Exploring the Role of Genetics in Sarcoidosis and Its Impact on the Development of Cardiac Sarcoidosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Diagnosis of Sarcoidosis
3.2. Epidemiology of Sarcoidosis
3.3. Pathogenesis
3.4. Cardiac Sarcoidosis
3.5. Genetics
The Role of Genetics in the Development of Cardiac Sarcoidosis
3.6. Treatment of Sarcoidosis
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calender, A.; Weichhart, T.; Valeyre, D.; Pacheco, Y. Current Insights in Genetics of Sarcoidosis: Functional and Clinical Impacts. J. Clin. Med. 2020, 9, 2633. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.R.; Dahy, A.; Dibas, M.; Abbas, A.S.; Ghozy, S.; El-Qushayri, A.E. Association between sarcoidosis and cardiovascular comorbidity: A systematic review and meta-analysis. Heart Lung 2020, 49, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gomez, N.; Peters, J.I.; Nambiar, A.M. Diagnosis and Management of Sarcoidosis. Am. Fam. Physician 2016, 93, 840–848. [Google Scholar] [PubMed]
- Kobak, S. Catch the rainbow: Prognostic factor of sarcoidosis. Lung India 2020, 37, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Cozier, Y.C. Sarcoidosis epidemiology: Recent estimates of incidence, prevalence and risk factors. Curr. Opin. Pulm. Med. 2020, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Westney, G.E.; Judson, M.A. Racial and ethnic disparities in sarcoidosis: From genetics to socioeconomics. Clin. Chest Med. 2006, 27, 453–462. [Google Scholar] [CrossRef]
- Valeyre, D.; Prasse, A.; Nunes, H.; Uzunhan, Y.; Brillet, P.Y.; Müller-Quernheim, J. Sarcoidosis. Lancet 2014, 383, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.; Aslam, S.; Osborne, M.; Abbasi, S.; Bittencourt, M.S.; Blankstein, R. Cardiac sarcoidosis-state of the art review. Cardiovasc. Diagn. Ther. 2016, 6, 50–63. [Google Scholar] [CrossRef]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef]
- Lehtonen, J.; Uusitalo, V.; Pöyhönen, P.; Mäyränpää, M.I.; Kupari, M. Cardiac sarcoidosis: Phenotypes, diagnosis, treatment, and prognosis. Eur. Heart J. 2023, 44, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Morimoto, S.; Hiramitsu, S.; Kato, Y.; Ito, T.; Hishida, H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am. Heart J. 1999, 138 Pt 1, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Trivieri, M.G.; Spagnolo, P.; Birnie, D.; Liu, P.; Drake, W.; Kovacic, J.C.; Baughman, R.; Fayad, Z.A.; Judson, M.A. Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1878–1901. [Google Scholar] [CrossRef] [PubMed]
- Bernardinello, N.; Petrarulo, S.; Balestro, E.; Cocconcelli, E.; Veltkamp, M.; Spagnolo, P. Pulmonary Sarcoidosis: Diagnosis and Differential Diagnosis. Diagnostics 2021, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- McKinzie, B.P.; Bullington, W.M.; Mazur, J.E.; Judson, M.A. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am. J. Med. Sci. 2010, 339, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Voortman, M.; Hendriks, C.M.R.; Elfferich, M.D.P.; Bonella, F.; Møller, J.; De Vries, J.; Costabel, U.; Drent, M. The Burden of Sarcoidosis Symptoms from a Patient Perspective. Lung 2019, 197, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Carmona, E.M.; Crowson, C.S.; Matteson, E.L. Diagnostic Utility of Angiotensin-Converting Enzyme in Sarcoidosis: A Population-Based Study. Lung 2016, 194, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vanmaris, R.M.M.; Rijkers, G.T. Biological role of the soluble interleukin-2 receptor in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kraaijvanger, R.; Janssen Bonás, M.; Vorselaars, A.D.M.; Veltkamp, M. Biomarkers in the Diagnosis and Prognosis of Sarcoidosis: Current Use and Future Prospects. Front. Immunol. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Prabhakar, H.B.; Rabinowitz, C.B.; Gibbons, F.K.; O’Donnell, W.J.; Shepard, J.A.; Aquino, S.L. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am. J. Roentgenol. 2008, 190 (Suppl. S3), S1–S6. [Google Scholar] [CrossRef]
