Modern Technologies of Hydrogen Production
Abstract
1. Introduction
2. Steam Methane Reforming
3. Partial Oxidation of Methane
4. Carbon Dioxide Reforming
5. Methane Pyrolysis
| Catalyst | T (°C) | Feed Gas Composition | Conversion of CH4 (%) | Reference |
|---|---|---|---|---|
| Ni/SiO2 | 650 | CH4 | 85 | [162] |
| 55% Ni − 15% Cu/MgO·Al2O3 | 675 | CH4 | 80 | [163] |
| 12.5% Ni − 12.4% Co/La2O3 | 700 | N2:CH4 = 1:9 | 82 | [164] |
| 50% Ni − 10% Fe/Al2O3 | 675 | N2:CH4 = 7:3 | 68 | [144] |
| 50% Ni − 10% Pd/Al2O3 | 675 | N2:CH4 = 7:3 | 75 | [165] |
| 20% Fe/WO3 + ZrO2 | 800 | N2:CH4 = 1:2 | 90 | [166] |
| Fe − 5.1% Mo/Al2O3 | 750 | CH4 | 69 | [167] |
| 65% Fe/Al2O3 | 750 | CH4 | 70 | [168] |
| Fe sponge | 1000 | CH4 | 85 | [169] |
6. Reforming of Biomass and Bio-Alcohols
7. Reversible Hydrogen Carriers
8. Hydrogen Purification and Membrane Catalysis
9. Water Electrolysis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurasz, J.; Canales, F.A.; Kies, A.; Guezgouz, M.; Beluco, A. A review on the complementarity of renewable energy sources: Concept, metrics, application and future research directions. Solar Energy 2020, 195, 703–724. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Digital management of solar energy en route to energy self-sufficiency. Glob. Chall. 2019, 3, 1800105. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Pal, K. Review on hydrogen storage materials and methods from an electrochemical viewpoint. J. Energy Storage 2019, 23, 234–249. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Golubenko, D.V. Membrane materials for energy production and storage. Pure Appl. Chem. 2020, 92, 1147–1157. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Popel’, O.S.; Tarasenko, A.B.; Filippov, S.P. Fuel cell based power-generating installations: State of the art and future prospects. Therm. Eng. 2018, 65, 859–874. [Google Scholar] [CrossRef]
- Gielen, D.; Taibi, E.; Miranda, R. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019; 52p. [Google Scholar]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- van Renssen, S. The hydrogen solution? Nat. Clim. Chang. 2020, 10, 799–801. [Google Scholar] [CrossRef]
- The Future of Hydrogen: Seizing Today’s Opportunities; International Energy Agency: Paris, France, 2019; 200p, Available online: https://read.oecd-ilibrary.org/energy/the-future-of-hydrogen_1e0514c4-en#page7 (accessed on 10 August 2022).
- Energy Technology Perspectives; International Energy Agency: Paris, France, 2020; 398p, Available online: https://iea.blob.core.windows.net/assets/7f8aed40-89af-4348-be19-c8a67df0b9ea/Energy_Technology_Perspectives_2020_PDF.pdf (accessed on 10 August 2022).
- Hydrogen Economy Outlook: Key Messages; Bloomberg Finance L.P.: New York, NY, USA, 2020; 12p, Available online: https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf (accessed on 10 August 2022).
- Lesmana, H.; Zhang, Z.; Li, X.; Zhu, M.; Xu, W.; Zhang, D. NH3 as a Transport Fuel in Internal Combustion Engines: A Technical Review. J. Energy Res. Technol. 2019, 141, 070703. [Google Scholar] [CrossRef]
- Oh, S.; Park, C.; Kim, S.; Kim, Y.; Choi, Y.; Kim, C. Natural gas–ammonia dual-fuel combustion in spark-ignited engine with various air–fuel ratios and split ratios of ammonia under part load condition. Fuel 2021, 290, 120095. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels towards Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Karchiyappan, T.A. Review on hydrogen energy production from electrochemical system: Benefits and challenges. Energy Sources A 2019, 41, 902–909. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, D.; Wei, Z.; Chen, Z.; Mirzayev, M.; Chen, Y.; Chen, S. Coal decarbonization: A state-of-the-art review of enhanced hydrogen production in underground coal gasification. Energy Rev. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Filippov, S.P.; Keiko, A.V. Coal gasification: At the crossroad. Technological Factors. Therm. Eng. 2021, 68, 209–220. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Z.; Ali Farouq, S.M. Thermal-hydro-chemical-mechanical alteration of coal pores in underground coal gasification. Fuel 2020, 262, 116543. [Google Scholar] [CrossRef]
- Steinberg, M. Fossil fuel decarbonization technology for mitigating global warming. Int. J. Hydrogen Energy 1999, 24, 771–777. [Google Scholar] [CrossRef]
- Rand, D.A.J. A journey on the electrochemical road to sustainability. J. Solid State Electrochem. 2011, 15, 1579–1622. [Google Scholar] [CrossRef]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Abanades, A. Low carbon production of hydrogen by methane decarbonization. In Production of Hydrogen from Renewable Resources; Fang, Z., Smith, J.R.L., Qi, X., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 149–177. [Google Scholar]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Machhammer, O.; Bode, A.; Hormuth, W. Financial and ecological evaluation of hydrogen production processes on large scale. Chem. Eng. Technol. 2016, 39, 1185–1193. [Google Scholar] [CrossRef]
- Pinsky, R.; Sabharwall, P.; Hartvigsen, J.; O’Brien, J. Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Prog. Nucl. Energy 2020, 123, 103317. [Google Scholar] [CrossRef]
- Jokar, S.; Farokhnia, M.A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The recent areas of applicability of palladium based membrane technologies for hydrogen production from methane and natural gas: A review. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Chen, L.N.; Li, H.Q.; Yan, M.W.; Yuan, C.F.; Zhan, W.W.; Jiang, Y.Q.; Xie, Z.X.; Kuang, Q.; Zheng, L.S. Ternary alloys encapsulated within different MOFs via a self-sacrificing template process: A potential platform for the investigation of size-selective catalytic performances. Small 2017, 13, 1700683. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced steam methane reforming for combined CO2 capture and hydrogen production: A State-of-the-Art Review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Tang, Y.; Wei, Y.; Wang, Z.; Zhang, S.; Li, Y.; Nguyen, L.; Li, Y.; Zhou, Y.; Shen, W.; Tao, F.F.; et al. Synergy of single-atom Ni1 and Ru1 sites on CeO2 for dry reforming of CH4. J. Am. Chem. Soc. 2019, 141, 7283–7293. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef]
