Electrical Characterization and Micro X-ray Computed Tomography Analysis of Next-Generation Silicon Alloy Lithium-Ion Cells
Abstract
1. Introduction
2. Experimental
2.1. Investigated Cells
2.2. Electrical Testing
2.3. Micro X-ray Computed Tomography
3. Results and Discussion
3.1. Electrical Characterization
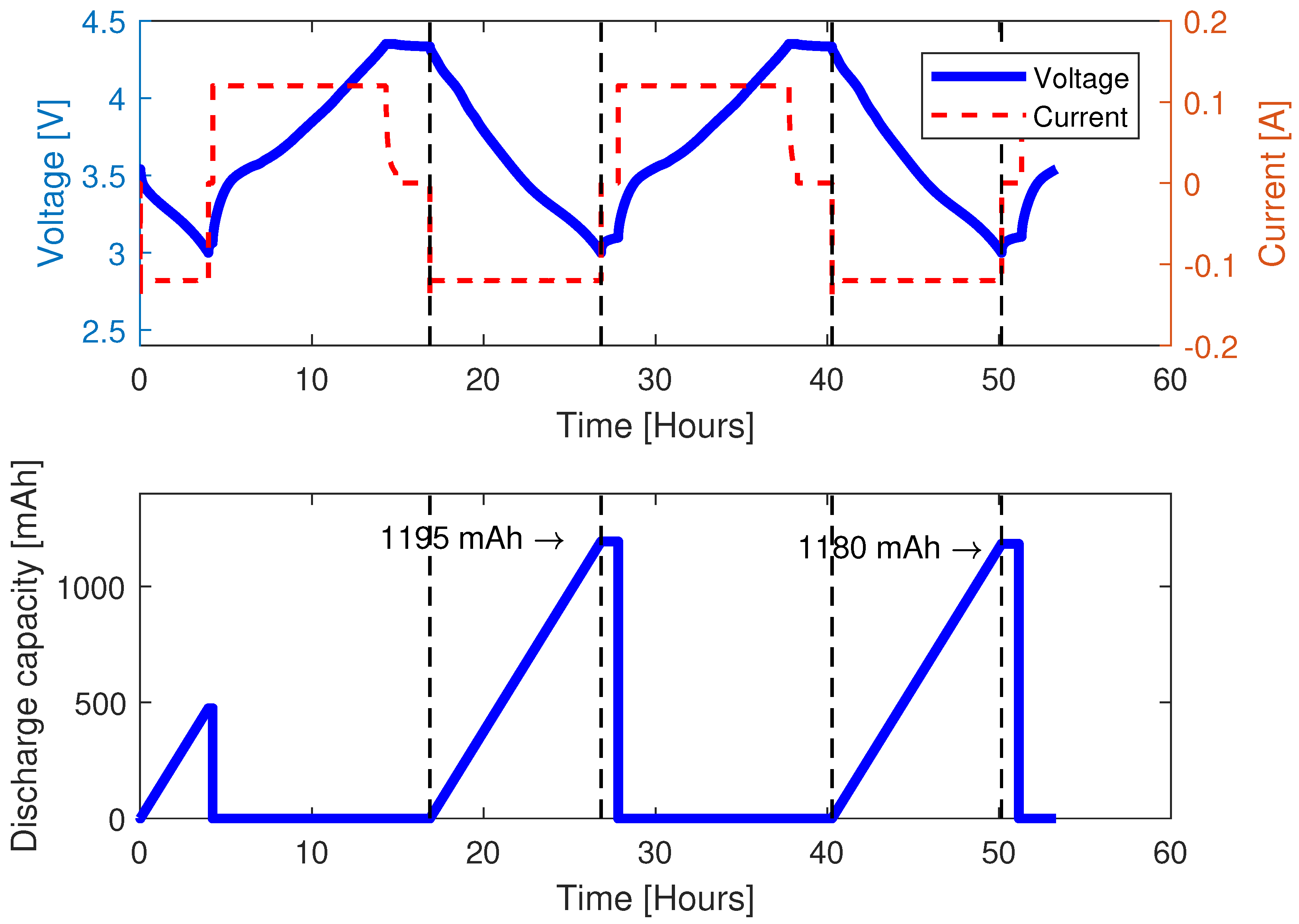
3.1.1. Preconditioning
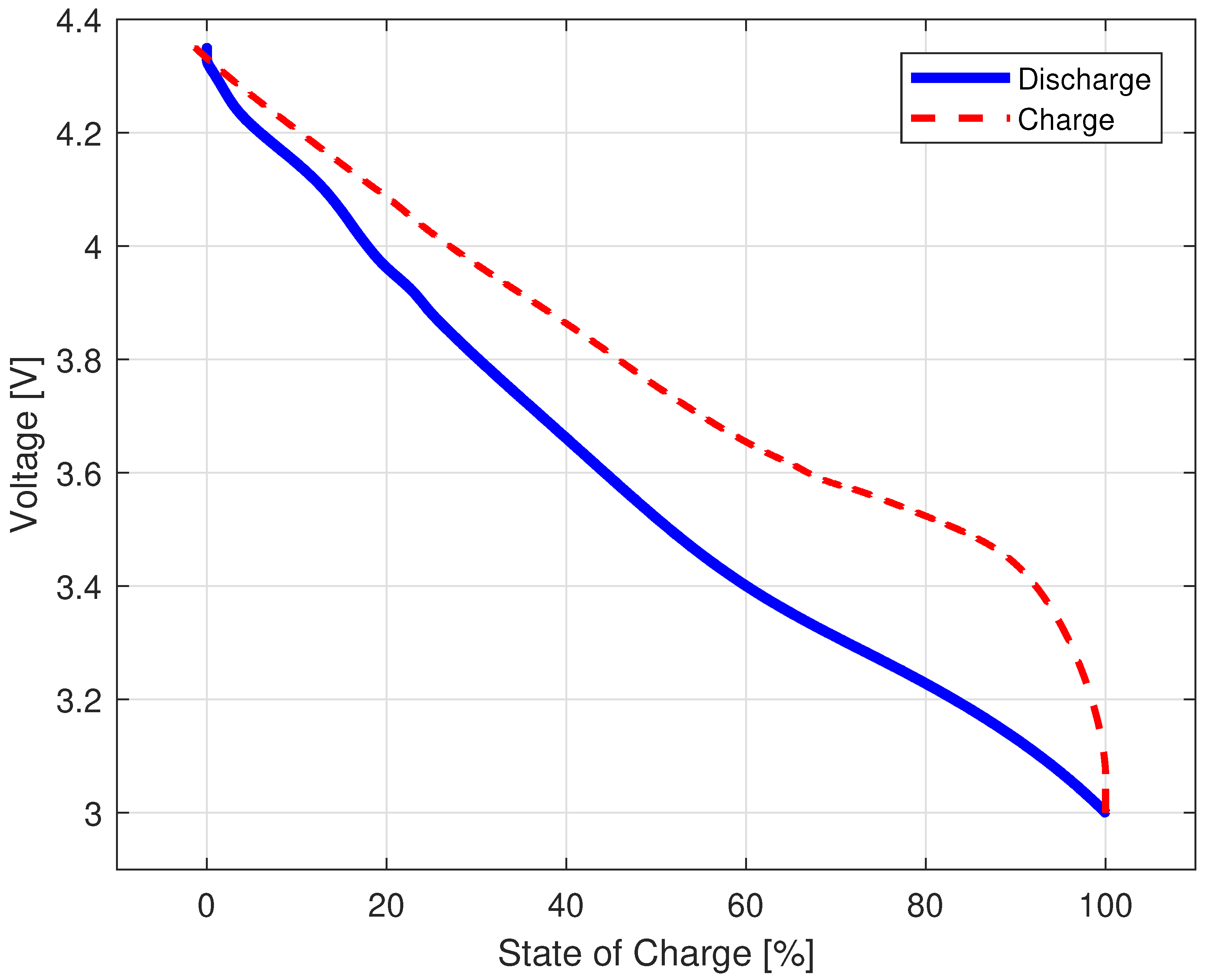
3.1.2. Quasi-Open-Circuit Voltage
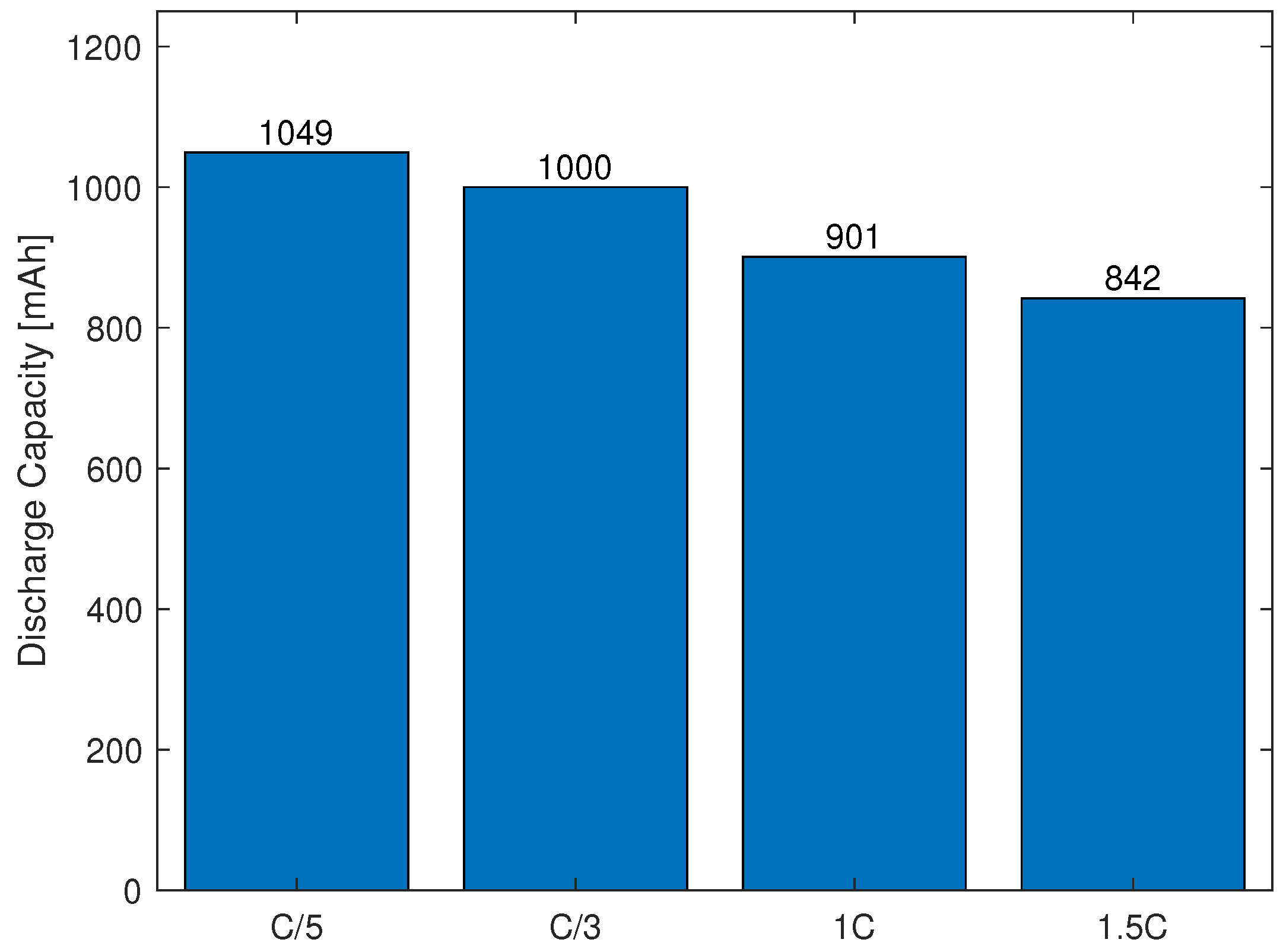
3.1.3. Capacity Test
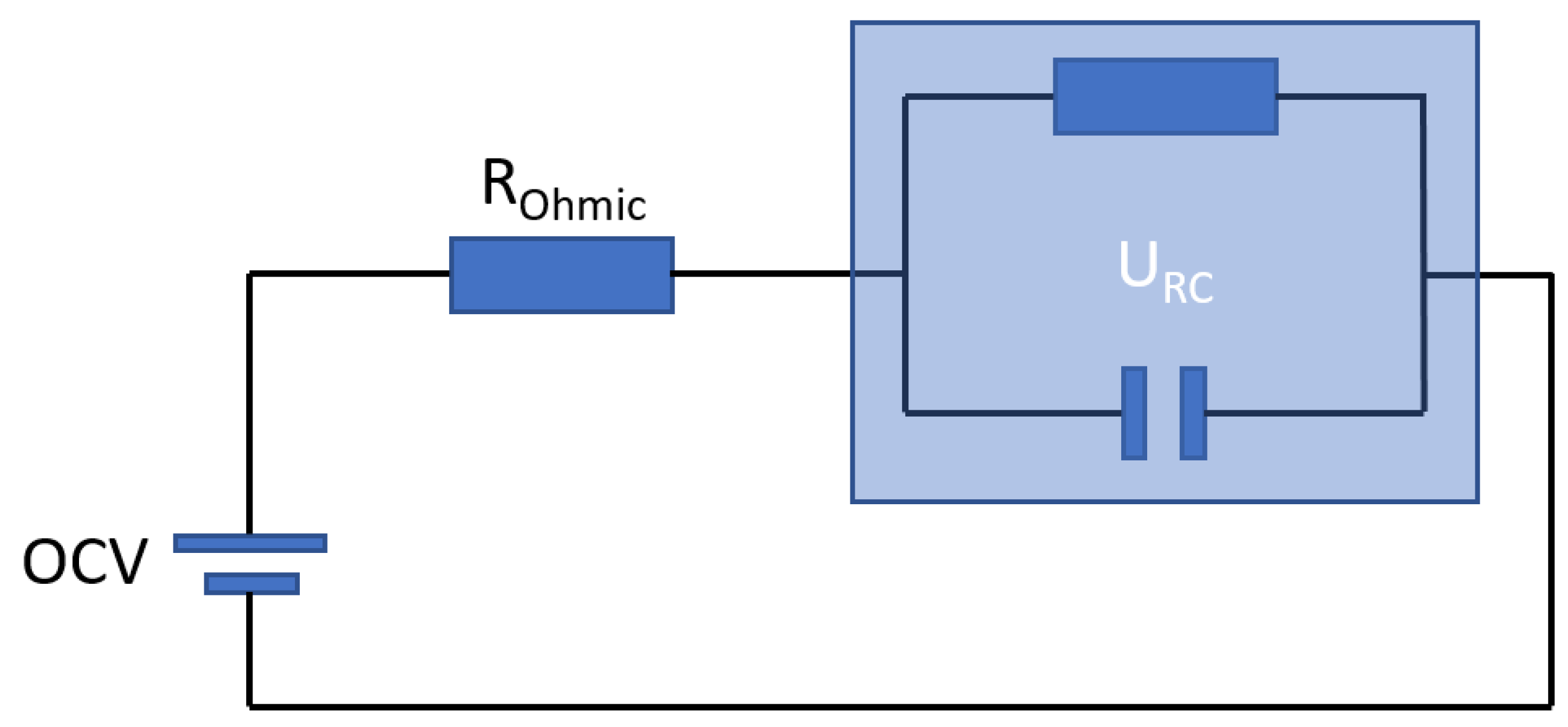
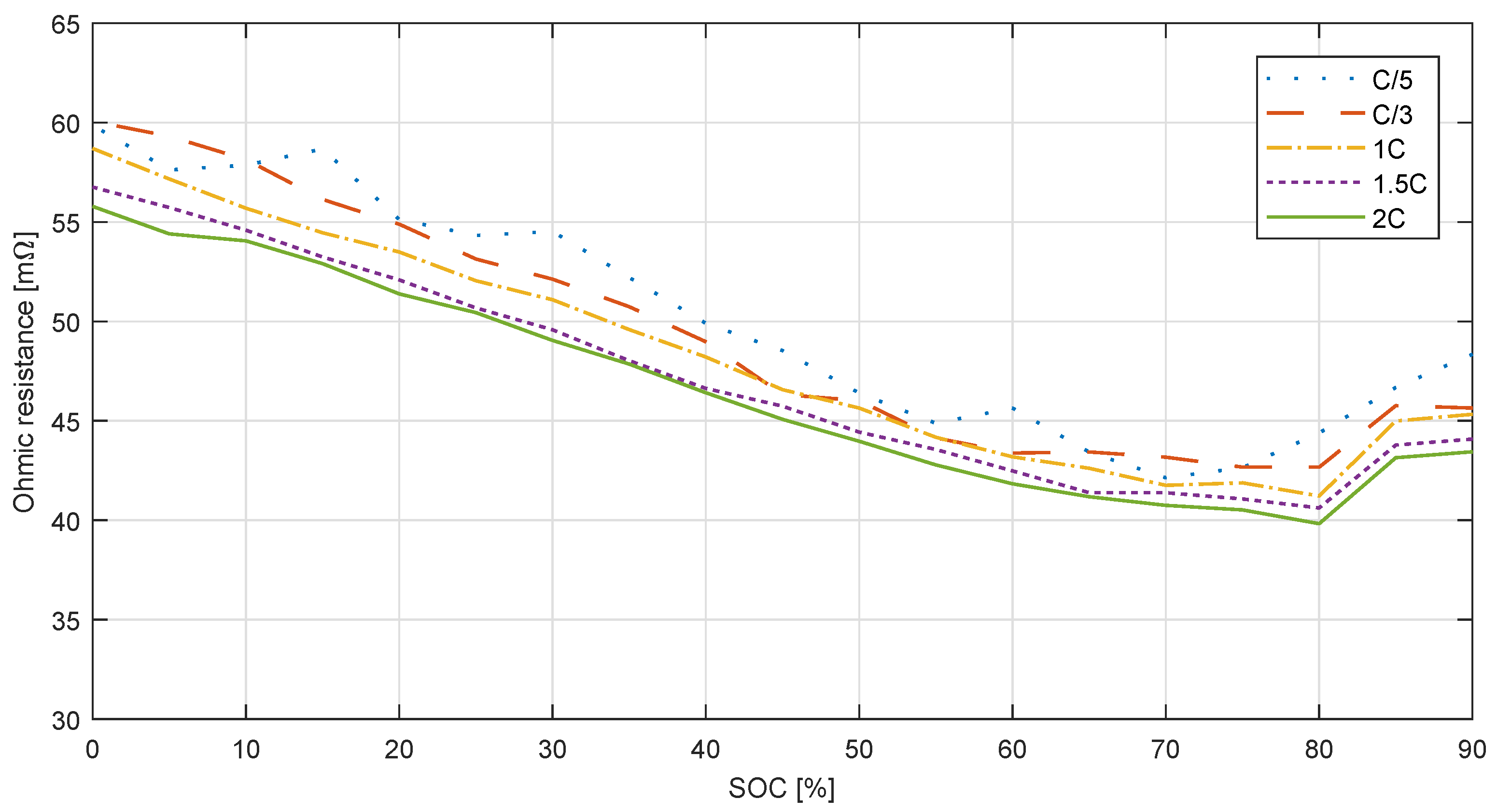