- Casella, M.; Dello Russo, A.; Bergonti, M.; Catto, V.; Conte, E.; Sommariva, E.; Gasperetti, A.; Vettor, G.; Tundo, F.; Sicuso, R.; et al. Diagnostic Yield of Electroanatomic Voltage Mapping in Guiding Endomyocardial Biopsies. Circulation 2020, 142, 1249–1260. [Google Scholar] [CrossRef]
- Benz, D.C.; Gräni, C.; Antiochos, P.; Heydari, B.; Gissler, M.C.; Ge, Y.; Cuddy, S.A.M.; Dorbala, S.; Kwong, R.Y. Cardiac magnetic resonance biomarkers as surrogate endpoints in cardiovascular trials for myocardial diseases. Eur. Heart J. 2023, 44, 4738–4747. [Google Scholar] [CrossRef]
- Marschner, C.A.; Aloufi, F.; Aitken, M.; Cheung, E.; Thavendiranathan, P.; Iwanochko, R.M.; Balter, M.; Moayedi, Y.; Duero Posada, J.; Hanneman, K. Combined FDG PET/MRI versus Standard-of-Care Imaging in the Evaluation of Cardiac Sarcoidosis. Radiol. Cardiothorac. Imaging 2023, 5, e220292. [Google Scholar] [CrossRef]
- Kurashima, S.; Kitai, T.; Xanthopoulos, A.; Skoularigis, J.; Triposkiadis, F.; Izumi, C. Diagnosis of cardiac sarcoidosis: Histological evidence vs. imaging. Expert. Rev. Cardiovasc. Ther. 2023, 21, 693–702. [Google Scholar] [CrossRef]
- Hervier, E.; Glessgen, C.; Nkoulou, R.; François Deux, J.; Vallee, J.P.; Adamopoulos, D. Hybrid PET/MR in Cardiac Imaging. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 613–624. [Google Scholar] [CrossRef]
- Shrivastav, R.; Hajra, A.; Krishnan, S.; Bandyopadhyay, D.; Ranjan, P.; Fuisz, A. Evaluation and Management of Cardiac Sarcoidosis with Advanced Imaging. Heart Fail. Clin. 2023, 19, 475–489. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Isted, A.; Hinojar, R.; Foote, L.; Carr-White, G.; Nagel, E. T1 and T2 Mapping in Recognition of Early Cardiac Involvement in Systemic Sarcoidosis. Radiology 2017, 285, 63–72. [Google Scholar] [CrossRef]
- Okafor, J.; Khattar, R.; Sharma, R.; Kouranos, V. The Role of Echocardiography in the Contemporary Diagnosis and Prognosis of Cardiac Sarcoidosis: A Comprehensive Review. Life 2023, 13, 1653. [Google Scholar] [CrossRef]
- Dweck, M.R.; Abgral, R.; Trivieri, M.G.; Robson, P.M.; Karakatsanis, N.; Mani, V.; Palmisano, A.; Miller, M.A.; Lala, A.; Chang, H.L.; et al. Hybrid Magnetic Resonance Imaging and Positron Emission Tomography with Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2018, 11, 94–107. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Terasaki, F.; Azuma, A.; Anzai, T.; Ishizaka, N.; Ishida, Y.; Isobe, M.; Inomata, T.; Ishibashi-Ueda, H.; Eishi, Y.; Kitakaze, M.; et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis—Digest Version. Circ. J. 2019, 83, 2329–2388. [Google Scholar] [CrossRef]
- Savale, L.; Huitema, M.; Shlobin, O.; Kouranos, V.; Nathan, S.D.; Nunes, H.; Gupta, R.; Grutters, J.C.; Culver, D.A.; Post, M.C.; et al. WASOG statement on the diagnosis and management of sarcoidosis-associated pulmonary hypertension. Eur. Respir. Rev. 2022, 31, 210165. [Google Scholar] [CrossRef]
- Bokhari, S.; Sheikh, T. Cardiac sarcoidosis: Advantages and limitations of advanced cardiac imaging. J. Nucl. Cardiol. 2022, 29, 2145–2148. [Google Scholar] [CrossRef]
- Pöyhönen, P.; Nordenswan, H.K.; Lehtonen, J.; Syväranta, S.; Shenoy, C.; Kupari, M. Cardiac magnetic resonance in giant cell myocarditis: A matched comparison with cardiac sarcoidosis. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 404–412. [Google Scholar] [CrossRef]
- Ekström, K.; Räisänen-Sokolowski, A.; Lehtonen, J.; Nordenswan, H.K.; Mäyränpää, M.I.; Kupari, M. Idiopathic giant cell myocarditis or cardiac sarcoidosis? A retrospective audit of a nationwide case series. ESC Heart Fail. 2020, 7, 1362–1370. [Google Scholar] [CrossRef]
- Trivieri, M.G.; Robson, P.M.; Vergani, V.; LaRocca, G.; Romero-Daza, A.M.; Abgral, R.; Devesa, A.; Azoulay, L.-D.; Karakatsanis, N.A.; Parikh, A.; et al. Hybrid Magnetic Resonance Positron Emission Tomography Is Associated with Cardiac-Related Outcomes in Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2024, 17, 411–424. [Google Scholar] [CrossRef]