- Zhou, L.; Martirez, J.M.P.; Finzel, J.; Zhang, C.; Swearer, D.F.; Tian, S.; Robatjazi, H.; Lou, M.; Dong, L.; Henderson, L.; et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 2020, 5, 61–70. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, C.; Tian, H.; Liu, T.; Zhang, X.; Chen, S.; Xiao, Q.; Wang, X.; Gong, J. Role of Fe Species of Ni-Based Catalysts for Efficient Low-Temperature Ethanol Steam Reforming. JACS Au 2021, 1, 1459–1470. [Google Scholar] [CrossRef]
- Sun, P.; Young, B.; Elgowainy, A.; Lu, Z.; Wang, M.; Morelli, B.; Hawkins, T. Criteria air pollutants and greenhouse gas emissions from hydrogen production in US steam methane reforming facilities. Environ. Sci. Technol. 2019, 53, 7103–7113. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure sensitivity in steam and dry methane reforming over nickel: Activity and carbon formation. ACS Catal. 2019, 10, 1428–1438. [Google Scholar] [CrossRef]
- Aragao, I.B.; Ro, I.; Liu, Y.; Ball, M.; Huber, G.W.; Zanchet, D.; Dumesic, J.A. Catalysts synthesized by selective deposition of Fe onto Pt for the water-gas shift reaction. Appl. Catal. B 2018, 222, 182–190. [Google Scholar] [CrossRef]
- Mitchell, S.; Perez-Ramirez, J. Single atom catalysis: A decade of stunning progress and the promise for a bright future. Nat. Commun. 2020, 11, 4302. [Google Scholar] [CrossRef]
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-atom alloy catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-X.; Lin, J.; Liu, J.; Wang, X.; Zhang, T.; Li, J. Dual metal active sites in an Ir1/FeOx single-atom catalyst: A Redox mechanism for the water-gas shift reaction. Angew. Chem. Int. Ed. 2020, 59, 12868–12875. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Ruocco, C.; Cortese, M.; Renda, S.; Meloni, E.; Festa, G.; Martino, M. Platinum based catalysts in the water gas shift reaction: Recent advances. Metals 2020, 10, 866. [Google Scholar] [CrossRef]
- Angeli, S.D.; Turchetti, L.; Monteleone, G.; Lemonidou, A.A. Catalyst development for steam reforming of methane and model biogas at low temperature. Appl. Catal. B 2016, 181, 34–46. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhong, H.; Wang, H.; Ma, K.; Pan, L. Effect of support morphology and size of nickel metal ions on hydrogen production from methane steam reforming. Chem. Phys. Lett. 2020, 746, 137291. [Google Scholar] [CrossRef]
- Lai, G.-H.; Lak, J.H.; Tsai, D.-H. Hydrogen Production via Low-Temperature Steam–Methane Reforming Using Ni–CeO2–Al2O3 Hybrid Nanoparticle Clusters as Catalysts. ACS Appl. Energy Mater. 2019, 2, 7963–7971. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Aghaali, M.H.; Firoozi, S. Enhancing the catalytic performance of Co substituted NiAl2O4 spinel by ultrasonic spray pyrolysis method for steam and dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 357–373. [Google Scholar] [CrossRef]
- Kim, D.H.; Youn, J.-R.; Seo, J.-C.; Kim, S.B.; Kim, M.-J.; Lee, K. One-pot synthesis of NiCo/MgAl2O4 catalyst for high coke-resistance in steam methane reforming: Optimization of Ni/Co ratio. Catal. Today, 2022; in press. [Google Scholar] [CrossRef]
- Santos, D.B.L.; Noronha, F.B.; Hori, C.E. Bi-reforming of methane for hydrogen production using LaNiO3/CexZr1−xO2 as precursor material. Int. J. Hydrogen Energy 2020, 45, 13947–13959. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Li, R.-j.; Zhang, J.-p.; Shi, J.; Li, K.-z.; Liu, H.-l.; Zhu, X. Regulation of metal-support interface of Ni/CeO2 catalyst and the performance of low temperature chemical looping dry reforming of methane. J. Fuel Chem. Technol. 2022, 50, 1458–1470. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, S.; Wang, Z.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry reforming of methane on single-site Ni/MgO catalysts: Importance of site confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Yang, Y.; Liu, B. High-performance Ni–Fe redox catalysts for selective CH4 to syngas conversion via chemical looping. ACS Catal. 2018, 8, 1748–1756. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Dou, B.; Wang, C.; Song, Y.; Chen, H.; Jiang, B.; Yang, M.; Xu, Y. Solid sorbents for in-situ CO2 removal during sorption-enhanced steam reforming process: A review. Renew. Sustain. Energy Rev. 2016, 53, 536–546. [Google Scholar] [CrossRef]
- Yan, Y.; Manovic, V.; Anthony, E.J.; Clough, P.T. Techno-economic analysis of low-carbon hydrogen production by sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 226, 113530. [Google Scholar] [CrossRef]
- Nkulikiyinka, P.; Yan, Y.; Gulec, F.; Manovic, V.; Clough, P.T. Prediction of sorption enhanced steam methane reforming products from machine learning based soft-sensor models. Energy AI 2020, 2, 100037. [Google Scholar] [CrossRef]
- Alent’ev, A.Y.; Volkov, A.V.; Vorotyntsev, I.V.; Maksimov, A.L.; Yaroslavtsev, A.B. Membrane Technologies for Decarbonization. Membr. Membr. Technol. 2021, 3, 255–273. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Z.; Cheng, Z.; Fang, X. Sol-gel-derived, CaZrO3-stabilized Ni/CaO-CaZrO3 bifunctional catalyst for sorption-enhanced steam methane reforming. Appl. Catal. B 2016, 196, 16–26. [Google Scholar] [CrossRef]
- Papalas, T.; Antzaras, A.N.; Lemonidou, A.A. Intensified steam methane reforming coupled with Ca-Ni looping in a dual fluidized bed reactor system: A conceptual design. Chem. Eng. J. 2020, 382, 122993. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob. Energy Intercon. 2019, 2, 436–443. [Google Scholar] [CrossRef]