3.1.4. Hybrid Pulse Power Characterization Test
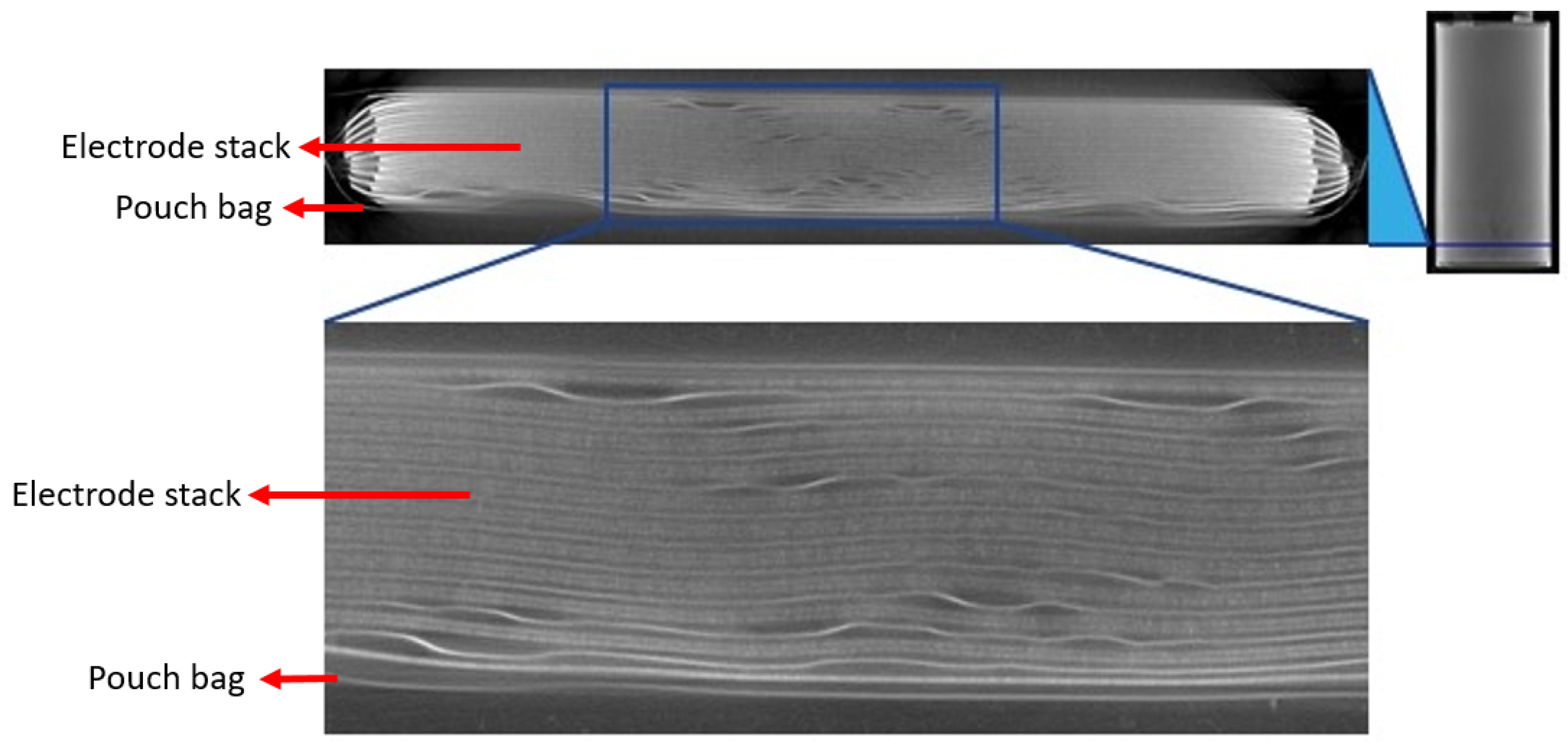
3.2. 3D X-ray Computed Tomography Analysis
3.2.1. Investigation of Pouch Cells Directly after Cell Formation
3.2.2. Investigation of Pouch Cells after BoL Tests
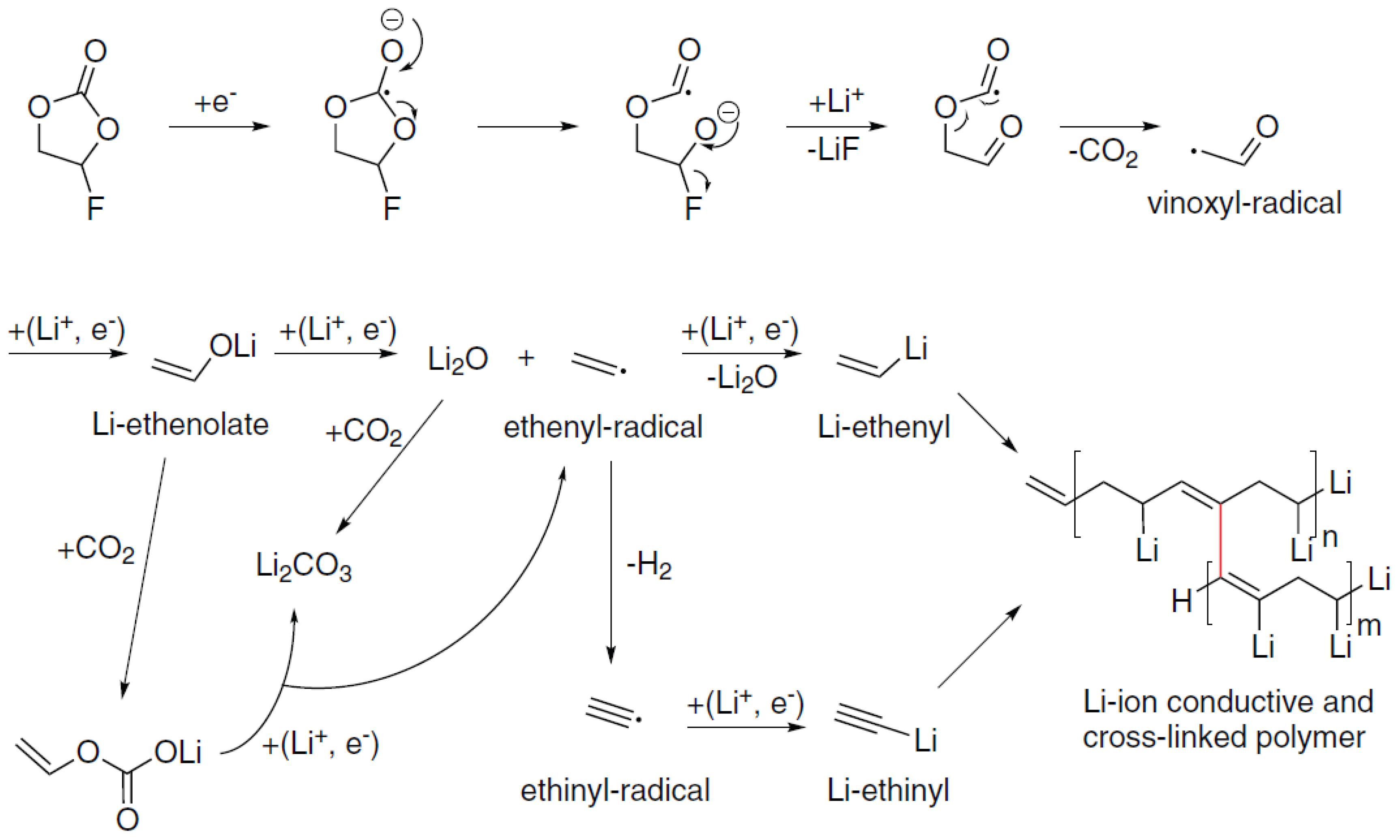
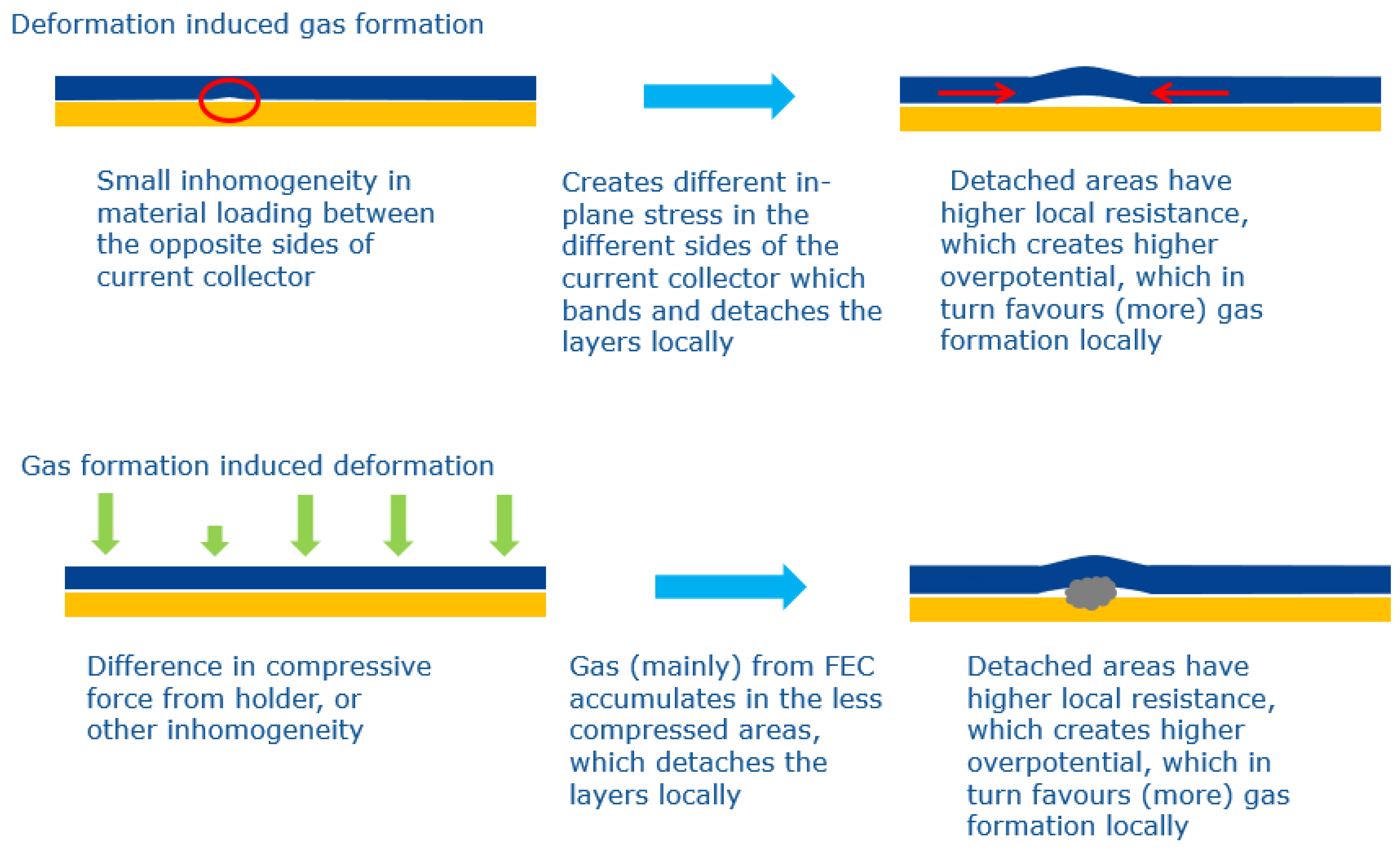
3.3. Discussion
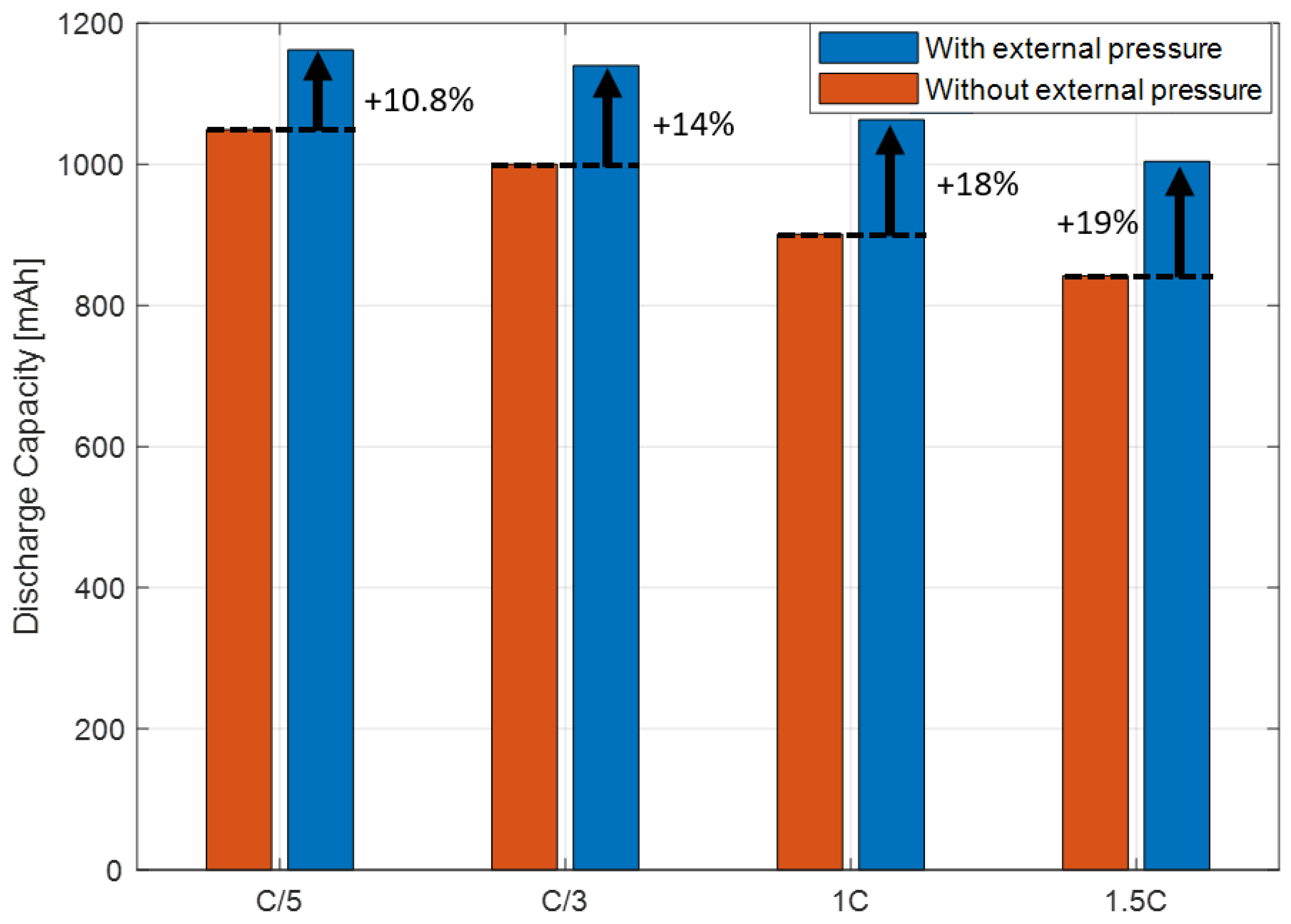
3.4. Investigation of the Effect of External Pressure
4. Conclusions
5. Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- COP21. The 21st Annual Conference of Parties (COP21). Available online: http://www.cop21paris.org/about/cop21/ (accessed on 20 July 2018).