- Jeudy, J.; Burke, A.P.; White, C.S.; Kramer, G.B.; Frazier, A.A. Cardiac sarcoidosis: The challenge of radiologic-pathologic correlation: From the radiologic pathology archives. Radiographics 2015, 35, 657–679. [Google Scholar] [CrossRef]
- Morimoto, T.; Azuma, A.; Abe, S.; Usuki, J.; Kudoh, S.; Sugisaki, K.; Oritsu, M.; Nukiwa, T. Epidemiology of sarcoidosis in Japan. Eur. Respir. J. 2008, 31, 372–379. [Google Scholar] [CrossRef]
- Birnbaum, A.D.; Rifkin, L.M. Sarcoidosis: Sex-dependent variations in presentation and management. J. Ophthalmol. 2014, 2014, 236905. [Google Scholar] [CrossRef]
- Giblin, G.T.; Murphy, L.; Stewart, G.C.; Desai, A.S.; Di Carli, M.F.; Blankstein, R.; Givertz, M.M.; Tedrow, U.B.; Sauer, W.H.; Hunninghake, G.M.; et al. Cardiac Sarcoidosis: When and How to Treat Inflammation. Card. Fail. Rev. 2021, 7, e17. [Google Scholar] [CrossRef]
- Zhang, H.; Costabel, U.; Dai, H. The Role of Diverse Immune Cells in Sarcoidosis. Front. Immunol. 2021, 12, 788502. [Google Scholar] [CrossRef]
- Schnerch, J.; Prasse, A.; Vlachakis, D.; Schuchardt, K.L.; Pechkovsky, D.V.; Goldmann, T.; Gaede, K.I.; Müller-Quernheim, J.; Zissel, G. Functional Toll-Like Receptor 9 Expression and CXCR3 Ligand Release in Pulmonary Sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 749–757. [Google Scholar] [CrossRef]
- Chahal, C.A.A.; Brady, P.A.; Cooper LTJr Lin, G.; Somers, V.K.; Crowson, C.S.; Matteson, E.L.; Ungprasert, P. Risk of Sudden Death in a General Unbiased Epidemiological Cohort of Sarcoidosis. J. Am. Heart Assoc. 2022, 11, e025479. [Google Scholar] [CrossRef]
- Narasimhan, B.; Patel, N.; Ho, K.; Amgai, B.; Okada, D.R.; Bandyopadhyay, D.; Krittanawong, C.; Wu, L.; Bhatia, K.; Shah, R.; et al. Incidence and Predictors of Sudden Cardiac Arrest in Sarcoidosis: A Nationwide Analysis. JACC Clin. Electrophysiol. 2021, 7, 1087–1095. [Google Scholar] [CrossRef]
- Ekström, K.; Lehtonen, J.; Nordenswan, H.K.; Mäyränpää, M.I.; Räisänen-Sokolowski, A.; Kandolin, R.; Simonen, P.; Pietilä-Effati, P.; Alatalo, A.; Utriainen, S.; et al. Sudden death in cardiac sarcoidosis: An analysis of nationwide clinical and cause-of-death registries. Eur. Heart J. 2019, 40, 3121–3128. [Google Scholar] [CrossRef]
- Okada, D.R.; Bravo, P.E.; Vita, T.; Agarwal, V.; Osborne, M.T.; Taqueti, V.R.; Skali, H.; Chareonthaitawee, P.; Dorbala, S.; Stewart, G.; et al. Isolated cardiac sarcoidosis: A focused review of an under-recognized entity. J. Nucl. Cardiol. 2018, 25, 1136–1146. [Google Scholar] [CrossRef]
- Smedema, J.P.; van Geuns, R.J.; Ainslie, G.; Ector, J.; Heidbuchel, H.; Crijns, H.J.G.M. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Fail. 2017, 4, 535–544. [Google Scholar] [CrossRef]
- Okada, D.R.; Smith, J.; Derakhshan, A.; Gowani, Z.; Misra, S.; Berger, R.D.; Calkins, H.; Tandri, H.; Chrispin, J. Ventricular Arrhythmias in Cardiac Sarcoidosis. Circulation 2018, 138, 1253–1264. [Google Scholar] [CrossRef]
- Vij, O.; Dey, M.; Morrison, K.; Kouranloo, K. Incidence, management and prognosis of new-onset sarcoidosis post COVID-19 infection. Sarcoidosis Vasc. Diffus. Lung Dis. 2024, 41, e2024004. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T., Jr. COVID-19, Myocarditis and Pericarditis. Circ. Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef]
- Zadeh, A.V.; Redondo, A.; Carrero, R.C.; Collado, E.; Larned, J.M. The impact of sarcoidosis on the risk of COVID-19-related myocarditis. J. Am. Coll. Cardiol. 2024, 83 (Suppl. 113), 678. [Google Scholar] [CrossRef]
- Bollano, E.; Polte, C.L.; Mäyränpää, M.I.; Oldfors, A.; Bergh, N.; Lehtonen, J.; Kandolin, R. Cardiac sarcoidosis and giant cell myocarditis after COVID-19 infection. ESC Heart Fail. 2022, 9, 4298–4303. [Google Scholar] [CrossRef]