- Liu, F.Q.; Li, G.-H.; Luo, S.-W.; Li, W.-H.; Huang, Z.-G.; Li, W.; Su, F.; Li, C.-Q.; Ding, Z.-B.; Jiang, Q. Ultrafast carbon dioxide sorption kinetics using morphology controllable lithium zirconate. ACS Appl. Mater. Interfaces 2019, 11, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Ahlen, M.; Cheung, O.; Sanna, A. Tuning Na2ZrO3 for fast and stable CO2 adsorption by solid state synthesis. Chem. Eng. J. 2020, 388, 124284. [Google Scholar] [CrossRef]
- Wang, Y.; Memon, M.Z.; AliSeelro, M.; Fu, W.; Gao, Y.; Dong, Y.; Ji, G. A review of CO2 sorbents for promoting hydrogen production in the sorption-enhanced steam reforming process. Int. J. Hydrogen Energy 2021, 46, 23358–23379. [Google Scholar] [CrossRef]
- Schwartz, N.; Harrington, J.; Ziegler, K.; Cox, P. Effects of structure and chemistry on electrochemical transport properties of anion exchange membranes for separation of CO2. Sep. Sci. Technol. 2023, 58, 212–219. [Google Scholar] [CrossRef]
- di Giuliano, A.; Gallucci, K. Sorption enhanced steam methane reforming based on nickel and calcium looping: A review. Chem. Eng. Process. 2018, 130, 240–252. [Google Scholar] [CrossRef]
- Cherbanski, R.; Molga, E. Sorption-enhanced steam methane reforming (SE-SMR)—A review: Reactor types, catalyst and sorbent characterization, process modeling. Chem. Process Eng. 2018, 39, 427–448. [Google Scholar]
- Osman, A.I. Catalytic hydrogen production from methane partial oxidation: Mechanism and kinetic study. Chem. Eng. Technol. 2020, 43, 641–648. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Cuenya, B.R.; Fierro, J.L.G. Partial oxidation of methane to syngas over nickel-based catalysts: Influence of support type, addition of rhodium, and preparation method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; MD Dostagir, N.H.; Shrotri, A.; Fukuoka, A.; Kobayashi, H. Partial oxidation of methane to syngas via formate Intermediate found for a ruthenium–rhenium bimetallic catalyst. ACS Catal. 2021, 11, 3782–3789. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Long, G.; Li, J.; Hu, X.; Ye, Z.; Wang, Z.; Buckley, C.E.; Dong, D. Synergistic promotion effect of MgO and CeO2 on nanofibrous Ni/Al2O3 catalysts for methane partial oxidation. Fuel 2019, 258, 116103. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2018, 346, 1–27. [Google Scholar] [CrossRef]
- Moiseev, I.I.; Loktev, A.S.; Shlyakhtin, O.A.; Mazo, G.N.; Dedov, A.G. New approaches to the design of nickel, cobalt, and Nickel–cobalt catalysts for partial oxidation and dry reforming of methane to synthesis gas. Petr. Chem. 2019, 59, S1–S20. [Google Scholar] [CrossRef]
- Ha, Q.L.M.; Lund, H.; Kreyenschulte, C.; Bartling, S.; Atia, H.; Vuong, T.H.; Wohlrab, S.; Armbruster, U. Development of highly stable Low Ni content catalyst for dry reforming of CH4-rich feedstocks. ChemCatChem 2020, 12, 1562–1568. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Llorc, J.; Bimbela, F.; Gandía, L.M. Partial oxidation of methane to syngas using Co/Mg and Co/Mg-Al oxide supported catalysts. Catal. Today 2019, 333, 259–267. [Google Scholar] [CrossRef]
- Khatun, R.; Bhandari, S.; Poddar, M.K.; Samanta, C.; Khan, T.S.; Khurana, D.; Bal, R. Partial oxidation of methane over high coke-resistant bimetallic Pt-Ni/CeO2 catalyst: Profound influence of Pt addition on stability. Int. J. Hydrogen Energy 2022, 47, 38895–38909. [Google Scholar] [CrossRef]
- Wachter, P.; Hödl, P.; Raic, J.; Gaber, C.; Demuth, M.; Hochenauer, C. Towards thermochemical recuperation applying combined steam reforming and partial oxidation of methane: Thermodynamic and experimental considerations. Energy Convers. Manag. 2022, 251, 114927. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A review of recent advances in water-gas shift catalysis for hydrogen production. Emergent Mater. 2020, 3, 881–917. [Google Scholar] [CrossRef]
- Uvarov, V.I.; Kapustin, R.D.; Fedotov, A.S.; Kirillov, A.O. Synthesis of porous ceramic materials for catalytically active membranes by technological combustion and sintering. Glass Ceram. 2020, 77, 221–225. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Borodi, G.; Lazar, M.D. Combined steam and dry reforming of methane for syngas production from biogas using bimodal pore catalysts. Catal. Today 2021, 366, 87–96. [Google Scholar] [CrossRef]
- Guilhaume, N.; Bianchi, D.; Wandawa, R.A.; Yin, W.; Schuurman, Y. Study of CO2 and H2O adsorption competition in the combined dry / steam reforming of biogas. Catal. Today 2021, 375, 282–289. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Laffir, F.; Curtin, T.; Thompson, J.M.; Rooney, D.W. A bimetallic catalyst on a dual component support for low temperature total methane oxidation. Appl. Catal. B 2016, 187, 408–418. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; McLaren, M.; Laffir, F.; Rooney, D.W. Characterisation of robust combustion catalyst from aluminium foil waste. Chem. Select 2018, 3, 1545–1550. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Y.; Liu, Y.; Sakao, T.; Huisingh, D.; Almeida, C.M.V.B. A comprehensive review of big data analytics throughout product lifecycle to support sustainable smart manufacturing: A framework, challenges and future research directions. J. Clean. Prod. 2019, 210, 1343–1365. [Google Scholar] [CrossRef]
- Leclerc, C.A.; Gudgila, R. Short Contact Time Catalytic Partial Oxidation of Methane over Rhodium Supported on Ceria Based 3-D Printed Supports. Ind. Eng. Chem. Res. 2019, 58, 14632–14637. [Google Scholar] [CrossRef]
