Chen, S.-A.; Chen, Y.-J. Pericardial fat is associated with the risk of ventricular arrhythmia in asian patients. Circ. J. 2016, 80, 1726–1733. [Google Scholar] [CrossRef]
- Kanat, S.; Karaduman, B.D.; Tütüncü, A.; Tenekecioğlu, E.; Mutluer, F.O.; Bayram, N.A. Effect of echocardiographic epicardial adipose tissue thickness on success rates of premature ventricular contraction ablation. Balk. Med. J. 2019, 36, 324–330. [Google Scholar] [CrossRef]
- Quisi, A.; Şentürk, S.E.; Harbalıoğlu, H.; Baykan, A.O. The relationship between echocardiographic epicardial adipose tissue, P-wave dispersion, ana corrected QT interval. Turk. Kardiyol. Dern. Ars. Arch. Turk. Soc. Cardiol. 2018, 46, 471–478. [Google Scholar] [CrossRef]
- Hung, W.-C.; Tang, W.-H.; Wang, C.-P.; Lu, L.-F.; Chung, F.-M.; Lu, Y.-C.; Hsu, C.-C.; Tsai, I.-T.; Jhuo, S.-J.; Lai, W.-T.; et al. Increased epicardial adipose tissue volume is associated with PR interval prolongation. Clin. Investig. Med. 2015, 38, E45–E52. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, Y.; Doğan, S.; Durakoğlugil, M.E.; Balcıoğlu, A.S.; Erdoğan, T.; Şatıroğlu, Ö.; Karadağ, Z.; Duman, H.; Bostan, M. The relationship between epicardial adipose tissue and P wave and QT dispersions. Turk. Kardiyol. Dern. Ars. Arch. Turk. Soc. Cardiol. 2015, 43, 621–629. [Google Scholar] [CrossRef][Green Version]
- Shen, J.; Zhu, D.; Chen, L.; Cang, J.; Zhao, Z.; Ji, Y.; Liu, S.; Miao, H.; Liu, Y.; Zhou, Q.; et al. Relationship between epicardial adipose tissue measured by computed tomography and premature ventricular complexes originating from different sites. Europace 2023, 25, euad102, Erratum in Europace 2023, 25, euad138. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.R.; Harte, A.L.; Howell, N.; Pritlove, D.C.; Ranasinghe, A.M.; Da Silva, N.F.; Youssef, E.M.; Khunti, K.; Davies, M.J.; Bonser, R.S.; et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J. Clin. Endocrinol. Metab. 2009, 94, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.S.; Schoene, K.; Stauber, A.; Darma, A.; Dagres, N.; Dinov, B.; Bertagnolli, L.; Hilbert, S.; Müssigbrodt, A.; Husser, D.; et al. Epicardial adipose tissue thickness as an independent predictor of ventricular tachycardia recurrence following ablation. Heart Rhythm. 2019, 16, 1492–1498. [Google Scholar] [CrossRef]
- Bekar, L.; Kalçık, M.; Çelik, O.; Alp, Ç.; Yetim, M.; Doğan, T.; Ekinözü, I.; Karaarslan, O.; Çamkıran, V.; Karavelioğlu, Y.; et al. Presence of fragmented QRS is associated with increased epicardial adipose tissue thickness in hypertensive patients. J. Clin. Ultrasound 2019, 47, 345–350. [Google Scholar] [CrossRef]
- Rubart, M.; Zipes, D.P. Genesis of cardiac arrhythmias: Electrophysiological considerations. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 7th ed.; Zipes, D.P., Libby, P., Bonow, R.O., Braunwald, E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 683–685. [Google Scholar]
- Rämö, J.T.; Kany, S.; Hou, C.R.; Friedman, S.F.; Roselli, C.; Nauffal, V.; Koyama, S.; Karjalainen, J.; Maddah, M.; Palotie, A.; et al. The Cardiovascular Impact and Genetics of Pericardial Adiposity. medRxiv 2023, 2023.07.16.23292729. [Google Scholar] [CrossRef]
- Sousa, J.A.; Mendonça, M.I.; Serrão, M.; Borges, S.; Henriques, E.; Freitas, S.; Tentem, M.; Santos, M.; Freitas, P.; Ferreira, A.; et al. Epicardial Adipose Tissue: The Genetics Behind an Emerging Cardiovascular Risk Marker. Clin. Med. Insights Cardiol. 2021, 15, 11795468211029244. [Google Scholar] [CrossRef]
- Chechi, K.; Vijay, J.; Voisine, P.; Mathieu, P.; Bossé, Y.; Tchernof, A.; Grundberg, E.; Richard, D. UCP1 expression–associated gene signatures of human epicardial adipose tissue. JCI Insight 2019, 4, e123618. [Google Scholar] [CrossRef]
- Vacca, M.; Di Eusanio, M.; Cariello, M.; Graziano, G.; D’Amore, S.; Petridis, F.D.; D’Orazio, A.; Salvatore, L.; Tamburro, A.; Folesani, G.; et al. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc. Res. 2016, 109, 228–239. [Google Scholar] [CrossRef]