- Ueberham, L.; Hagendorff, A.; Klingel, K.; Paetsch, I.; Jahnke, C.; Kluge, T.; Ebbinghaus, H.; Hindricks, G.; Laufs, U.; Dinov, B. Pathophysiological Gaps, Diagnostic Challenges, and Uncertainties in Cardiac Sarcoidosis. J. Am. Heart Assoc. 2023, 12, e027971. [Google Scholar] [CrossRef]
- Sverrild, A.; Backer, V.; Kyvik, K.O.; Kaprio, J.; Milman, N.; Svendsen, C.B.; Thomsen, S.F. Heredity in sarcoidosis: A registry-based twin study. Thorax 2008, 63, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Hena, K.M. Sarcoidosis Epidemiology: Race Matters. Front. Immunol. 2020, 11, 537382. [Google Scholar] [CrossRef]
- Iannuzzi, M.C.; Iyengar, S.K.; Gray-McGuire, C.; Elston, R.C.; Baughman, R.P.; Donohue, J.F.; Hirst, K.; Judson, M.A.; Kavuru, M.S.; Maliarik, M.J.; et al. Genome-wide search for sarcoidosis susceptibility genes in African Americans. Genes Immun. 2005, 6, 509–518. [Google Scholar] [CrossRef]
- Semenzato, G. ACCESS: A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 83–86. [Google Scholar]
- Sikorova, K.; Osoegawa, K.; Kocourkova, L.; Strnad, A.; Petrkova, J.; Fernández-Viña, M.A.; Doubkova, M.; Petrek, M. Association between sarcoidosis and HLA polymorphisms in a Czech population from Central Europe: Focus on a relationship with clinical outcome and treatment. Front. Med. 2023, 10, 1094843. [Google Scholar] [CrossRef]
- Voorter, C.E.; Drent, M.; van den Berg-Loonen, E.M. Severe pulmonary sarcoidosis is strongly associated with the haplotype HLA-DQB1*0602-DRB1*150101. Hum. Immunol. 2005, 66, 826–835. [Google Scholar] [CrossRef]
- Becker, C.D.; Sridhar, P.; Iannuzzi, M.C. Cardiac sarcoidosis associated with BTNL2. Cardiology 2009, 112, 76–79. [Google Scholar] [CrossRef]
- Rossman, M.D.; Thompson, B.; Frederick, M.; Maliarik, M.; Iannuzzi, M.C.; Rybicki, B.A.; Pandey, J.P.; Newman, L.S.; Magira, E.; Beznik-Cizman, B.; et al. HLA-DRB1*1101: A significant risk factor for sarcoidosis in blacks and whites. Am. J. Hum. Genet. 2003, 73, 720–735. [Google Scholar] [CrossRef]
- Dehghan, A. Genome-Wide Association Studies. Methods Mol. Biol. 2018, 1793, 37–49. [Google Scholar] [CrossRef]
- Jaganathan, D.; Bohra, A.; Thudi, M.; Varshney, R.K. Fine mapping and gene cloning in the post-NGS era: Advances and prospects. Theor. Appl. Genet. 2020, 133, 1791–1810. [Google Scholar] [CrossRef]
- Zorzetto, M.; Bombieri, C.; Ferrarotti, I.; Medaglia, S.; Agostini, C.; Tinelli, C.; Malerba, G.; Carrabino, N.; Beretta, A.; Casali, L.; et al. Complement receptor 1 gene polymorphisms in sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2002, 27, 17–23. [Google Scholar] [CrossRef]
- Hill, M.R.; Papafili, A.; Booth, H.; Lawson, P.; Hubner, M.; Beynon, H.; Read, C.; Lindahl, G.; Marshall, R.P.; McAnulty, R.J.; et al. Functional prostaglandin-endoperoxide synthase 2 polymorphism predicts poor outcome in sarcoidosis. Am. J. Respir. Crit. Care Med. 2006, 174, 915–922. [Google Scholar] [CrossRef]
- Lahtela, E.; Kankainen, M.; Sinisalo, J.; Selroos, O.; Lokki, M.L. Exome Sequencing Identifies Susceptibility Loci for Sarcoidosis Prognosis. Front. Immunol. 2019, 10, 2964. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, D.; Lim, L.L.; Allada, G.; Smith, J.R.; Austin, C.R.; Doyle, T.M.; Goodwin, K.A.; Rosenbaum, J.T.; Martin, T.M. Association of interleukin 23 receptor gene with sarcoidosis. Dis. Markers 2011, 31, 17–24. [Google Scholar] [CrossRef]
- Spagnolo, P.; Maier, L.A. Genetics in sarcoidosis. Curr. Opin. Pulm. Med. 2021, 27, 423–429. [Google Scholar] [CrossRef]
- Karakaya, B.; van Moorsel, C.H.M.; Veltkamp, M.; Roodenburg-Benschop, C.; Kazemier, K.M.; van der Helm-van Mil, A.H.M.; Huizinga, T.W.J.; Grutters, J.C.; Rijkers, G.T. A Polymorphism in C-C Chemokine Receptor 5 (CCR5) Associates with Löfgren’s Syndrome and Alters Receptor Expression as well as Functional Response. Cells 2021, 10, 1967. [Google Scholar] [CrossRef]
- Garman, L.; Pezant, N.; Pastori, A.; Savoy, K.A.; Li, C.; Levin, A.M.; Iannuzzi, M.C.; Rybicki, B.A.; Adrianto, I.; Montgomery, C.G. Genome-Wide Association Study of Ocular Sarcoidosis Confirms HLA Associations and Implicates Barrier Function and Autoimmunity in African Americans. Ocul. Immunol. Inflamm. 2021, 29, 244–249. [Google Scholar] [CrossRef]