- Electric Vehicles. European Automobile Manufacturers Association. Available online: https://www.acea.be/industry-topics/tag/category/electric-vehicles (accessed on 2 July 2018).
- van Mierlo, J. The World Vehicle Journal, The Open Access for Electric the e-Mobility Scene Journal for the e-Mobility Scene. World Electr. Veh. J. 2018, 9, 1. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; van Mierlo, J. Cost projection of state of the art lithium-ion batteries for electric vehicles up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Gao, P.; Kaas, H.W.; Mohr, D.; Wee, D. Disruptive Trends That Will Transform the Auto Industry; Technical Report; McKinsey & Company: New York, NY, USA, 2016. [Google Scholar]
- Smekens, J.; Gopalakrishnan, R.; Van den Steen, N.; Omar, N.; Hegazy, O.; Hubin, A.; Van Mierlo, J. Influence of electrode density on the performance of Li-ion batteries: Experimental and simulation results. Energies 2016, 9, 104. [Google Scholar] [CrossRef]
- Mekonnen, Y.; Sundararajan, A.; Sarwat, A.I. A Review of Cathode and Anode Materials for Lithium-Ion Batteries. In Proceedings of the SoutheastCon 2016, Norfolk, VA, USA, 30 March–3 April 2016; pp. 2–7. