Study on a New Static Sealing Method and Sealing Performance Evaluation Model for PEMFC
Abstract
:1. Introduction
1.1. Pre-Compressing Static Sealing
1.2. Adhesive Static Sealing
- (1)
- In terms of principle, hydrogen and oxygen are fuels of positive and negative electrodes, respectively, and these fuels are stored in gas storage tanks. The positive and negative electrodes are separated by a proton exchange membrane. Under operating conditions, fuels are transported to electrodes, and then electric energy can be generated by electrochemical reactions. PEMFCs are a type of safe and durable power battery with clean fuels and low carbon emissions. In RFBs, positive and negative liquid electrolytes (containing metal ions) are stored in two electrolyte tanks which are arranged outside of the cells. The positive and negative electrodes are separated by an ion exchange membrane. Under operating conditions, liquid electrolytes are transported to electrodes [15].
- (2)
- In terms of structure, the single cells of PEMFCs include a membrane electrode (MEA), bipolar plates and a sealing gasket, as shown in Figure 4a. The single cells of RFBs include an ion exchange membrane, electrodes, plates and a sealing gasket [18,19], as shown in Figure 4b [20]. These sealing gaskets can effectively prevent the leakage of fuel gases and liquid electrolytes.
- (3)
- In terms of sealing difficulties, the difficulties of these two types of batteries are similar as well. There are four main issues. (1) The total length of the sealing line is long, and the amount of sealing is large. (2) Sealing performance degrades over time with the operation of batteries as they are exposed to complex and corrosive working environments. (3) There are disassembly and reassembly requirements during the stacking process. (4) The lifetime of seals should be consistent with the service time of