- Drent, M.; van den Berg, R.; Haenen, G.R.; van den Berg, H.; Wouters, E.F.; Bast, A. NF-kappaB activation in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2001, 18, 50–56. [Google Scholar]
- Gray-McGuire, C.; Sinha, R.; Iyengar, S.; Millard, C.; Rybicki, B.A.; Elston, R.C.; Iannuzzi, M.C.; SAGA Study Consortium. Genetic characterization and fine mapping of susceptibility loci for sarcoidosis in African Americans on chromosome 5. Hum. Genet. 2006, 120, 420–430. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Ozturk, O.G.; Durmaz, A.; Othman Hasan, O.; Guzelbaba, B.; Seydaoglu, G.; Kuleci, S.; Hanta, I.; Erken, E.; Kocabas, A. Early prediction of sarcoidosis prognosis with HLA typing: A 5 year follow-up study. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 184–191. [Google Scholar] [CrossRef]
- Hedfors, E.; Lindström, F. HLA-B8/DR3 in sarcoidosis. Correlation to acute onset disease with arthritis. Tissue Antigens 1983, 22, 200–203. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, L.; Zhang, Y.; Jiang, D.; Li, H. Human leukocyte antigen-A, -B, and -DRB1 alleles and sarcoidosis in Chinese Han subjects. Hum. Immunol. 2011, 72, 571–575. [Google Scholar] [CrossRef]
- Sato, H.; Woodhead, F.A.; Ahmad, T.; Grutters, J.C.; Spagnolo, P.; van den Bosch, J.M.; Maier, L.A.; Newman, L.S.; Nagai, S.; Izumi, T.; et al. Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum. Mol. Genet. 2010, 19, 4100–4111. [Google Scholar] [CrossRef]
- Liao, S.Y.; Jacobson, S.; Hamzeh, N.Y.; Culver, D.A.; Barkes, B.Q.; Mroz, M.; Macphail, K.; Pacheco, K.; Patel, D.C.; Wasfi, Y.S.; et al. Genome-wide association study identifies multiple HLA loci for sarcoidosis susceptibility. Hum. Mol. Genet. 2023, 32, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Naruse, T.K.; Matsuzawa, Y.; Ota, M.; Katsuyama, Y.; Matsumori, A.; Hara, M.; Nagai, S.; Morimoto, S.; Sasayama, S.; Inoko, H. HLA-DQB1*0601 is primarily associated with the susceptibility to cardiac sarcoidosis. Tissue Antigens 2000, 56, 52–57. [Google Scholar] [CrossRef]
- Levin, A.M.; Adrianto, I.; Datta, I.; Iannuzzi, M.C.; Trudeau, S.; Li, J.; Drake, W.P.; Montgomery, C.G.; Rybicki, B.A. Association of HLA-DRB1 with Sarcoidosis Susceptibility and Progression in African Americans. Am. J. Respir. Cell Mol. Biol. 2015, 53, 206–216. [Google Scholar] [CrossRef]
- Darlington, P.; Gabrielsen, A.; Sörensson, P.; Tallstedt, L.; Padyukov, L.; Eklund, A.; Grunewald, J. HLA-alleles associated with increased risk for extra-pulmonary involvement in sarcoidosis. Tissue Antigens 2014, 83, 267–272. [Google Scholar] [CrossRef]
- Li, Y.; Wollnik, B.; Pabst, S.; Lennarz, M.; Rohmann, E.; Gillissen, A.; Vetter, H.; Grohé, C. BTNL2 gene variant and sarcoidosis. Thorax 2006, 61, 273–274. [Google Scholar] [CrossRef]
- Meyer, T.; Lauschke, J.; Ruppert, V.; Richter, A.; Pankuweit, S.; Maisch, B. Isolated cardiac sarcoidosis associated with the expression of a splice variant coding for a truncated BTNL2 protein. Cardiology 2008, 109, 117–121. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Koscinska, K.; Suchnicki, K.; Lange, A. HSP70-hom gene single nucleotide (+2763 G/A and +2437 C/T) polymorphisms in sarcoidosis. Int. J. Immunogenet. 2006, 33, 135–140. [Google Scholar] [CrossRef]
- Casanova, N.G.; Gonzalez-Garay, M.L.; Sun, B.; Bime, C.; Sun, X.; Knox, K.S.; Crouser, E.D.; Sammani, N.; Gonzales, T.; Natt, B.; et al. Differential transcriptomics in sarcoidosis lung and lymph node granulomas with comparisons to pathogen-specific granulomas. Respir. Res. 2020, 21, 321. [Google Scholar] [CrossRef]
- McDougal, K.E.; Fallin, M.D.; Moller, D.R.; Song, Z.; Cutler, D.J.; Steiner, L.L.; Cutting, G.R.; ACCESS Research Group. Variation in the lymphotoxin-alpha/tumor necrosis factor locus modifies risk of erythema nodosum in sarcoidosis. J. Investig. Dermatol. 2009, 129, 1921–1926. [Google Scholar] [CrossRef]