- Siang, T.J.; Jalil, A.A.; Liew, S.Y.; Owgi, A.H.K.; Rahman, A.F.A. A review on state-of-the-art catalysts for methane partial oxidation to syngas production. Catal. Rev. 2022, 11, 1–57. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Y.; Zhao, Y.-X.; He, S.-G. Conversion of Methane with Oxygen to Produce Hydrogen Catalyzed by Triatomic Rh3–Cluster Anion. J. Am. Chem. Soc. 2022, 2, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Boukha, Z.; Gil-Calvo, M.; de Rivas, B.; Gonzalez-Velasco, J.R.; Gutierrez-Ortiz, J.I.; Lopez-Fonseca, R. Behaviour of Rh supported on hydroxyapatite catalysts in partial oxidation and steam reforming of methane: On the role of the speciation of the Rh particles. Appl. Catal. A 2018, 556, 191–203. [Google Scholar] [CrossRef]
- Cheephat, C.; Daorattanachai, P.; Devahastin, S.; Laosiripojana, N. Partial oxidation of methane over monometallic and bimetallic Ni-, Rh-, Re-based catalysts: Effects of Re addition, co-fed reactants and catalyst support. Appl. Catal. A 2018, 563, 1–8. [Google Scholar] [CrossRef]
- Rogozhnikov, V.N.; Snytnikov, P.V.; Salanov, A.N.; Kulikov, A.V.; Ruban, N.V.; Potemkin, D.I.; Sobyanin, V.A.; Kharton, V.V. Rh/θ-Al2O3/FeCrAlloy wire mesh composite catalyst for partial oxidation of natural gas. Mater. Lett. 2019, 236, 316–319. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Guo, S.; Ma, Z.; Li, Y.; Ma, L.; Zhang, K. Abundant hydrogen production over well dispersed nickel nanoparticles confined in mesoporous metal oxides in partial oxidation of methane. Int. J. Hydrogen Energy 2019, 44, 30171–30184. [Google Scholar] [CrossRef]
- Dossumov, K.; Kurokawa, H.; Yergaziyeva, Y.; Myltykbayeva, L.; Tayrabekova, S. Nickel Oxide Catalysts for Partial Oxidation of Methane to Synthesis Gas. Eurasian Chem.-Technol. J. 2016, 18, 25–30. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mondal, K.C.; Choudhary, T.V. Partial oxidation of methane to syngas with or without simultaneous steam or CO2 reforming over a high-temperature stable-NiCoMgCeOx supported on zirconia–hafnia catalyst. Appl. Catal. A 2016, 306, 45–50. [Google Scholar] [CrossRef]
- Karaismailoglu, M.; Figen, H.E.; Baykara, S.Z. Hydrogen production by catalytic methane decomposition over yttria doped nickel based catalysts. Int. J. Hydrogen Energy 2019, 44, 9922–9929. [Google Scholar] [CrossRef]
- Osman, A.I.; Meudal, J.; Laffir, F.; Thompson, J.; Rooney, D. Enhanced catalytic activity of Ni on η-Al2O3 and ZSM-5 on addition of ceria zirconia for the partial oxidation of methane. Appl. Catal. B 2017, 212, 68–79. [Google Scholar] [CrossRef]
- Alam, S.; Kumar, J.P.; Rani, K.Y.; Sumana, C. Self-sustained process scheme for high purity hydrogen production using sorption enhanced steam methane reforming coupled with chemical looping combustion. J. Clean. Prod. 2017, 162, 687–701. [Google Scholar] [CrossRef]
- Yan, Y.; Thanganadar, D.; Clough, P.T.; Mukherjee, S.; Patchigolla, K.; Manovic, V.; Anthony, E.J. Process simulations of blue hydrogen production by upgraded sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 222, 113144. [Google Scholar] [CrossRef]
- Saithong, N.; Authayanun, S.; Patcharavorachot, Y.; Arpornwichanop, A. Thermodynamic analysis of the novel chemical looping process for two-grade hydrogen production with CO2 capture. Energy Convers. Manag. 2019, 180, 325–337. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Nedolivko, V.V.; Zasypalov, G.O.; Vutolkina, A.V.; Gushchin, P.A.; Vinokurov, V.A.; Kulikov, L.A.; Egazar’yants, S.V.; Karakhanov, E.A.; Maksimov, A.L.; Glotov, A.P. Carbon dioxide reforming of methane. Russ. J. Appl. Chem. 2020, 93, 765–787. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Chen, L.; Gangadharan, P.; Lou, H.H. Sustainability assessment of combined steam and dry reforming versus tri-reforming of methane for syngas production. Asia-Pac. J. Chem. Eng. 2018, 13, e2168. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Rosli, S.N.A.; Abidin, S.Z.; Osazuwa, O.U.; Fan, X.; Jiao, Y. The effect of oxygen mobility/vacancy on carbon gasification in nano catalytic dry reforming of methane: A review. J. CO2 Util. 2022, 63, 102109. [Google Scholar] [CrossRef]
- Niu, J.; Guo, F.; Ran, J.; Qi, W.; Yang, Z. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations. Int. J. Hydrogen Energy 2020, 45, 30267–30287. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5901–5928. [Google Scholar] [CrossRef]
- Fedotov, A.S.; Tsodikov, M.V.; Yaroslavtsev, A.B. Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters. Processes 2022, 10, 2060. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B. 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Bakhtiari, K.; Kootenaei, A.S.; Maghsoodi, S.; Azizi, S.; Ghomsheh, S.M.T. Synthesis of high sintering-resistant Ni-modified halloysite based catalysts containing La, Ce, and Co for dry reforming of methane. Ceram. Int. 2022, 48, 37394–37402. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, J.; Xu, R.; Zhang, R.; Ge, J. Highly dispersed Ni/MgO-mSiO2 catalysts with excellent activity and stability for dry reforming of methane. Nano Res. 2022, 15, 5004–5013. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Li, K.; Chang, X.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Assabumrungrat, S.; Zhao, Z.-J.; Zeng, L.; Gong, J. Ordered mesoporous Ni/La2O3 catalysts with interfacial synergism towards CO2 activation in dry reforming of methane. Appl. Catal. B 2019, 259, 118092. [Google Scholar] [CrossRef]