stacks. Therefore, more reliable sealability is necessary and urgent for the stable operation of PEMFCs and RFBs.

2. Theoretical Models
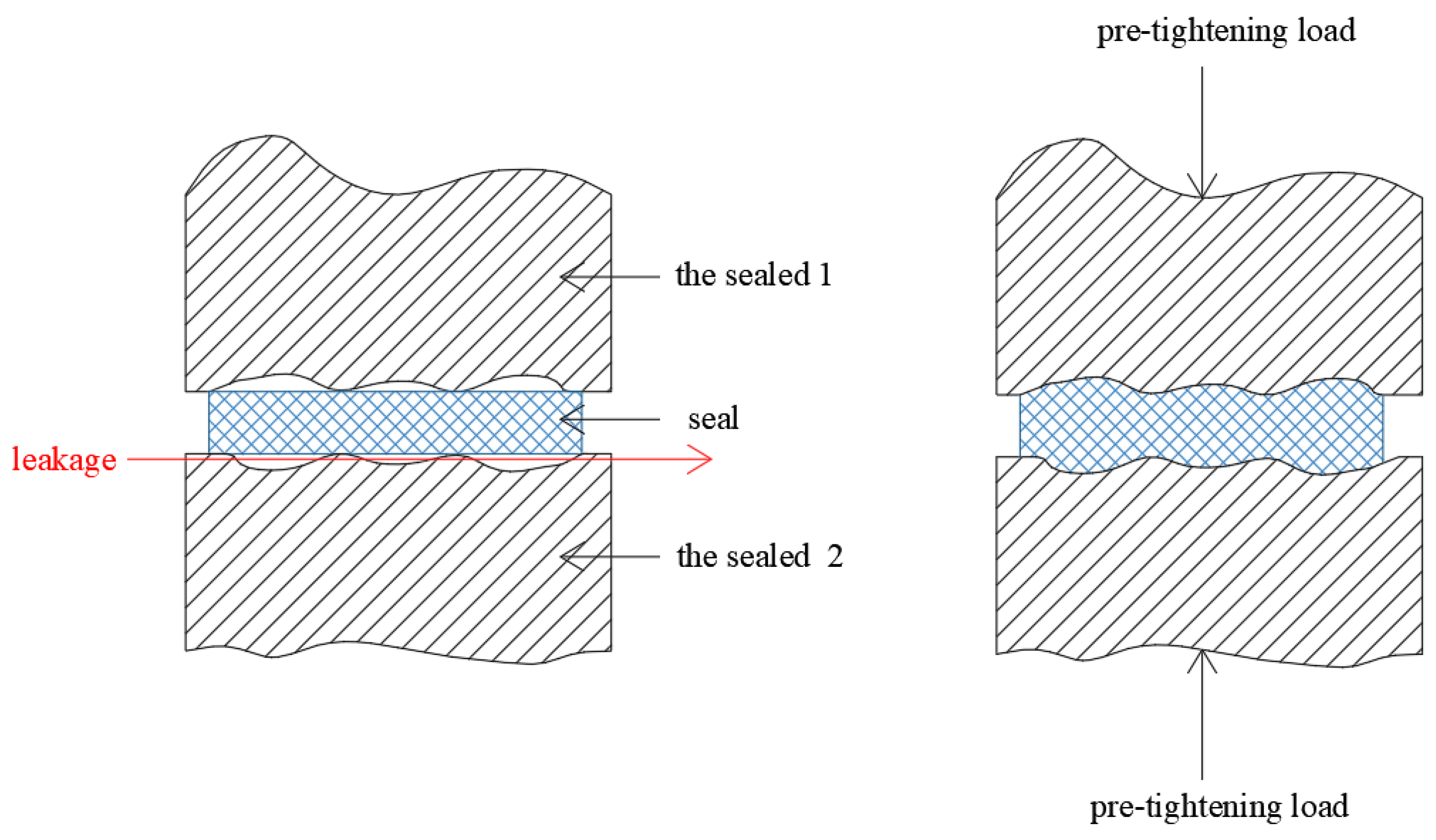
2.1. The Sealing Processes of Alterable Static Sealing
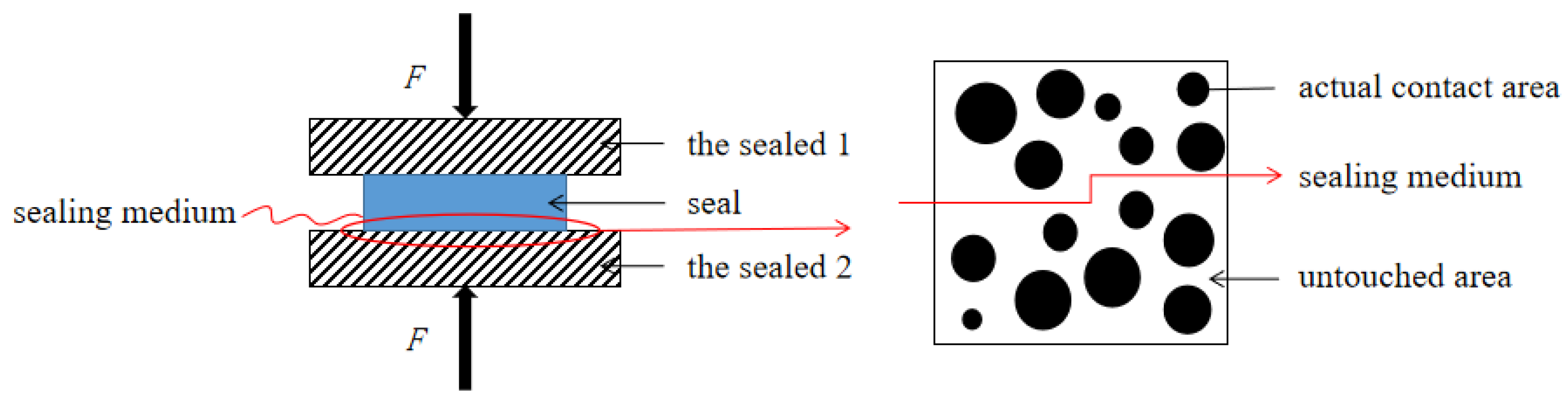
2.2. The Relationship between Sealing Performance and the Actual Contact Area Ratio r on Pre-Compressing Static Sealing
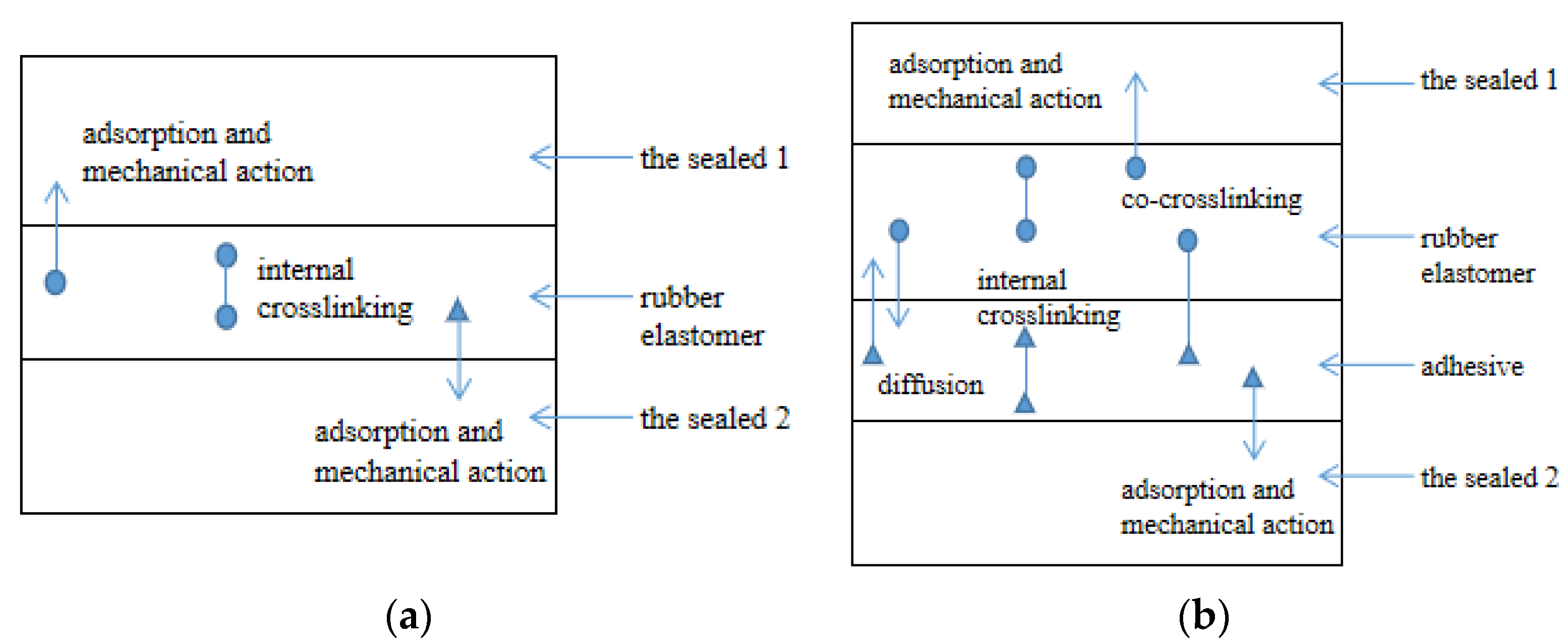
2.3. The Bonding Model of Interpenetration Region around the Interface
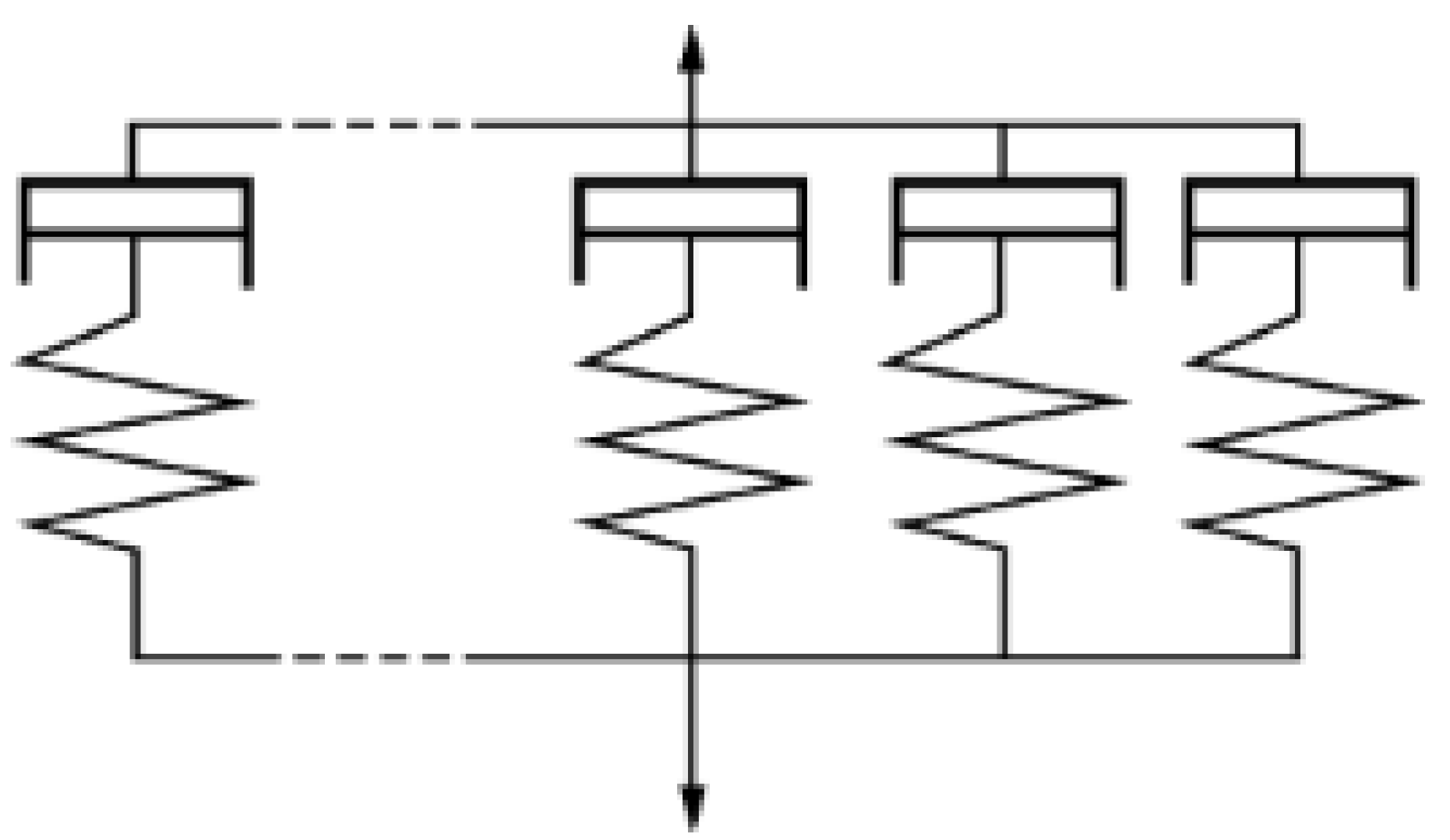
2.3.1. Model of Covalent Polymer Networks
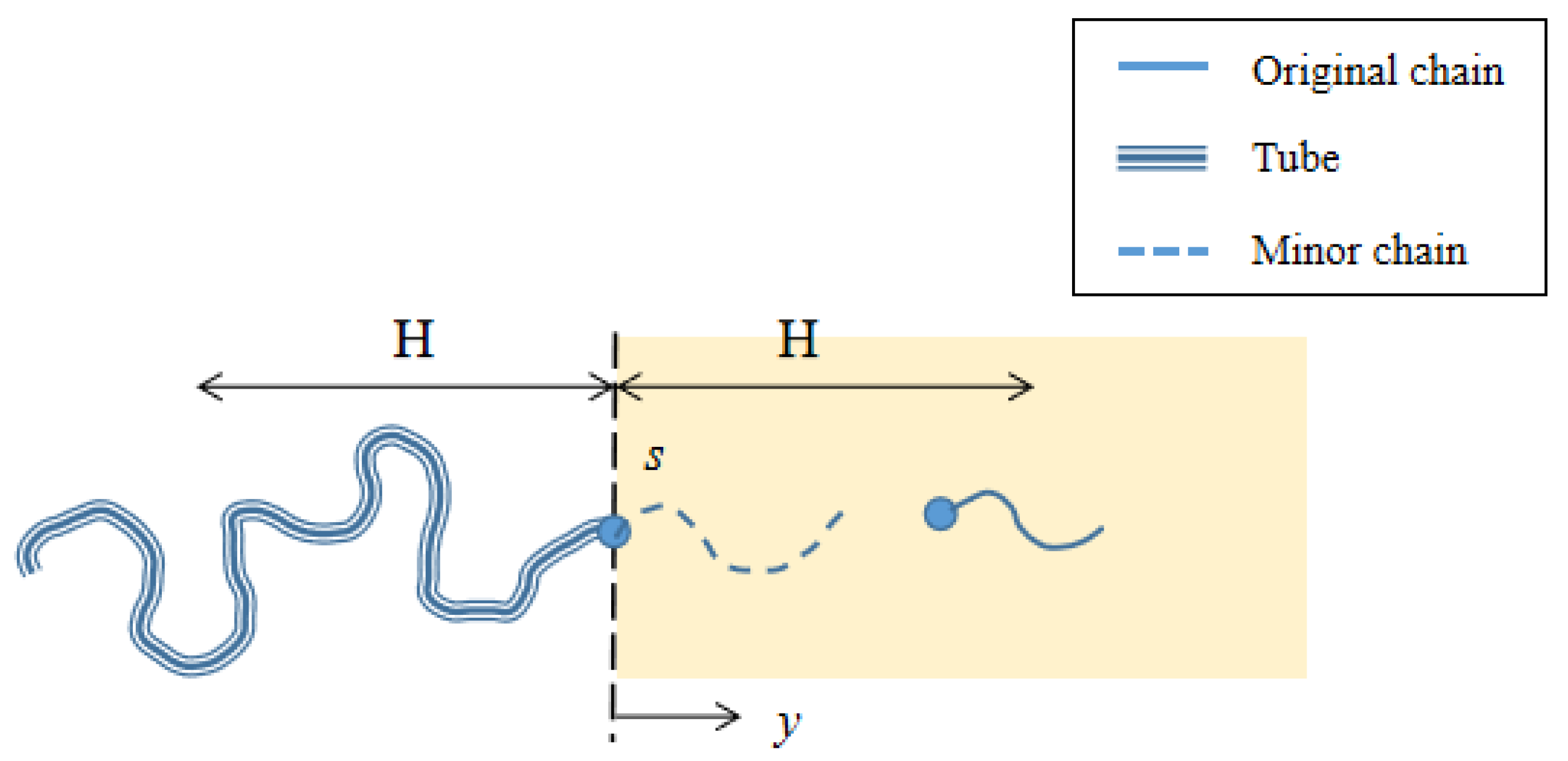