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Kheirabadi, N.; Shafiekhani, A. Graphene/Li-ion battery. J. Appl. Phys. 2012, 112. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X. Silicon-based nanomaterials for lithium-ion batteries. Chin. Sci. Bull. 2012, 57, 4104–4110. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Sealy, C. Lithium-ion batteries charge to the next level. Mater. Today 2018, 21, 231–240. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based anode materials for li-ion batteries: A mini review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- FiveVB. FiveVB. 2015. Available online: http://www.fivevb.eu (accessed on 20 July 2018).
- Marinaro, M.; Yoon, D.; Gabrielli, G.; Stegmaier, P.; Figgemeier, E.; Spurk, P.C.; Nelis, D.; Schmidt, G.; Chauveau, J.; Axmann, P.; et al. High performance 1.2 Ah Si-alloy/Graphite|LiNi0.5Mn0.3Co0.2O2prototype Li-ion battery. J. Power Sources 2017, 357, 188–197. [Google Scholar] [CrossRef]
- Mobi. Battery Innovation Centre Infrastructure. 2 Battery Testers at Cell Level (PEC—SBT 0550). Available online: http://mobi.vub.ac.be/mobi/research-infra/battery-innovation-centre-infrastructure (accessed on 15 July 2018).
- International Organization for Standardization. ISO 12405-2: Lectrically Propelled Road Vehicles—Test Specification for Lithium-Ion Traction Battery Packs and Systems—Part 2: High-Energy Applications. Available online: https://www.iso.org/standard/55854.html (accessed on 2 August 2018).
- International Electrotechnical Commission. IEC 62660-1: Secondary Lithium-Ion Cells for the Propulsion of Electric Road Vehicles—Part 1: Performance Testing. Available online: https://webstore.iec.ch/publication/7331 (accessed on 2 August 2018).