- Davoudi, S.; Chang, V.S.; Navarro-Gomez, D.; Stanwyck, L.K.; Sevgi, D.D.; Papavasileiou, E.; Ren, A.; Uchiyama, E.; Sullivan, L.; Lobo, A.M.; et al. Association of genetic variants in RAB23 and ANXA11 with uveitis in sarcoidosis. Mol. Vis. 2018, 24, 59–74. [Google Scholar]
- Foley, P.J.; Lympany, P.A.; Puscinska, E.; Zielinski, J.; Welsh, K.I.; du Bois, R.M. Analysis of MHC encoded antigen-processing genes TAP1 and TAP2 polymorphisms in sarcoidosis. Am. J. Respir. Crit. Care Med. 1999, 160, 1009–1014. [Google Scholar] [CrossRef]
- Medica, I.; Kastrin, A.; Maver, A.; Peterlin, B. Role of genetic polymorphisms in ACE and TNF-alpha gene in sarcoidosis: A meta-analysis. J. Hum. Genet. 2007, 52, 836–847. [Google Scholar] [CrossRef]
- Gialafos, E.; Triposkiadis, F.; Kouranos, V.; Rapti, A.; Kosmas, I.; Manali, E.; Giamouzis, G.; Elezoglou, A.; Peros, I.; Anagnostopoulou, O.; et al. Relationship between tumor necrosis factor-α (TNFA) gene polymorphisms and cardiac sarcoidosis. In Vivo 2014, 28, 1125–1129. [Google Scholar]
- Meguro, A.; Ishihara, M.; Petrek, M.; Yamamoto, K.; Takeuchi, M.; Mrazek, F.; Kolek, V.; Benicka, A.; Yamane, T.; Shibuya, E.; et al. Genetic control of CCL24, POR, and IL23R contributes to the pathogenesis of sarcoidosis. Commun. Biol. 2020, 3, 465. [Google Scholar] [CrossRef]
- Akahoshi, M.; Ishihara, M.; Remus, N.; Uno, K.; Miyake, K.; Hirota, T.; Nakashima, K.; Matsuda, A.; Kanda, M.; Enomoto, T.; et al. Association between IFNA genotype and the risk of sarcoidosis. Hum. Genet. 2004, 114, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Pabst, S.; Baumgarten, G.; Stremmel, A.; Lennarz, M.; Knüfermann, P.; Gillissen, A.; Vetter, H.; Grohé, C. Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin. Exp. Immunol. 2006, 143, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Calender, A.; Lim, C.X.; Weichhart, T.; Buisson, A.; Besnard, V.; Rollat-Farnier, P.A.; Bardel, C.; Roy, P.; Cottin, V.; Devouassoux, G.; et al. Exome sequencing and pathogenicity-network analysis of five French families implicate mTOR signalling and autophagy in familial sarcoidosis. Eur. Respir. J. 2019, 54, 1900430. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.L.; Rybicki, B.A.; Iannuzzi, M.C.; Elston, R.C.; Iyengar, S.K.; Gray-McGuire, C.; Sarcoidosis Genetic Analysis Consortium (SAGA). Reduction of sample heterogeneity through use of population substructure: An example from a population of African American families with sarcoidosis. Am. J. Hum. Genet. 2006, 79, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Ohchi, T.; Shijubo, N.; Kawabata, I.; Ichimiya, S.; Inomata, S.; Yamaguchi, A.; Umemori, Y.; Itoh, Y.; Abe, S.; Hiraga, Y.; et al. Polymorphism of Clara cell 10-kD protein gene of sarcoidosis. Am. J. Respir. Crit. Care Med. 2004, 169, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Schmid, B.; Ellinghaus, D.; Nothnagel, M.; Gaede, K.I.; Schürmann, M.; Lipinski, S.; Rosenstiel, P.; Zissel, G.; Höhne, K.; et al. A novel sarcoidosis risk locus for Europeans on chromosome 11q13. 1. Am. J. Respir. Crit. Care Med. 2012, 186, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Stjepanovic, M.I.; Mihailovic-Vucinic, V.; Spasovski, V.; Milin-Lazovic, J.; Skodric-Trifunovic, V.; Stankovic, S.; Andjelkovic, M.; Komazec, J.; Momcilovic, A.; Santric-Milicevic, M.; et al. Genes and metabolic pathway of sarcoidosis: Identification of key players and risk modifiers. Arch. Med. Sci. 2019, 15, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Ellinghaus, D.; Nutsua, M.; Hofmann, S.; Montgomery, C.G.; Iannuzzi, M.C.; Rybicki, B.A.; Petrek, M.; Mrazek, F.; Pabst, S.; et al. Identification of Immune-Relevant Factors Conferring Sarcoidosis Genetic Risk. Am. J. Respir. Crit. Care Med. 2015, 192, 727–736. [Google Scholar] [CrossRef]