- La Parola, V.; Liotta, L.F.; Pantaleo, G.; Testa, M.L.; Venezia, A.M. CO2 reforming of CH4 over Ni supported on SiO2 modified by TiO2 and ZrO2: Effect of the support synthesis procedure. Appl. Catal. A 2022, 642, 118704. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Zhang, Q.; Yang, Z.; Sun, Y.; Zou, G. Methane dry reforming over activated carbon supported Ni-catalysts prepared by solid phase synthesis. J. Clean. Prod. 2020, 274, 122256. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, G.; Li, M.; Wu, Y.; Nie, H.; Li, D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Pupulin, E.; Menegazzo, F.; Ghedini, E.; Di Michele, A.; Mattarelli, M.; Cruciani, G.; Signoretto, M. Nickel based catalysts for methane dry reforming. Effect of supports on catalytic activity and stability. Int. J. Hydrogen Energy 2019, 44, 28065–28076. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Chin, C.Y.; Setiabudi, H.D.; Vo, D.V.N. Tailoring the properties and catalytic activities of Ni/SBA-15 via different TEOS/P123 mass ratios for CO2 reforming of CH4. J. Environ. Chem. Eng. 2017, 5, 3122–3128. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Yang, W.; Fan, X.; Pan, Q.; Chen, H. Development of Ni-Co supported on SBA-15 catalysts for non-thermal plasma assisted co-conversion of CO2 and CH4: Results and lessons learnt. Carbon Capture Sci. Technol. 2022, 5, 100067. [Google Scholar] [CrossRef]
- Kiani, P.; Meshksar, M.; Rahimpour, M.R.; Iulianelli, A. CO2 utilization in methane reforming using La-doped SBA-16 catalysts prepared via pH adjustment method. Fuel 2022, 322, 124248. [Google Scholar] [CrossRef]
- Valderrama, G.; Urbina De Navarro, C.; Goldwasser, M.R. CO2 reforming of CH4 over Co-La-based perovskite-type catalyst precursors. J. Power Source 2013, 234, 31–37. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane pyrolysis for CO2-free H2 production: A green process to overcome renewable energies unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Noskov, A.S.; Parmon, V.N. Prospects for the direct catalytic conversion of methane into useful chemical products. Catal. Ind. 2017, 9, 283–298. [Google Scholar] [CrossRef]
- Parfenov, V.E.; Nikitchenko, N.V.; Pimenov, A.A.; Kuz’min, A.E.; Kulikova, M.V.; Chupichev, O.B.; Maksimov, A.L. Methane pyrolysis for hydrogen production: Specific features of using molten metals. Russ. J. Appl. Chem. 2020, 93, 625–632. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Arshad, F.; Hassan, I.U.; Tabook, M.A.; Pedram, M.Z.; Mustaqeem, M.; Tabassum, H.; Ahmed, W.; Rezakazemi, M. Thermocatalytic hydrogen production through decomposition of methane. A review. Front. Chem. 2021, 9, 736801. [Google Scholar] [CrossRef]
- Weger, L.; Abánades, A.; Butler, T. Methane cracking as a bridge technology to the hydrogen economy. Int. J. Hydrogen Energy 2017, 42, 720–731. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.M.; Zhou, L. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: A review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Sanchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane pyrolysis for zero-emission hydrogen production: A potential bridge technology from fossil fuels to a renewable and sustainable hydrogen economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Banu, A.; Bicer, Y. Integration of methane cracking and direct carbon fuel cell with CO2 capture for hydrogen carrier production. Int. J. Hydrogen Energy 2022, 47, 19502–19516. [Google Scholar] [CrossRef]
- Korányi, T.I.; Németh, M.; Beck, A.; Horváth, A. Recent Advances in Methane Pyrolysis: Turquoise Hydrogen with Solid Carbon Production. Energies 2022, 15, 6342. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Sinha, B.; Pant, K.K.; Gupta, R.B. Kinetic study and modeling of homogeneous thermocatalytic decomposition of methane over a Ni−Cu−Zn/Al2O3 catalyst for the production of hydrogen and bamboo-shaped carbon nanotubes. Ind. Eng. Chem. Res. 2016, 55, 11672–11680. [Google Scholar] [CrossRef]
- Yadav, M.D.; Patwardhan, A.W.; Joshi, J.B.; Dasgupta, K. Kinetic study of multi-walled carbon nanotube synthesis by thermocatalytic decomposition of methane using floating catalyst chemical vapour deposition. Chem. Eng. J. 2019, 377, 119895. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Zhou, Y.; Yu, G.; Jin, L.; Hu, H. Mechanism of methane decomposition with hydrogen addition over activated carbon via in-situ pyrolysis-electron impact ionization time-of-flight mass spectrometry. Fuel 2020, 263, 116734. [Google Scholar] [CrossRef]
- Chen, Q.; Lua, A.C. Kinetic reaction and deactivation studies on thermocatalytic decomposition of methane by electroless nickel plating catalyst. Chem. Eng. J. 2020, 389, 124366. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Hydrogen and carbon nanofibers synthesis by methane decomposition over Ni-Pd/Al2O3 catalyst. Int. J. Hydrogen Energy 2016, 41, 5494–5503. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Jia, Q.M.; Takriff, M.S. Catalytic decomposition of undiluted methane into hydrogen and carbon nanotubes over Pt promoted Ni/CeO2 catalysts. New J. Chem. 2018, 42, 14843–14856. [Google Scholar] [CrossRef]
- Kutteri, D.A.; Wang, I.-W.; Samanta, A.; Li, L.L.; Hu, J.L. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3d transition metal catalysts. Catal. Sci. Technol. 2018, 8, 858–869. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Seshan, K.; AlOtaibi, R.L.; Al-Fatesh, A.S. Hydrogen production via catalytic methane decomposition over alumina supported iron catalyst. Arab. J. Chem. 2018, 11, 405–414. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Rezaei, M.; Meshkani, F.; Zhang, K.F.; Zhao, X.T.; Pei, W.B.; Liu, Y.X.; Deng, J.G.; Arandiyan, H.; Dai, H.X. Mesoporous Ni/MeOx (Me = Al, Mg, Ti, and Si): Highly efficient catalysts in the decomposition of methane for hydrogen production. Appl. Surf. Sci. 2019, 478, 581–593. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Sun, J.B.; Gao, W.M.; Cui, Y.B. Effect of metal additives on the catalytic performance of Ni/Al2O3 catalyst in thermocatalytic decomposition of methane. Int. J. Hydrogen Energy 2019, 44, 7205–7215. [Google Scholar] [CrossRef]
- Ouyang, M.Z.; Boldrin, P.; Maher, R.C.; Chen, X.L.; Liu, X.H.; Cohen, L.F.; Brandon, N.P. A mechanistic study of the interactions between methane and nickel supported on doped ceria. Appl. Catal. B 2019, 248, 332–340. [Google Scholar] [CrossRef]