- Baccouche, I.; Jemmali, S.; Manai, B.; Omar, N.; Essoukri Ben Amara, N. Improved OCV model of a Li-ion NMC battery for online SOC estimation using the extended Kalman filter. Energies 2017, 10, 764. [Google Scholar] [CrossRef]
- Somakettarin, N.; Funaki, T. Study on Factors for Accurate Open Circuit Voltage Characterizations in Mn-Type Li-Ion Batteries. Batteries 2017, 3, 8. [Google Scholar] [CrossRef]
- Farmann, A.; Sauer, D.U. A study on the dependency of the open-circuit voltage on temperature and actual aging state of lithium-ion batteries. J. Power Sources 2017, 347, 1–13. [Google Scholar] [CrossRef]
- Nikolian, A.; Firouz, Y.; Gopalakrishnan, R.; Timmermans, J.M.; Omar, N.; van den Bossche, P.; van Mierlo, J. Lithium ion batteries-development of advanced electrical equivalent circuit models for nickel manganese cobalt lithium-ion. Energies 2016, 9, 360. [Google Scholar] [CrossRef]
- He, H.; Xiong, R.; Fan, J. Evaluation of lithium-ion battery equivalent circuit models for state of charge estimation by an experimental approach. Energies 2011, 4, 582–598. [Google Scholar] [CrossRef]
- Pfrang, A.; Kersy, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Käbitz, S.; Figgemeier, E. Long-Term Cycling Induced Jelly Roll Deformation in Commercial 18650 Cells. J. Power Sources 2018, 392, 168–175. [Google Scholar] [CrossRef]
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.; Knoblauch, V.; Wohlfahrt-Mehrens, M. A Mechanical Aging Mechanism in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]
- Bach, T.C.; Schuster, S.F.; Fleder, E.; Müller, J.; Brand, M.J.; Lorrmann, H.; Jossen, A.; Sextl, G. Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. J. Energy Storage 2016, 5, 212–223. [Google Scholar] [CrossRef]
- Lindgren, F.; Xu, C.; Niedzicki, L.; Marcinek, M.; Gustafsson, T.; Björefors, F.; Edström, K.; Younesi, R. SEI Formation and Interfacial Stability of a Si Electrode in a LiTDI-Salt Based Electrolyte with FEC and VC Additives for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15758–15766. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.F.; Chevalier, J.; Lucas, I.T.; Kostecki, R. HF Formation in LiPF6-Based Organic Carbonate Electrolytes. ECS Electrochem. Lett. 2013, 2, A121–A123. [Google Scholar] [CrossRef]
- Hammami, A.; Raymond, N.; Armand, M. Runaway risk of forming toxic compounds. Nature 2003, 301, 1063–1063. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.F.; Lucas, I.T.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef]
- Schroder, K.W.; Dylla, A.G.; Harris, S.J.; Webb, L.J.; Stevenson, K.J. Role of surface oxides in the formation of solid-electrolyte interphases at silicon electrodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 21510–21524. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved performance of the silicon anode for li-ion batteries: Understanding the surface modification mechanism of fluoroethylene carbonate as an effective electrolyte additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Leung, K.; Rempe, S.B.; Foster, M.E.; Ma, Y.; Martinez del la Hoz, J.M.; Sai, N.; Balbuena, P.B. Modeling Electrochemical Decomposition of Fluoroethylene Carbonate on Silicon Anode Surfaces in Lithium Ion Batteries. J. Electrochem. Soc. 2013, 161, A213–A221. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Haering, D.; Solchenbach, S.; Marino, C.; Tsiouvaras, N.; Stinner, C.; Gasteiger, H.A. Consumption of fluoroethylene Carbonate (FEC) on Si-C Composite Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1705–A1716. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Chon, M.J.; Shimshak, M.; Srinivasan, V.; Guduru, P.R. In situ measurements of stress evolution in silicon thin films during electrochemical litiation and delithiation. J. Power Sources 2010, 195, 5062–5066. [Google Scholar] [CrossRef]

| Anode | Cathode | |
|---|---|---|
| Electrode composition | 55% Si alloy (3M) | 93% NMC 5:3:2 |
| 33% SMG-A3 | 4% Carbon | |
| 10% LiPAA | 3% HSV 900+ ADX 161 (Arkema) | |
| 2% Super PLi | ||
| Loading (mg/cm) | 6.5 | 17.5 |
| Electrode density (g/cc) | 1.7 | 3.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berckmans, G.; De Sutter, L.; Kersys, A.; Kriston, A.; Marinaro, M.; Kasper, M.; Axmann, P.; Smekens, J.; Wohlfahrt-Mehrens, M.; Pfrang, A.; et al. Electrical Characterization and Micro X-ray Computed Tomography Analysis of Next-Generation Silicon Alloy Lithium-Ion Cells. World Electr. Veh. J. 2018, 9, 43. https://doi.org/10.3390/wevj9030043
Berckmans G, De Sutter L, Kersys A, Kriston A, Marinaro M, Kasper M, Axmann P, Smekens J, Wohlfahrt-Mehrens M, Pfrang A, et al. Electrical Characterization and Micro X-ray Computed Tomography Analysis of Next-Generation Silicon Alloy Lithium-Ion Cells. World Electric Vehicle Journal. 2018; 9(3):43. https://doi.org/10.3390/wevj9030043
Chicago/Turabian StyleBerckmans, Gert, Lysander De Sutter, Algirdas Kersys, Akos Kriston, Mario Marinaro, Michael Kasper, Peter Axmann, Jelle Smekens, Margret Wohlfahrt-Mehrens, Andreas Pfrang, and et al. 2018. "Electrical Characterization and Micro X-ray Computed Tomography Analysis of Next-Generation Silicon Alloy Lithium-Ion Cells" World Electric Vehicle Journal 9, no. 3: 43. https://doi.org/10.3390/wevj9030043
APA StyleBerckmans, G., De Sutter, L., Kersys, A., Kriston, A., Marinaro, M., Kasper, M., Axmann, P., Smekens, J., Wohlfahrt-Mehrens, M., Pfrang, A., Jaguemont, J., Van Mierlo, J., & Omar, N. (2018). Electrical Characterization and Micro X-ray Computed Tomography Analysis of Next-Generation Silicon Alloy Lithium-Ion Cells. World Electric Vehicle Journal, 9(3), 43. https://doi.org/10.3390/wevj9030043