- Hofmann, S.; Fischer, A.; Nothnagel, M.; Jacobs, G.; Schmid, B.; Wittig, M.; Franke, A.; Gaede, K.I.; Schürmann, M.; Petrek, M.; et al. Genome-wide association analysis reveals 12q13.3-q14.1 as new risk locus for sarcoidosis. Eur. Respir. J. 2013, 41, 888–900. [Google Scholar] [CrossRef]
- Pabst, S.; Fränken, T.; Schönau, J.; Stier, S.; Nickenig, G.; Meyer, R.; Skowasch, D.; Grohé, C. Transforming growth factor-{beta} gene polymorphisms in different phenotypes of sarcoidosis. Eur. Respir. J. 2011, 38, 169–175. [Google Scholar] [CrossRef]
- Lareau, C.A.; Adrianto, I.; Levin, A.M.; Iannuzzi, M.C.; Rybicki, B.A.; Montgomery, C.G. Fine mapping of chromosome 15q25 implicates ZNF592 in neurosarcoidosis patients. Ann. Clin. Transl. Neurol. 2015, 2, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, S.; Navarro-Gomez, D.; Shen, L.; Ung, C.; Ren, A.; Sullivan, L.; Kwong, M.; Janessian, M.; Comander, J.; Gai, X.; et al. NOD2 genetic variants and sarcoidosis-associated uveitis. Am. J. Ophthalmol. Case Rep. 2016, 3, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.M.; Iannuzzi, M.C.; Montgomery, C.G.; Trudeau, S.; Datta, I.; Adrianto, I.; Chitale, D.A.; McKeigue, P.; Rybicki, B.A. Admixture fine-mapping in African Americans implicates XAF1 as a possible sarcoidosis risk gene. PLoS ONE 2014, 9, e92646. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.A.; Adrianto, I.; Dumancas, G.G.; Levin, A.M.; Iannuzzi, M.C.; Rybicki, B.A.; Montgomery, C. Role of NOD2 Pathway Genes in Sarcoidosis Cases with Clinical Characteristics of Blau Syndrome. Am. J. Respir. Crit. Care Med. 2015, 192, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Reichel, P.; Müller-Myhsok, B.; Schlaak, M.; Müller-Quernheim, J.; Schwinger, E. Results from a genome-wide search for predisposing genes in sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 164, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, M.C.; Rybicki, B.A. Genetics of sarcoidosis: Candidate genes and genome scans. Proc. Am. Thorac. Soc. 2007, 4, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Garman, L.; Montgomery, C.G.; Rivera, N.V. Recent advances in sarcoidosis genomics: Epigenetics, gene expression, and gene by environment (G×E) interaction studies. Curr. Opin. Pulm. Med. 2020, 26, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Giuliani, L.; Di Toro, A. Genetic Basis of Myocarditis: Myth. or Reality? In Myocarditis; Published 7 March 2020; Springer: Cham, Switzerland, 2020; pp. 45–89. [Google Scholar] [CrossRef]
- Lota, A.S.; Hazebroek, M.R.; Theotokis, P.; Wassall, R.; Salmi, S.; Halliday, B.P.; Tayal, U.; Verdonschot, J.; Meena, D.; Owen, R.; et al. Genetic Architecture of Acute Myocarditis and the Overlap with Inherited Cardiomyopathy. Circulation 2022, 146, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Korthals, D.; Bietenbeck, M.; Könemann, H.; Doldi, F.; Ventura, D.; Schäfers, M.; Mohr, M.; Wolfes, J.; Wegner, F.; Yilmaz, A.; et al. Cardiac Sarcoidosis-Diagnostic and Therapeutic Challenges. J. Clin. Med. 2024, 13, 1694. [Google Scholar] [CrossRef]
- Sohn, D.W.; Park, J.B. Cardiac sarcoidosis. Heart 2023, 109, 1132–1138. [Google Scholar] [CrossRef]
- Serei, V.D.; Fyfe, B. The Many Faces of Cardiac Sarcoidosis. Am. J. Clin. Pathol. 2020, 153, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Takashige, N.; Naruse, T.K.; Matsumori, A.; Hara, M.; Nagai, S.; Morimoto, S.; Hiramitsu, S.; Sasayama, S.; Inoko, H. Genetic polymorphisms at the tumour necrosis factor loci (TNFA and TNFB) in cardiac sarcoidosis. Tissue Antigens 1999, 54, 191–193. [Google Scholar] [CrossRef]
- Fischer, A.; Rybicki, B.A. Granuloma genes in sarcoidosis: What is new? Curr. Opin. Pulm. Med. 2015, 21, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Scheel, P.J., 3rd; Cartella, I.; Murray, B.; Gilotra, N.A.; Ammirati, E. Role of genetics in inflammatory cardiomyopathy. Int. J. Cardiol. 2024, 400, 131777. [Google Scholar] [CrossRef] [PubMed]