- Wang, J.F.; Jin, L.J.; Li, Y.; Hu, H.Q. Preparation of Fedoped carbon catalyst for methane decomposition to hydrogen. Ind. Eng. Chem. Res. 2017, 56, 11021–11027. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E.; Khan, W.U. Methane decomposition over iron catalyst for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 7593–7600. [Google Scholar] [CrossRef]
- Wang, I.-W.; Kutteri, D.A.; Gao, B.Y.; Tian, H.J.; Hu, J.L. Methane pyrolysis for carbon nanotubes and COx-free H2 over transition-metal catalysts. Energy Fuels 2019, 33, 197–205. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Methane decomposition over Ni, Co and Fe based monometallic catalysts supported on sol gel derived SiO2 microflakes. Chem. Eng. J. 2015, 262, 1009–1021. [Google Scholar] [CrossRef]
- Silva, R.R.C.M.; Oliveira, H.A.; Guarino, A.C.P.F.; Toledo, B.B.; Moura, M.B.T.; Oliveira, B.T.M.; Passos, F.B. Effect of support on methane decomposition for hydrogen production over cobalt catalysts. Int. J. Hydrogen Energy 2016, 41, 6763–6772. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Kadier, A.; Takriff, M.S.; Hassan, N.S.M. One-pot sol–gel synthesis of Ni/TiO2 catalysts for methane decomposition into COx free hydrogen and multiwalled carbon nanotubes. Int. J. Hydrogen Energy 2017, 42, 16495–16513. [Google Scholar] [CrossRef]
- Garcia-Sancho, C.; Guil-López, R.; Sebastian-Lopez, A.; Navarro, R.M.; Fierro, J.L.G. Hydrogen production by methane decomposition: A comparative study of supported and bulk ex-hydrotalcite mixed oxide catalysts with Ni, Mg and Al. Int. J. Hydrogen Energy 2018, 43, 9607–9621. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Barama, S.; Ibrahim, A.A.; Barama, A.; Khan, W.U.; Fakeeha, A. Study of methane decomposition on Fe/MgO-based catalyst modified by Ni, Co, and Mn additives. Chem. Eng. Commun. 2017, 204, 739–749. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Meshkani, F.; Rezaei, M. Thermocatalytic decomposition of methane over mesoporous nanocrystalline promoted Ni/MgO•Al2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 16476–16488. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Co-, Cu- and Fe-doped Ni/Al2O3 catalysts for the catalytic decomposition of methane into hydrogen and carbon nanofibers. Catalysts 2018, 8, 300. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Screening of Ni-Cu bimetallic catalysts for hydrogen and carbon nanofilaments production via catalytic decomposition of methane. Appl. Catal. A 2018, 559, 10–19. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Abbas, H.F.; Abnis, F.; Kudo, S.; Hayashi, J.-I.; Wan Daud, W.M.A. Methane decomposition with a minimal catalyst: An optimization study with response surface methodology over Ni/SiO2 nanocatalyst. Int. J. Hydrogen Energy 2020, 45, 14383–14395. [Google Scholar] [CrossRef]
- Rategarpanah, A.; Meshkani, F.; Wang, Y.; Arandiyan, H.; Rezaei, M. Thermocatalytic conversion of methane to highly pure hydrogen over Ni–Cu/MgO·Al2O3catalysts: Influence of noble metals (Pt and Pd) on the catalytic activity and stability. Energy Convers. Manag. 2018, 166, 268–280. [Google Scholar] [CrossRef]
- Khan, W.U.; Fakeeha, A.H.; Al-Fatesh, A.S.; Ibrahim, A.A.; Abasaeed, A.E. La2O3 supported bimetallic catalysts for the production of hydrogen and carbon nanomaterials from methane. Int. J. Hydrogen Energy 2016, 41, 976–983. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane decomposition over Ni-Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber. Int. J. Hydrogen Energy 2016, 41, 1574–1584. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.; Kasim, S.; Ibrahim, A.A.; Al-Awadi, A.S.; Fakeeha, A.H.; Al-Fatesh, A.S. H2 Production from Catalytic Methane Decomposition Using Fe/x-ZrO2 and Fe-Ni/(x-ZrO2) (x = 0, La2O3, WO3) Catalysts. Catalysts 2020, 10, 793. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; L’azaro, M.J.; Moliner, R.; Suelves, I. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: Thermogravimetric analysis and scaling-up of Fe-Mo catalysts. Int. J. Hydrogen Energy 2014, 39, 3698–3709. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-l.; Li, J.; Wei, N.; Gary, D.; et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl. Catal. B 2017, 208, 44–59. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Grigorenko, A.V.; Gromov, A.A.; Kumar, V.; Dudoladov, A.O.; Slavkina, O.V.; Darishchev, V.I. Methane pyrolysis on sponge iron powder for sustainable hydrogen production. Res. Eng. 2022, 15, 100598. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, H.; Qi, M.; Zhang, G.; Hu, H.; Ma, X. Hydrogen production by catalytic methane decomposition: Carbon materials as catalysts or catalyst supports. Int. J. Hydrogen Energy 2017, 42, 19755–19775. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, X.; Xie, W.T.; Hao, Q.Q.; Chen, H.Y.; Ma, X. K2CO3-promoted methane pyrolysis on nickel/coal-char hybrids. J. Anal. Appl. Pyrolysis 2018, 136, 53–61. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Marzbali, M.H.; Chiang, K.; Surapaneni, A.; Shah, K. Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int. J. Hydrogen Energy 2020, 45, 29978–29992. [Google Scholar] [CrossRef]
- Nishii, H.; Miyamoto, D.; Umeda, Y.; Hamaguchi, H.; Suzuki, M.; Tanimoto, T.; Harigai, T.; Takikawa, H.; Suda, Y. Catalytic activity of several carbons with different structures for methane decomposition and by-produced carbons. Appl. Surf. Sci. 2019, 473, 291–297. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lua, A.C. Hydrogen production by thermocatalytic methane decomposition. Heat Transf. Eng. 2013, 34, 896–903. [Google Scholar] [CrossRef]
- Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic methane pyrolysis in molten MnCl2 -KCl. Appl. Catal. B 2019, 254, 659–666. [Google Scholar]
- Leal Pérez, B.J.; Medrano Jiménez, J.A.; Bhardwaj, R.; Goetheer, E.; van Sint Annaland, M.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrogen Energy 2021, 46, 4917–4935. [Google Scholar]