- Tan, L.; Xu, Q.; Wang, Q.; Shi, R.; Zhang, G. Identification of key genes and pathways affected in epicardial adipose tissue from patients with coronary artery disease by integrated bioinformatics analysis. PeerJ 2020, 8, e8763. [Google Scholar] [CrossRef] [PubMed]
- Reiner, L.; Mazzoleni, A.; Rodriguez, F.L. Statistical analysis of the epicardial fat weight in human hearts. AMA Arch. Pathol. 1955, 60, 369–373. [Google Scholar] [PubMed]
- Mancio, J.; Pinheiro, M.; Ferreira, W.; Carvalho, M.; Barros, A.; Ferreira, N.; Vouga, L.; Ribeiro, V.G.; Leite-Moreira, A.; Falcao-Pires, I.; et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int. J. Cardiol. 2017, 249, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Groh, W.J.; Lowe, M.R.; Zipes, D.P. Severity of cardiac conduction involvement and arrhythmias in myotonic dystrophy type 1 correlates with age and CTG repeat length. J. Cardiovasc. Electrophysiol. 2002, 13, 444–448. [Google Scholar] [CrossRef]
- Russo, A.D.; Pelargonio, G.; Parisi, Q.; Santamaria, M.; Messano, L.; Sanna, T.; Casella, M.; Martino, G.D.; Ponti, R.D.; Pace, M.; et al. Widespread electroanatomic alternations of right cardiac chambers in patients with myotonic dystrophy type. J. Cardiovasc. Electrophysiol. 2006, 17, 34–40. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Wolfe, J.T.; Holmes, D.R.; Edwards, W.D. Pathology of the cardiac conduction system in myotonic dystrophy: A study of 12 cases. J. Am. Coll. Cardiol. 1988, 11, 662–671. [Google Scholar] [CrossRef]
- Awad, M.M.; Calkins, H.; Judge, D.P. Mechanisms of disease: Molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 258–267. [Google Scholar] [CrossRef]
- Chan, B.; Adam, D.N. A Review of Fabry Disease. Skin. Therapy Lett. 2018, 23, 4–6. [Google Scholar]
- del Pino, M.; Andrés, A.; Bernabéu, A.Á.; de Juan-Rivera, J.; Fernández, E.; Díaz, J.d.D.G.; Hernández, D.; Luño, J.; Fernández, I.M.; Paniagua, J.; et al. Fabry Nephropathy: An Evidence-Based Narrative Review. Kidney Blood Press. Res. 2018, 43, 406–421. [Google Scholar] [CrossRef]
- Frustaci, A.; Morgante, E.; Russo, M.A.; Scopelliti, F.; Grande, C.; Verardo, R.; Franciosa, P.; Chimenti, C. Pathology and function of conduction tissue in Fabry disease cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2015, 8, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Birket, M.J.; Raibaud, S.; Lettieri, M.; Adamson, A.D.; Letang, V.; Cervello, P.; Redon, N.; Ret, G.; Viale, S.; Wang, B.; et al. A Human stem cell model of Fabry disease implicates LIMP-2 accumulation in cardiomyocyte pathology. Stem Cell Rep. 2019, 13, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, A.V.; Pitale, S.U.; Sivanesan, S.D.; Deshpande, P.K.; Deshpande, S.P.; Daiwile, A. Pathogenic gene expression of epicardial adipose tissue in patients with coronary artery disease. Indian. J. Med. Res. 2020, 151, 554–561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, A.R.; da Silva, N.F.; Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef]
- Kremen, J.; Dolinkova, M.; Krajickova, J.; Blaha, J.; Anderlova, K.; Lacinova, Z.; Haluzikova, D.; Bosanska, L.; Vokurka, M.; Svacina, S.; et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: Possible role in postoperative insulin resistance. J. Clin. Endocrinol. Metab. 2006, 91, 4620–4627. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Chu, C.-S.; Lee, K.-T.; Lin, T.-H.; Hsieh, C.-C.; Chiu, C.-C.; Voon, W.-C.; Sheu, S.-H.; Lai, W.-T. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. 2008, 32, 268–274. [Google Scholar] [CrossRef]
- Greif, M.; Becker, A.; von Ziegler, F.; Lebherz, C.; Lehrke, M.; Broedl, U.C.; Tittus, J.; Parhofer, K.; Becker, C.; Reiser, M.; et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arter. Thromb. Vasc. Biol. 2009, 29, 781–786. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Mach, F.; Steffens, S. The specific role of chemokines in atherosclerosis. Thromb. Haemost. 2007, 97, 714–721. [Google Scholar] [CrossRef]