- Castrichini, M.; Agboola, K.M.; Vyas, H.; Abou Ezzeddine, O.F.; Siontis, K.C.; Giudicessi, J.R.; Rosenbaum, A.N.; Pereira, N.L. Cardiac Sarcoidosis Mimickers: Genetic Testing in Undifferentiated Inflammatory Cardiomyopathies. Circ. Genom. Precis. Med. 2023, 16, 478–479. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
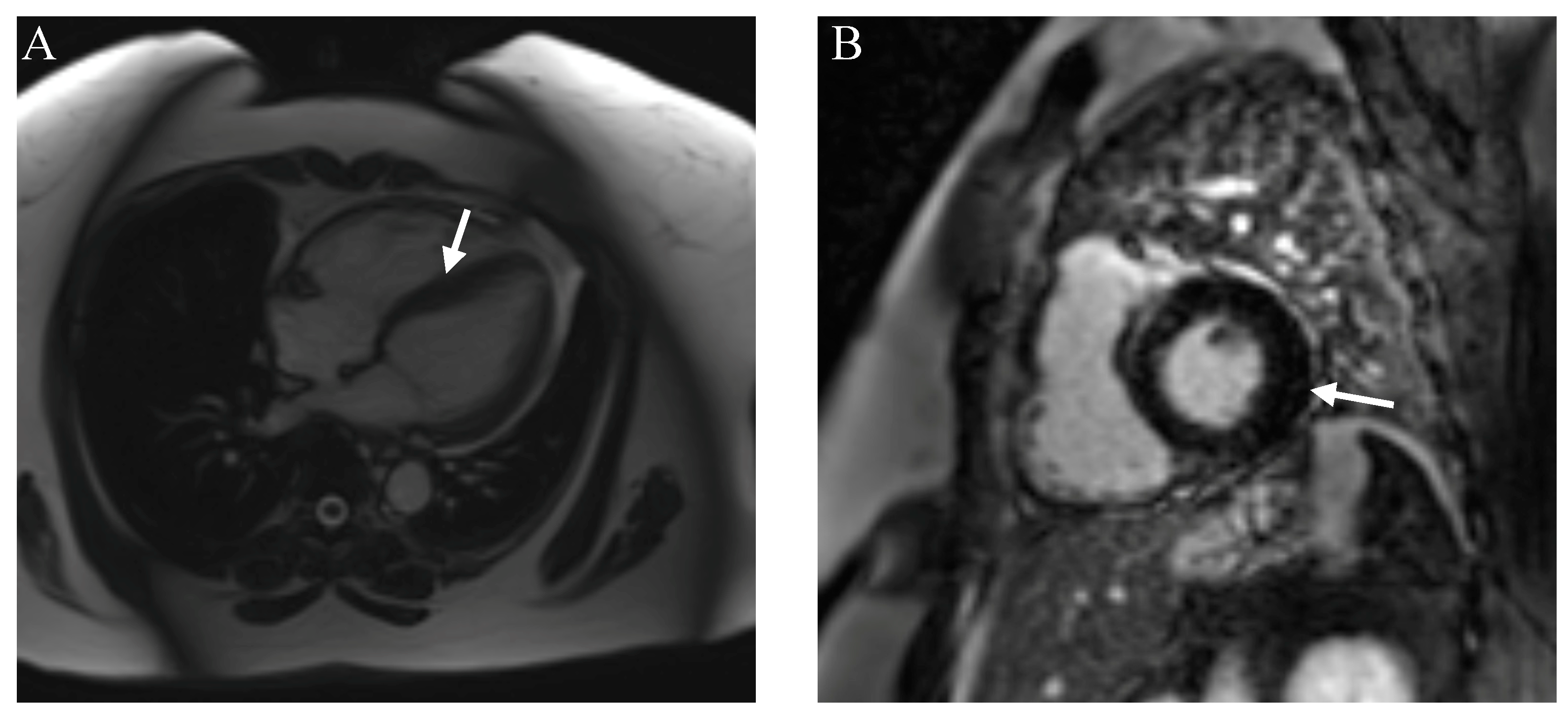
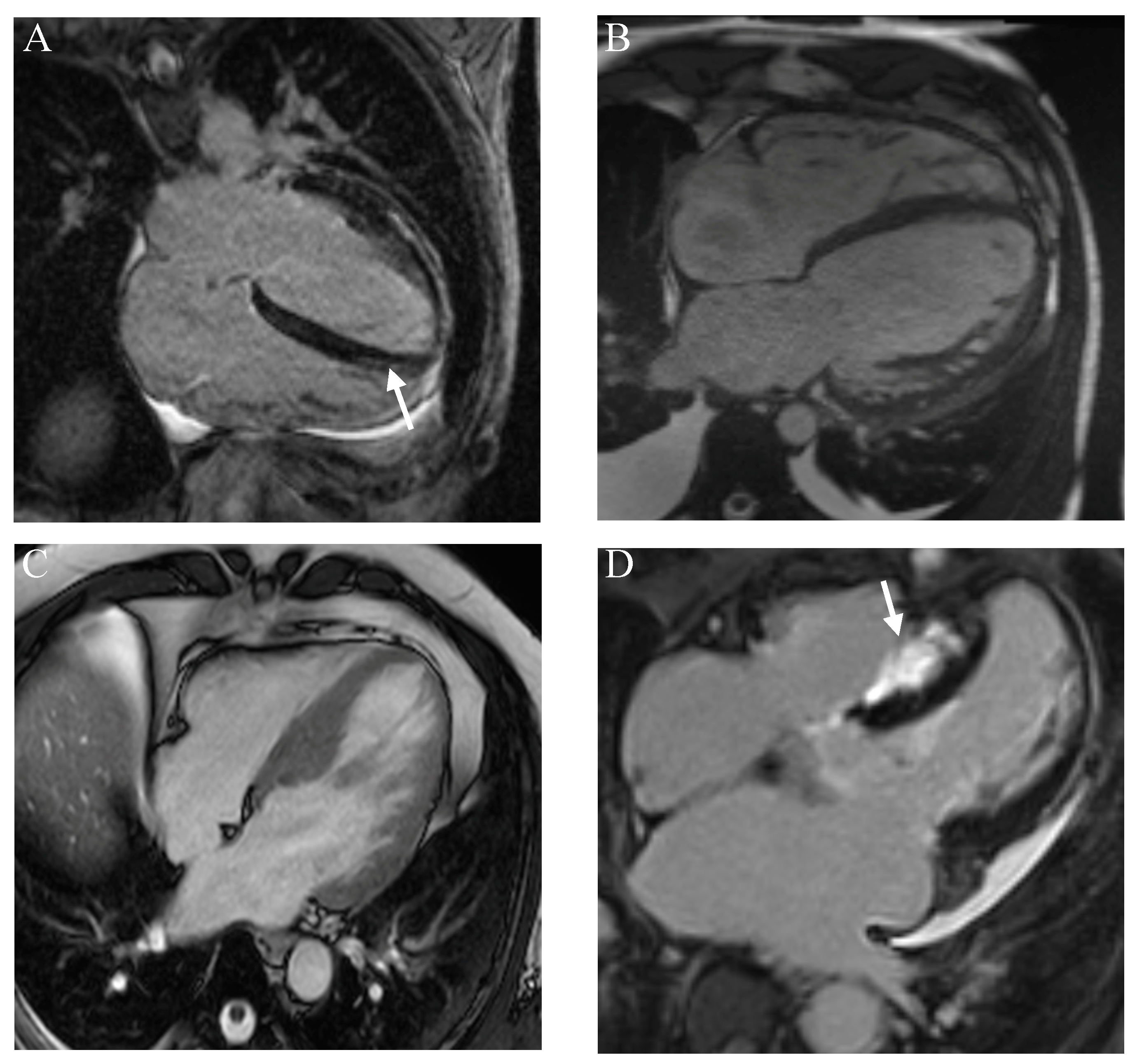

| Cardiac Disorder | Clinical Features | Imaging Features | Biochemical/Genetic Features |
|---|---|---|---|
| HCM |
|
|
|
| DCM |
|
|
|
| ACM |
|
|
|
| Amyloidosis |
|
|
|
| Myocarditis |
|
|
|
| Chromosome | Genetic Variant/Locus | Features |
|---|---|---|
| 1 | 1q32 [64] 1p22 [56] PTGS2 [65] AADACL3 [66] | Disease resolution |
| 1 | IL23 [67] | Nervous system involvement |
| 3 | 3p12-14 [68] CCR5 [69] MAGI1 [70] | Progression of lung disease Ocular involvement |
| 4 | NFKB1 [71] | |
| 5 | 5q11.2 [72] 5p15 [72] | Protective |
| 6 | HLA-B7 [73] HLA-B8 [74] HLA-B*51 [75] HLA-C [58] HLA-DPA1/DPB1 [58] HLA-DPB1 (*0101) [61] HLA-DQA1*0301 [76] HLA-DQA1*0501 [77]: HLA-DQA1*0505 [58] HLA-DQB1*0201 [76] HLA-DQB1*0302 [58] HLA-DQB1*0503/4 [76] HLA-DQB1*0601 [78]: HLA-DQB1*0602 [58] HLA-DQB1*0604 [58] HLA-DQB1*1501 [76] HLA-DRB1*0101 [76] HLA-DRB1*03 [58] HLA-DRB1*04 [58] HLA-DRB1*0301 [79]: HLA-DRB1*0302 [80] HLA-DRB1*04 [80] HLA-DRB1*08 [76] HLA-DRB1*14 [76] HLA-DRB1*15 [58] HLA-DRB1*1101 [61] HLA-DRB1*1201 [76] | Increased frequency in African Americans Associated with Lofgren Syndrome; (Han-Chinese) Protective Associated with Lofgren syndrome Chronic course Associated with Lofgren syndrome Protective Chronic; associated with cardiac sarcoidosis Chronic course Chronic course Protective Spontaneous resolution; associated with Lofgren syndrome Protective Decreased risk of extra-pulmonary manifestations; immune response to mycobacterial antigens Increased risk of extra thoracic and skin manifestations Increased risk of extra thoracic and skin manifestations; increased risk of uveitis Associated with left ventricular systolic dysfunction and chronic course Chronic course Chronic course |
| 6 | BTNL2 [81,82] HSPA1L [83] NOTCH4 [84] LTA [85] RAB23 [86]: TAP2 [87] TNFα [88,89] | Isolated cardiac sarcoidosis Associated in African American and European patients Associated with erythema nodosum in female Caucasians Associated with uveitis Associated in European patients Associated in European patients; cardiac sarcoidosis. |
| 7 | CCL24 [90] STYXL1/SRRM3 [90] | Associated with both disease development and spontaneous remission |
| 9 | 9p22 [91] 9q32 [92] 9q34 [72] | Chronic disease |
| 10 | ANXA11 (10q22.3) [86] DDIT4 [93] | Associated in European patients |
| 11 | 11p15 [94] 11q12-13 [95] CCDC88B (11q13) [96] | Associated with inflammatory bowel disease |
| 12 | CYP27B1 [97] SH2B3 [98] 12q13.3–q14.1 (OS9) [99] | Protective |
| 14 | TGF-β3 [100] | |
| 15 | ZNF592 [101] | Associated with neurological involvement in African American and European American patients |
| 16 | NOD2 [102] | Associated with early onset sarcoidosis and uveitis |
| 17 | 17q21 [100] XAF1 [103] | |
| 19 | DBP [97] KIR3DL1/KIRDS1 [65] | |
| 22 | TAB1-TAB2 [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivalokanathan, S. Exploring the Role of Genetics in Sarcoidosis and Its Impact on the Development of Cardiac Sarcoidosis. Cardiogenetics 2024, 14, 106-121. https://doi.org/10.3390/cardiogenetics14020009
Sivalokanathan S. Exploring the Role of Genetics in Sarcoidosis and Its Impact on the Development of Cardiac Sarcoidosis. Cardiogenetics. 2024; 14(2):106-121. https://doi.org/10.3390/cardiogenetics14020009
Chicago/Turabian StyleSivalokanathan, Sanjay. 2024. "Exploring the Role of Genetics in Sarcoidosis and Its Impact on the Development of Cardiac Sarcoidosis" Cardiogenetics 14, no. 2: 106-121. https://doi.org/10.3390/cardiogenetics14020009
APA StyleSivalokanathan, S. (2024). Exploring the Role of Genetics in Sarcoidosis and Its Impact on the Development of Cardiac Sarcoidosis. Cardiogenetics, 14(2), 106-121. https://doi.org/10.3390/cardiogenetics14020009