- Kang, D.; Palmer, C.; Mannini, D.; Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic Methane Pyrolysis in Molten Alkali Chloride Salts Containing Iron. ACS Catal. 2020, 10, 7032–7042. [Google Scholar] [CrossRef]
- Patzschke, C.F.; Parkinson, B.; Willis, J.J.; Nandi, P.; Love, A.M.; Raman, S.; Hellgardt, K. Co-Mn catalysts for H2 production via methane pyrolysis in molten salts. Chem. Eng. J. 2021, 414, 128730. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Methane cracking for hydrogen production: A review of catalytic and molten media pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Parkinson, B.; Patzschke, C.F.; Nikolis, D.; Raman, S.; Dankworth, D.C.; Hellgardt, K. Methane pyrolysis in monovalent alkali halide salts: Kinetics and pyrolytic carbon properties. Int. J. Hydrogen Energy 2021, 46, 6225–6238. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Ferreira-Pinto, L.; Parizi, M.P.S.; de Arau´jo, P.C.C.; Zanette, A.F.; Cardozo-Filho, L. Experimental basic factors in the production of H2 via supercritical water gasification. Int. J. Hydrogen Energy 2019, 44, 25365–25383. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas reforming to syngas: A review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Arapova, O.V.; Konstantinov, G.I.; Bukhtenko, O.V.; Ellert, O.G.; Nikolaev, S.A.; Vasil’kov, A.Y. The role of nanosized nickel particles in microwave-assisted dry reforming of lignin. Chem. Eng. J. 2017, 309, 628–637. [Google Scholar] [CrossRef]
- Shu, R.; Zhou, L.; Zhu, Z.; Luo, B.; You, H.; Zhong, Z.; He, Y. Enhanced hydrogenolysis of enzymatic hydrolysis lignin over in situ prepared RuNi bimetallic catalyst. Int. J. Hydrogen Energy 2022, 47, 41564–41572. [Google Scholar] [CrossRef]
- Weng, S.-F.; Hsieh, H.-C.; Lee, C.-S. Hydrogen production from oxidative steam reforming of ethanol on nickel-substituted pyrochlore phase catalysts. Int. J. Hydrogen Energy 2017, 42, 2849–2860. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Yaroslavtsev, A.B. Catalysts for the steam reforming and electrochemical oxidation of methanol. Inorg. Mater. 2018, 54, 1315–1329. [Google Scholar] [CrossRef]
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int. J. Hydrogen Energy 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 2022, 122, 385–441. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrogen Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2021, 387, 224–236. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Srisiriwat, N.; Therdthianwong, A.; Croiset, E. Reforming of bioethanol over Ni/Al2O3 and Ni/ CeZrO2/Al2O3 catalysts in supercritical water for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2877–2886. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Lytkina, A.A.; Ermilova, M.M.; Efimov, M.N.; Zemtsov, L.M.; Orekhova, N.V.; Karpacheva, G.P.; Bondarenko, G.N.; Yaroslavtsev, A.B.; Muraviev, D.N. Ethanol and methanol steam reforming on transition metal catalysts supported on detonation synthesis nanodiamonds for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 3557–3565. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic reforming of oxygenates: State of the art and future prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Zhang, L.; Li, Y.; Pan, L. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming. Int. J. Hydrogen Energy 2017, 42, 9930–9937. [Google Scholar] [CrossRef]
- Qing, S.-J.; Hou, X.-N.; Liu, Y.-J.; Wang, L.; Li, L.-D.; Gao, Z.-X. Catalytic performance of Cu-Ni-Al spinel for methanol steam reforming to hydrogen. J. Fuel Chem. Technol. 2018, 46, 1210–1217. [Google Scholar] [CrossRef]
- Scotti, N.; Bossola, F.; Zaccheria, F.; Ravasio, N. Copper–zirconia catalysts: Powerful multifunctional catalytic tools to approach sustainable processes. Catalysts 2020, 10, 168. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Petriev, I.S.; Baryshev, M.G.; Yaroslavtsev, A.B. Ru-Rh based catalysts for hydrogen production via methanol steam reforming in conventional and membrane reactors. Int. J. Hydrogen Energy 2019, 44, 13310–13322. [Google Scholar] [CrossRef]
- Li, J.; Mei, X.; Zhang, L.; Yu, Z.; Liu, Q.; Wei, T.; Wu, W.; Dong, D.; Xu, L.; Hu, X. A comparative study of catalytic behaviors of Mn, Fe, Co, Ni, Cu and Zn-based catalysts in steam reforming of methanol, acetic acid and acetone. Int. J. Hydrogen Energy 2020, 45, 3815–3832. [Google Scholar] [CrossRef]
- Mosinska, M.; Stępińska, N.; Maniukiewicz, W.; Rogowski, J.; Mierczynska-Vasilev, A.; Vasilev, K.; Szynkowska, M.I.; Mierczyński, P. Hydrogen production on Cu-Ni catalysts via the oxy-steam reforming of methanol. Catalysts 2020, 10, 273. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Peng, X.; Chen, J.-L.; Pao, C.-W.; Zhang, X.; Dun, C.; Young, M.; Prendergast, D.; Urban, J.J.; et al. Insights into the mechanism of methanol steam reforming tandem reaction over CeO2 supported single-site Catalysts. J. Am. Chem. Soc. 2021, 143, 12074–12081. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Wichert, M.; Zapf, R.; Ziogas, A.; Kolb, G.; Klemm, E. Kinetic investigations of the steam reforming of methanol over a Pt/In2O3/Al2O3 catalyst in microchannels. Chem. Eng. Sci. 2016, 155, 201–209. [Google Scholar] [CrossRef]
- Greluk, M.; Słowik, G.; Rotko, M.; Machocki, A. Steam reforming and oxidative steam reforming of ethanol over PtKCo/CeO2 catalyst. Fuel 2016, 183, 518–530. [Google Scholar] [CrossRef]
- Prasongthum, N.; Xiao, R.; Zhang, H.; Tsubaki, N.; Natewong, P.; Reubroycharoen, P. Highly active and stable Ni supported on CNTs-SiO2 fiber catalysts for steam reforming of ethanol. Fuel Process. Technol. 2017, 160, 185–195. [Google Scholar] [CrossRef]