2.3.2. Diffusion Model of Polymer Chains
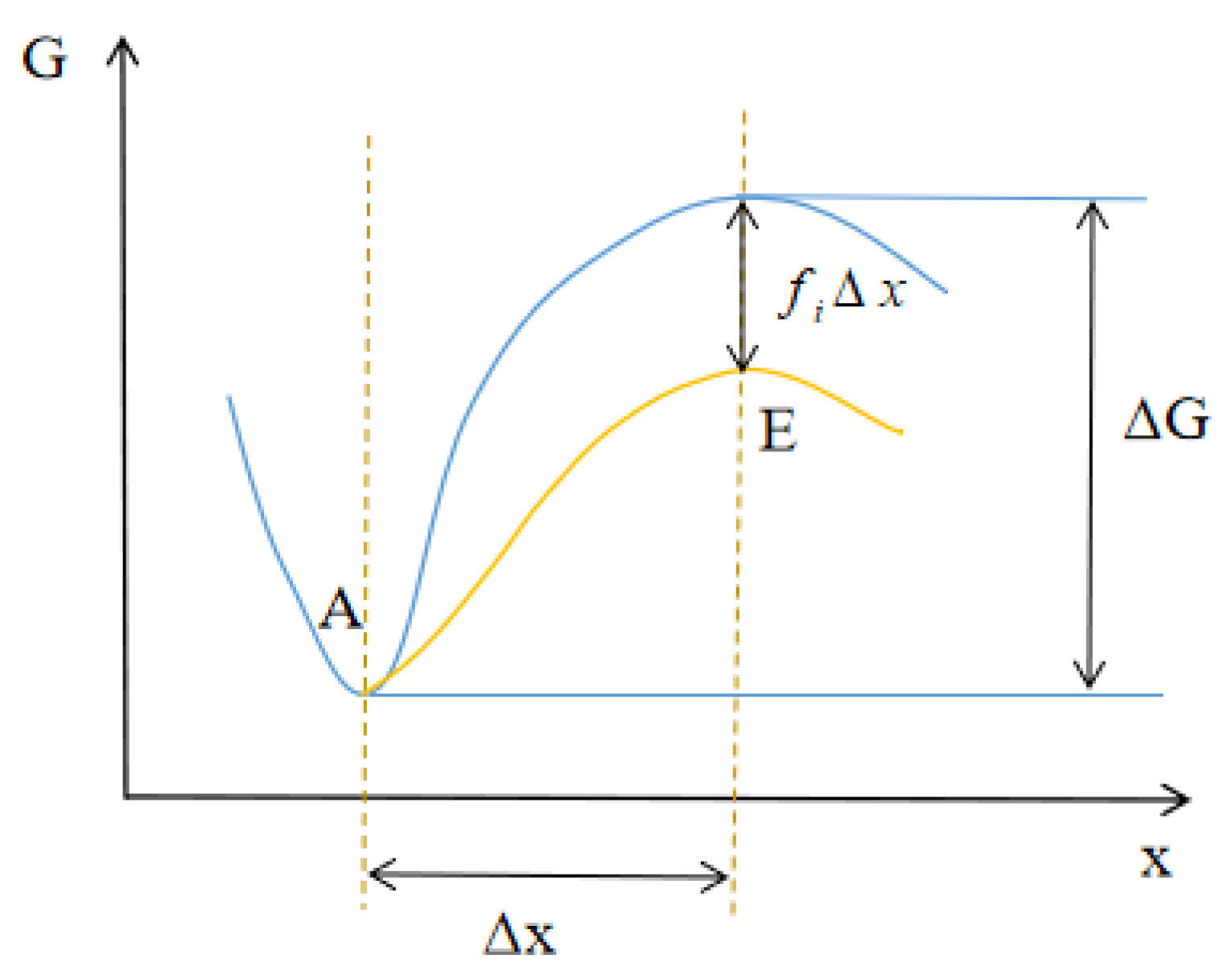
2.3.3. Diffusion–Reaction Model around the Interface
2.4. The Relationship between Interpenetration around the Interface and the Actual Contact Area Ratio r
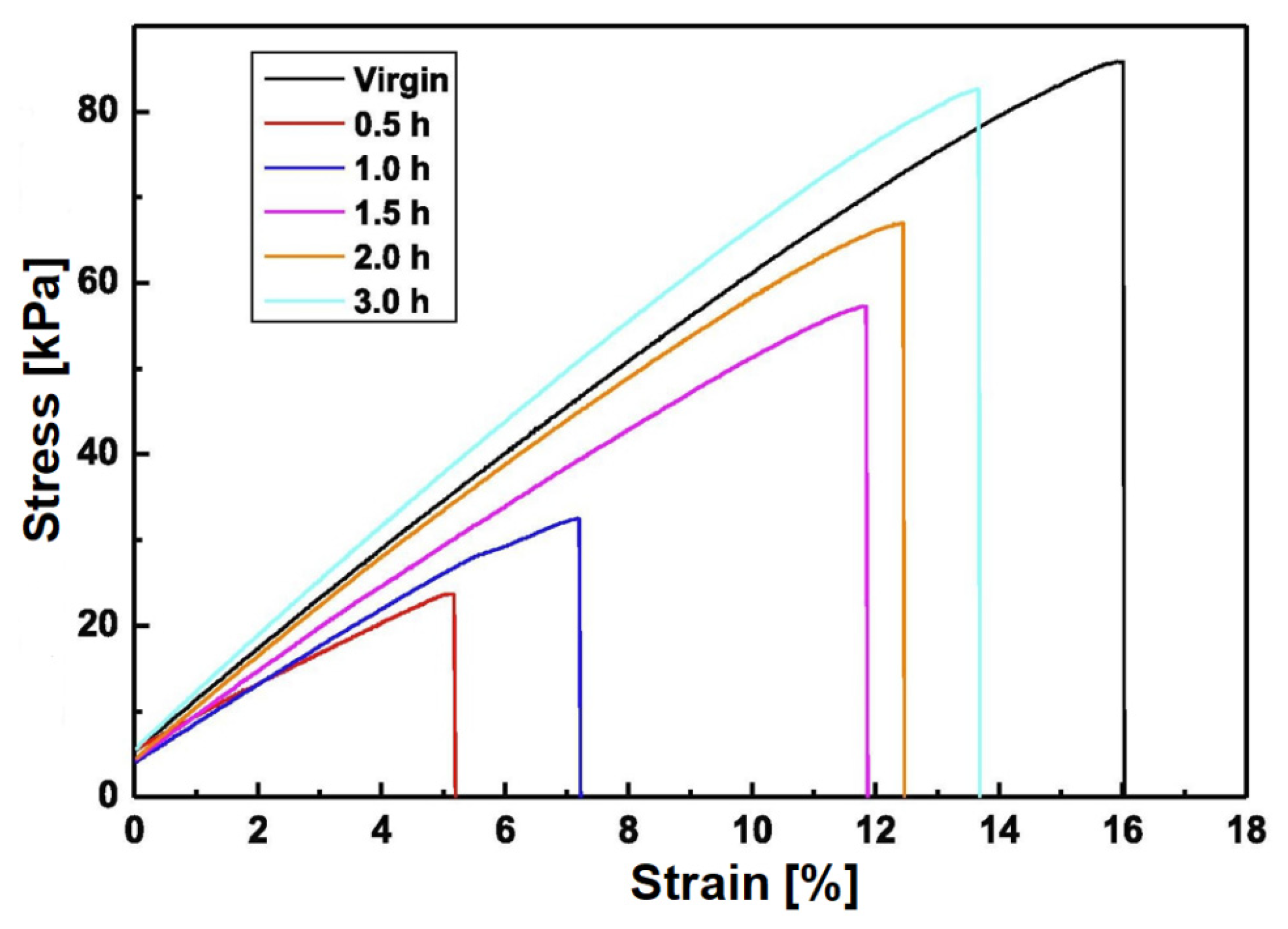
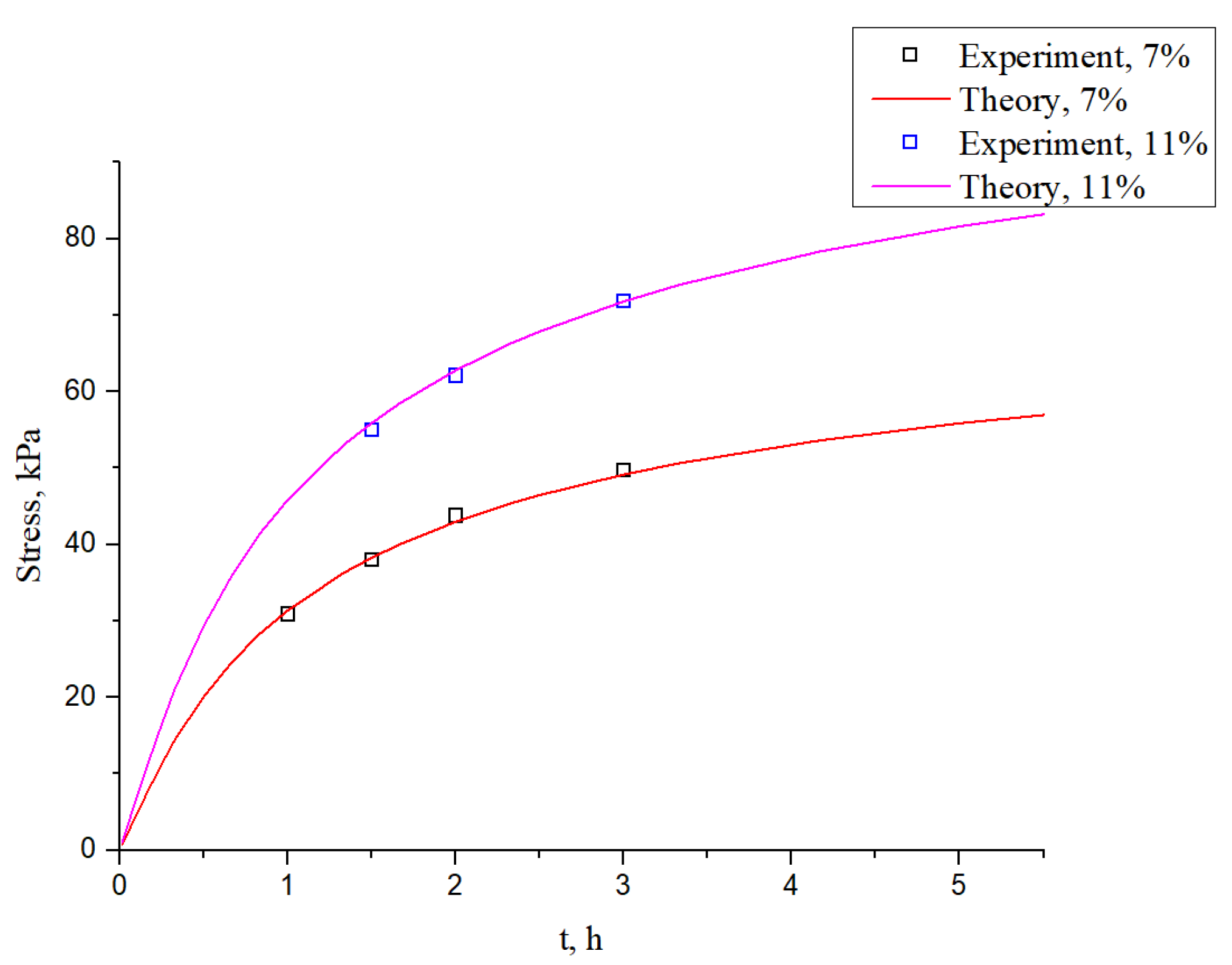
3. Results
- (1)
- The effect of bonding on the sealing performance is proposed, and there are some previous bonding theories [43]. In the sealing formed by the sealant and joint sealant, the sealing force is increased as the bonding between contact surfaces is achieved, and a stable bonding strength is formed. Meanwhile, the bonding area becomes the actual contact area [41,44]. The probability of leakage is reduced, and the sealing performance is improved.
- (2)
- According to the research of Persson, B.N.J. et al. [3,31,32,33], the leakage rate could be represented by the actual contact area ratio r, in order to represent the sealability. As long as the value of the actual contact area ratio r is bigger than the threshold , leakage channels can almost be ignored.
- (3)
- According to the research of Pang, M.H. et al. [34], the sealing force and r change in a power function law, and the exponent of the power function is .