- Gao, H.; Mejhert, N.; Fretz, J.A.; Arner, E.; Lorente-Cebrián, S.; Ehrlund, A.; Dahlman-Wright, K.; Gong, X.; Strömblad, S.; Douagi, I.; et al. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014, 19, 981–992. [Google Scholar] [CrossRef]
- Stine, R.R.; Shapira, S.N.; Lim, H.-W.; Ishibashi, J.; Harms, M.; Won, K.-J.; Seale, P. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol. Metab. 2016, 5, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef]
- Ahmad, F.; Li, D.; Karibe, A.; Gonzalez, O.; Tapscott, T.; Hill, R.; Weilbaecher, D.; Blackie, P.; Furey, M.; Gardner, M.; et al. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation 1998, 98, 2791–2795. [Google Scholar] [CrossRef]
- Melberg, A.; Oldfors, A.; Blomström-Lundqvist, C.; Stålberg, E.; Carlsson, B.; Larsson, E.; Lidell, C.; Eeg-Olofsson, K.E.; Wikström, G.; Henriksson, K.G.; et al. Autosomal dominant myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy linked to chromosome 10q. Ann. Neurol. 1999, 46, 684–692. [Google Scholar] [CrossRef]
- Nava, A.; Bauce, B.; Basso, C.; Muriago, M.; Rampazzo, A.; Villanova, C.; Daliento, L.; Buja, G.; Corrado, D.; Danieli, G.A.; et al. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 2000, 36, 2226–2233. [Google Scholar] [CrossRef]
- McKoy, G.; Protonotarios, N.; Crosby, A.; Tsatsopoulou, A.; Anastasakis, A.; Coonar, A.; Norman, M.; Baboonian, C.; Jeffery, S.; McKenna, W.J. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 2000, 355, 2119–2124. [Google Scholar] [CrossRef]
- Protonotarios, N.; Tsatsopoulou, A.; Anastasakis, A.; Sevdalis, E.; McKoy, G.; Stratos, K.; Gatzoulis, K.; Tentolouris, K.; Spiliopoulou, C.; Panagiotakos, D.; et al. Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J. Am. Coll. Cardiol. 2001, 38, 1477–1484. [Google Scholar] [CrossRef]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; Digioia, C. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef]
- Dalal, D.; Nasir, K.; Bomma, C.; Prakasa, K.; Tandri, H.; Piccini, J.; Roguin, A.; Tichnell, C.; James, C.; Russell, S.D.; et al. Arrhythmogenic right ventricular dysplasia: A United States experience. Circulation 2005, 112, 3823–3832. [Google Scholar] [CrossRef]
- Haan, A.D.D.; Tan, B.Y.; Zikusoka, M.N.; Lladó, L.I.; Jain, R.; Daly, A.; Tichnell, C.; James, C.; Amat-Alarcon, N.; Abraham, T.; et al. Analysis in North Americans With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ. Cardiovasc. Genet. 2009, 2, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Fabritz, L.; Zwiener, M.; Witt, H.; Schäfers, M.; Zellerhoff, S.; Paul, M.; Athai, T.; Hiller, K.-H.; Baba, H.A.; et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 2006, 114, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Merner, N.D.; Hodgkinson, K.A.; Haywood, A.F.; Connors, S.; French, V.M.; Drenckhahn, J.-D.; Kupprion, C.; Ramadanova, K.; Thierfelder, L.; McKenna, W.; et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am. J. Hum. Genet. 2008, 82, 809–821. [Google Scholar] [CrossRef]
- Christensen, A.H.; Bundgaard, H.; Schwartz, M.; Hansen, S.H.; Svendsen, J.H.; Judge, D.P.; Corrado, D.; Thiene, G.; Quarta, G.; Muir, A.; et al. Cardiac Myotonic Dystrophy mimicking arrhythmogenic right ventricular cardiomyopathy in a young sudden cardiac death victim. Circ. Arrhythmia Electrophysiol. 2008, 1, 317–320. [Google Scholar] [CrossRef]
- Otten, E.; Asimaki, A.; Maass, A.; van Langen, I.M.; van der Wal, A.; de Jonge, N.; van den Berg, M.P.; Saffitz, J.E.; Wilde, A.A.; Jongbloed, J.D.; et al. Desmin mutations as a cause of right ventricular heart failure affect the intercalated disks. Heart Rhythm. 2010, 7, 1058–1064. [Google Scholar] [CrossRef]
- Klauke, B.; Kossmann, S.; Gaertner, A.; Brand, K.; Stork, I.; Brodehl, A.; Dieding, M.; Walhorn, V.; Anselmetti, D.; Gerdes, D.; et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum. Mol. Genet. 2010, 19, 4595–4607. [Google Scholar] [CrossRef]