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kömmling, A.; Jaunich, M.; Pourmand, P.; Wolff, D.; Gedde, U.W. Influence of Ageing on Sealability of Elastomeric O-Rings. Macromol. Symp. 2017, 373, 1600157. [Google Scholar] [CrossRef]
- Sun, J. The Theory and Applied Researches on the Prediction of the Leakage Rate of Mechanical Seals. Ph.D. Thesis, Nanjing Tech University, Nanjing, China, 2006. [Google Scholar]
- Persson, B.N.J.; Albohr, O.; Creton, C.; Peveri, V. Contact Area between a Viscoelastic Solid and a Hard, Randomly Rough, Substrate. J. Chem. Phys. 2004, 120, 8779–8793. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.C. Research on Modeling and Mechanism of Static Sealing Based on Percolation Theory. Ph.D. Thesis, Beijing Institute of Technology, Beijing, China, 2015. [Google Scholar]
- Lau, K. The Brief Description of the O-ring Static Seal. Hydraul. Pneum. Seals 2013, 33, 70–72. [Google Scholar]
- Li, J. Prediction Model and Control Method of Leakage Rate of Gate Sealing Structure. Ph.D. Thesis, Huazhong University of Science & Technology, Wuhan, China, 2016. [Google Scholar]
- Li, C.F. Cause and Solution of Rubber and Metal Framework Failure. Chem. Eng. Des. Commun. 2019, 45, 67–68. [Google Scholar] [CrossRef] [Green Version]
- Simader, C.; Hörl, L.; Haas, W. The development of design guidelines for the application of formed-in-place gaskets. Seal. Technol. 2012, 2012, 7–11. [Google Scholar] [CrossRef]
- Zhi, J.; Wang, S.; Zhang, M.; Wang, H.; Lu, H.; Lin, W.; Qiao, C.; Hu, C.; Jia, Y. Numerical analysis of the dependence of rubber hysteresis loss and heat generation on temperature and frequency. Mech. Time-Depend. Mat. 2019, 23, 427–442. [Google Scholar] [CrossRef]
- Giannisa, S.; Adams, R.D. Failure of elastomeric sealants under tension and shear: Experiments and analysis. Int. J. Adhes. Adhes. 2019, 91, 77–91. [Google Scholar] [CrossRef]
- Someno, K. Japan’s policies and moves to create a decarbonized society by 2050. World Environ. 2021, 1, 42–46. [Google Scholar]
- Speech at the General Debate of the Seventy-Fifth United Nations General Assembly (Full Text). Available online: https://www.ccps.gov.cn/xxsxk/zyls/202009/t20200922_143558.shtml (accessed on 22 September 2020).
- Wen, X.M. Research and Prospect on Battery Technology of Electric Vehicles. Automob. Parts 2015, 11, 84–86. [Google Scholar]
- Jiang, C.S. Inhibition of Hydrogen’s Explosion Limits. Master’s Thesis, Shandong Jianzhu University, Jinan, China, 2017. [Google Scholar]
- Zhang, H.; Sun, C.Y. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Improved Redox Flow Batteries for Electric Cars. Available online: https://cordis.europa.eu/article/id/113921-improved-redox-flow-batteries-for-electric-cars/fr (accessed on 13 January 2021).
- Water-Based Flow Battery for EVs. Available online: https://arpa-e.energy.gov/technologies/projects/water-based-flow-battery-evs (accessed on 21 August 2013).
- Zhang, H.; Chen, N.; Sun, C.Y.; Luo, X.D. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2020, 44, 3839–3853. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Luo, X.D.; Sun, C.Y. SiO2-decorated graphite felt electrode by silicic acid etching for ironchromium redox flow battery. Electrochim. Acta 2020, 336, 135646. [Google Scholar] [CrossRef]
- Nam, S.; Lee, D.; Kim, J.; Lee, D.G. Development of a fluoroelastomer/glass fiber composite flow frame for a vanadium redox flow battery (VRFB). Compos. Struct. 2016, 145, 113–118. [Google Scholar] [CrossRef]
- Department of Energy, USA. Hydrogen and Fuel Cell Activities, Progress, and Plans: Report to Congress. Available online: http://www.hydrogen.energy.gov/pdfs/epact_report_sec811.pdf (accessed on 10 July 2021).
- 2018 DOE Hydrogen and Fuel Cells Program Review: Fuel Cell Systems Analysis. Available online: https://www.docin.com/p-2161522038.html (accessed on 10 July 2021).
- Cui, T.; Lin, C.W.; Chien, C.H.; Chao, Y.J.; Van Zee, J.W. Service life estimation of liquid silicone rubber seals in polymer electrolyte membrane fuel cell environment. J. Power Sources 2010, 196, 1216–1221. [Google Scholar] [CrossRef]
- Stukenbroeker, T.; Wang, W.; Winne, J.M.; Du Prez, F.E.; Nicolaÿ, R.; Leibler, L. Polydimethylsiloxane quenchable vitrimers. Polym. Chem. 2017, 43, 6590–6593. [Google Scholar] [CrossRef]
- Röttger, M.; Domenech, T.; van der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous urethane vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Wang, Y.; Jiang, Y.; Zhou, C.; Lei, J. Carbon Nanotubes-Polyurethane Vitrimer Nanocomposites with the Ability of Surface Welding Controlled by Heat and Near-Infrared Light. Macromol. Chem. Phys. 2017, 218, 1700265. [Google Scholar] [CrossRef]
- Hanna, J.M.; Nathalie, L.; Ange, N.; Kuruvilla, J.; Cherian, M.; Sabu, T. Stress relaxation behavior of organically modified montmorillonite filled natural rubber/nitrile rubber nanocomposites. Appl. Clay Sci. 2014, 87, 120–128. [Google Scholar]
- Cui, T.; Chao, Y.J.; Van Zee, J.W. Stress relaxation behavior of EPDM seals in polymer electrolyte membrane fuel cell environment. Int. J. Hydrog. Energy 2012, 37, 13478–13483. [Google Scholar] [CrossRef]
- Cui, T.; Chao, Y.J.; Chen, X.M.; Van Zee, J.W. Effect of water on life prediction of liquid silicone rubber seals in polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 9536–9543. [Google Scholar] [CrossRef]
- Dapp, W.B.; Lücke, A.; Persson, B.N.J.; Müser, M.H. Self-Affine Elastic Contacts: Percolation and Leakage. Phys. Rev. Lett. 2012, 108, 244301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, B.N.J.; Yang, C. Theory of the leak-rate of seals. J. Phys.-Condens. Mat. 2008, 20, 315011. [Google Scholar] [CrossRef]
- Persson, B.N.J. Theory of rubber friction and contact mechanics. J. Chem. Phys. 2001, 115, 3840–3861. [Google Scholar] [CrossRef] [Green Version]
- Pang, M.H.; Ma, L.J.; Nie, Y.F.; Wang, Z.K. Fluid wetting infiltration characteristics and mechanical driving mechanism in rough rubber-glass contact interface. Lubr. Sci. 2020, 32, 58–68. [Google Scholar] [CrossRef]
- Arruda, E.M.; Boyer, M.C. A three-dimensional constitutive model for the large stretch behavior of rubber elastic materials. J. Mech. Phys. Solids 1993, 41, 389–412. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.M.; Gao, Z.M. A constitutive model of nanocomposite hydrogels with nanoparticle crosslinkers. J. Mech. Phys. Solids 2016, 94, 127–147. [Google Scholar] [CrossRef]
- Wang, Q.M.; Gossweiler, G.R.; Craig, S.L.; Zhao, X. Mechanics of mechanochemically responsive elastomers. J. Mech. Phys. Solids 2015, 82, 320–344. [Google Scholar] [CrossRef] [Green Version]
- Xin, A.; Zhang, R.R.; Yu, K.H.; Wang, Q.M. Mechanics of electrophoresis-induced reversible hydrogel adhesion. J. Mech. Phys. Solids 2019, 125, 1–21. [Google Scholar] [CrossRef]
- Jordi, R.A.; Marx, D. Covalent mechanochemistry: Theoretical concepts and computational tools with applications to molecular nanomechanics. Chem. Rev. 2012, 112, 5412–5487. [Google Scholar]
- Hänggi, P.; Talkner, P.; Borkovec, M. Reaction-rate theory: Fifty years after Kramers. Rev. Mod. Phys. 1990, 62, 251–341. [Google Scholar] [CrossRef]
- Okada, H.; Itoh, T.; Suga, T. The influence of surface profiles on leakage in room temperature seal-bonding. Sensor Actuator A: Phys. 2008, 144, 124–129. [Google Scholar] [CrossRef]
- Xiang, H.P.; Yin, J.F.; Lin, J.H.; Liu, X.X.; Rong, M.Z.; Zhang, M.Q. Photo-crosslinkable, self-healable and reprocessable rubbers. Chem. Eng. J. 2019, 358, 878–890. [Google Scholar] [CrossRef]
- Yang, C.F. Study on Bonding Technology of Rubber Seal Ring Interface of Wind Power Bearing. Master Thesis, Dalian Jiaotong University, Dalian, China, 2019. [Google Scholar]
- Kim, J.; Zollinger, D.; Taylor, P.; Rasmussen, R.O. Effects of Shape and Bond Strength on Adhesive Failure of Joint Sealants. Transp. Res. Rec. 2021, 2675, 203–212. [Google Scholar] [CrossRef]
- Yu, K.H.; Xin, A.; Wang, Q.M. Mechanics of Self-healing Polymer Networks Crosslinked by Dynamic Bonds. J. Mech. Phys. Solids 2018, 121, 409–431. [Google Scholar] [CrossRef]




| Parameter | Definition | Simulation Data |
|---|---|---|
| Reaction rate | ||
| Distance along the energy landscape coordinate | ||
| Kuhn segment length | ||
| Rouse friction coefficient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhao, J.; Wang, Y.; Ke, Y.; Wang, Z. Study on a New Static Sealing Method and Sealing Performance Evaluation Model for PEMFC. World Electr. Veh. J. 2021, 12, 237. https://doi.org/10.3390/wevj12040237
Huang X, Zhao J, Wang Y, Ke Y, Wang Z. Study on a New Static Sealing Method and Sealing Performance Evaluation Model for PEMFC. World Electric Vehicle Journal. 2021; 12(4):237. https://doi.org/10.3390/wevj12040237
Chicago/Turabian StyleHuang, Xiaoyu, Jinghui Zhao, Yichun Wang, Yuchao Ke, and Zixi Wang. 2021. "Study on a New Static Sealing Method and Sealing Performance Evaluation Model for PEMFC" World Electric Vehicle Journal 12, no. 4: 237. https://doi.org/10.3390/wevj12040237
APA StyleHuang, X., Zhao, J., Wang, Y., Ke, Y., & Wang, Z. (2021). Study on a New Static Sealing Method and Sealing Performance Evaluation Model for PEMFC. World Electric Vehicle Journal, 12(4), 237. https://doi.org/10.3390/wevj12040237