- Hedberg, C.; Melberg, A.; Kuhl, A.; Jenne, D.; Oldfors, A. Autosomal dominant myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy 7 is caused by a DES mutation. Eur. J. Hum. Genet. 2012, 20, 984–985. [Google Scholar] [CrossRef]
- Jacob, K.A.; Noorman, M.; Cox, M.G.P.J.; Groeneweg, J.A.; Hauer, R.N.W.; van der Heyden, M.A.G. Geographical distribution of plakophilin-2 mutation prevalence in patients with arrhythmogenic cardiomyopathy. Neth. Heart J. 2012, 20, 234–239. [Google Scholar] [CrossRef]
- Chen, S.N.; Gurha, P.; Lombardi, R.; Ruggiero, A.; Willerson, J.T.; Marian, A.J. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ. Res. 2014, 114, 454–468. [Google Scholar] [CrossRef]
- Samanta, R.; Pouliopoulos, J.; Thiagalingam, A.; Kovoor, P. Role of adipose tissue in the pathogenesis of cardiac arrhythmias. Heart Rhythm. 2016, 13, 311–320. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Herman, H.K.; Quigley, P.C.; Shinnick, J.K.; Cundiff, C.A.; Caltharp, S.; Shehata, B.M. Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D): Review of 16 pediatric cases and a proposal of modified pediatric criteria. Pediatr. Cardiol. 2016, 37, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Sen-Chowdhry, S.; Prasad, S.K.; Syrris, P.; Wage, R.; Ward, D.; Merrifield, R.; Smith, G.C.; Firmin, D.N.; Pennell, D.J.; McKenna, W.J. Cardiovascular Magnetic Resonance in Arrhythmogenic Right Ventricular Cardiomyopathy Revisited: Comparison with Task Force Criteria and Genotype. J. Am. Coll. Cardiol. 2006, 48, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Norgett, E.E.; Hatsell, S.J.; Carvajal-Huerta, L.; Cabezas, J.C.; Common, J.; Purkis, P.E.; Whittock, N.; Leigh, I.M.; Stevens, H.P.; Kelsell, D.P. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 2000, 9, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Galvez, R.R.; Portano, J.D.M.; Cortes, R.T.; Alvarez, E.B.G.; Cubias, S.M.S.; Zelaya, S.M. Reduction of epicardial adipose tissue thickness with PCSK9 inhibitors. Eur. Heart J. 2020, 41, ehaa946.3008. [Google Scholar] [CrossRef]
- Andreasen, C.R.; Andersen, A.; Knop, F.K.; Vilsbøll, T. Understanding the place for GLP-1RA therapy: Translating guidelines for treatment of type 2 diabetes into everyday clinical practice and patient selection. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 40–52. [Google Scholar] [CrossRef]
- Giugliano, D.; Scappaticcio, L.; Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Ceriello, A.; Chiodini, P.; Esposito, K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: An updated meta-analysis of eight CVOTs. Cardiovasc. Diabetol. 2021, 20, 189. [Google Scholar] [CrossRef]
- Chen, J.; Xu, S.; Wang, L.; Zhou, W.; Li, P.; Deng, N.; Tang, Q.; Li, Y.; Wu, L.; Chen, J.; et al. Exendin-4 inhibits atrial arrhythmogenesis in a model of myocardial infarction-induced heart failure via the GLP-1 receptor signaling pathway. Exp. Ther. Med. 2020, 20, 3669–3678. [Google Scholar] [CrossRef]
- Kai, Z.; Yongbo, W.; Lin, Z.; Jie, G.; Daoqun, J.; Zhiqiang, C. Exendin-4 attenuates ische-mia-induced ventricular arrhythmias in rats. Cardiol. J. 2013, 20, 29–33. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Ye, H.; Wang, Y.; Wang, L.; Zhang, X. Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 910256. [Google Scholar] [CrossRef]
- Liu, Z.; Bian, N.; Wu, S.; Fan, Y.; Li, H.; Yu, J.; Guo, J.; Chen, D. A meta-analysis evaluating indirectly GLP-1 receptor agonists and ar-rhythmias in patients with type 2 diabetes and myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 1019120. [Google Scholar]
- Ang, R.; Mastitskaya, S.; Hosford, P.S.; Basalay, M.; Specterman, M.; Aziz, Q.; Li, Y.; Orini, M.; Taggart, P.; Lambiase, P.D.; et al. Modulation of Cardiac Ventricular Excitability by GLP-1 (Glucagon-Like Peptide-1). Circ. Arrhythmia Electrophysiol. 2018, 11, e006740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).