- Mulewa, W.; Tahir, M.; Amin, N.A.S. MMT-supported Ni/TiO2 nanocomposite for low temperature ethanol steam reforming toward hydrogen production. Chem. Eng. J. 2017, 326, 956–969. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Optimum operating conditions in ethanol steam reforming over a Ni/La2O3-αAl2O3 catalyst in a fluidized bed reactor. Fuel Process. Technol. 2018, 169, 207–216. [Google Scholar] [CrossRef]
- Tran, S.B.T.; Choi, H.; Oh, S.; Park, J.Y. Defective Nb2O5-supported Pt catalysts for CO oxidation: Promoting catalytic activity via oxygen vacancy engineering. J. Catal. 2019, 375, 124–134. [Google Scholar] [CrossRef]
- Ploner, K.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Zhang, L.; Armbrüster, M.; Obendorf, D.; Bernardi, J.; Klötzer, B.; et al. Reactive metal-support interaction in the Cu-In2O3 system: Intermetallic compound formation and its consequences for CO2-selective methanol steam reforming. Sci. Technol. Adv. Mater. 2019, 20, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. The influence of the support composition and structure (MxZr1−xO2-δ) of bimetallic catalysts on the activity in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 198–207. [Google Scholar] [CrossRef]
- Abrokwah, R.Y.; Deshmane, V.G.; Kuila, D. Comparative performance of M-MCM-41 (M: Cu, Co, Ni, Pd, Zn and Sn) catalysts for steam reforming of methanol. J. Molec. Catal. A 2016, 425, 10–20. [Google Scholar] [CrossRef]
- Khani, Y.; Tahay, P.; Bahadoran, F.; Safaria, N.; Soltanali, S.; Alavi, A. Synergic effect of heat and light on the catalytic reforming of methanol over Cu/x-TiO2(x=La, Zn, Sm, Ce) nanocatalysts. Appl. Catal. A 2020, 594, 117456. [Google Scholar] [CrossRef]
- Lytkina-Payen, A.; Tabachkova, N.; Yaroslavtsev, A. Methanol Steam Reforming on Bimetallic Catalysts Based on In and Nb Doped Titania or Zirconia: A Support Effect. Processes 2022, 10, 19. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Rudenko, A.O.; Lyagaeva, J.G.; Medvedev, D.A. Lanthanum-containing proton-conducting electrolytes with perovskite structures. Membr. Membr. Technol. 2021, 3, 73–97. [Google Scholar] [CrossRef]
- Zhang, J.; Su, D.S.; Blume, R.; Schlogl, R.; Wang, R.; Yang, X.; Gajovic, A. Surface Chemistry and Catalytic Reactivity of a Nanodiamond in the Steam-Free Dehydrogenation of Ethylbenzene. Angew. Chem. Int. Ed. 2010, 49, 8640–8644. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Belenov, S.V.; Guterman, V.E.; Efimov, M.N.; Yaroslavtsev, A.B. Bimetallic carbon nanocatalysts for methanol steam reforming in conventional and membrane reactors. Catal. Today 2016, 268, 60–67. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, X.; Liang, N.; Abdullah, A. Improvement of Biohydrogen Production from Dark Fermentation by Cocultures and Activated Carbon Immobilization. Energy Fuels 2017, 31, 12217–12222. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Potential of Bio-Hydrogen Production from Dark Fermentation of Crop Residues: A Review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an Energy Vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling fermentative synthesis of molecular hydrogen from biomass formation in Thermotoga maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef]
- Eroglu, E.; Melis, A. Photobiological Hydrogen Production: Recent Advances and State of the Art. Bioresour. Technol. 2011, 102, 8403–8413. [Google Scholar] [CrossRef]
- Baeyens, J.; JiapeiNie, H.; Appels, L.; Devil, R.; Ansart, R.; Deng, Y. Reviewing the potential of bio-hydrogen production by fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Sha-habuddin, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Advances in the Thermo-Chemical Production of Hydrogen from Biomass and Residual Wastes: Summary of Recent Techno-Economic Analyzes. Bioresour. Technol. 2020, 299, 122557. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Maksimov, A.L. Hydrogen storage using liquid organic carriers. Russ. J. Appl. Chem. 2020, 93, 1815–1830. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghi, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the current status of ammonia-blended hydrogen fuel engine development. Energies 2022, 15, 1023. [Google Scholar] [CrossRef]
- Wang, P.; Chang, F.; Gao, W.; Guo, J.; Wu, G.; He, T.; Chen, P. Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation. Nat. Chem. 2016, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Aika, K.-I. Role of alkali promoter in ammonia synthesis over ruthenium catalysts—Effect on reaction mechanism. Catal. Today 2017, 286, 14–20. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Ermilova, M.M.; Orekhova, N.V.; Tolkacheva, A.S.; Shkerin, S.N.; Yaroslavtsev, A.B. Transformations of ethanol on catalysts based on nanoporous calcium aluminate, mayenite (Ca12Al14O33),and mayenite doped by copper. Nanotechnol. Russia 2017, 12, 597–604. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Hassan, S.H.A.; Rahman, M.M.; Oh, S.-E. The effect of Nafion membrane fouling on the power generation of a microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 13643–13651. [Google Scholar] [CrossRef]
- Jeerh, G.; Zhang, M.; Tao, S. Recent progress in ammonia fuel cells and their potential applications. J. Mater. Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Geburtig, D.; Preuster, P.; Boesmann, A.; Mueller, K.; Wasserscheid, P. Chemical utilization of hydrogen from fluctuating energy sources—Catalytic transfer hydrogenation from charged Liquid Organic Hydrogen Carrier systems. Int. J. Hydrogen Energy 2016, 41, 1010–1017. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N.; Bogdan, V.I. Systems for accumulation, storage and release of hydrogen. Russ. Chem. Rev. 2020, 89, 897–916. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Li, L.; Zhu, T.; Chen, X.; Cheng, H. Study on reversible hydrogen uptake and release of 1,2-dimethylindole as a new liquid organic hydrogen carrier. Int. J. Hydrogen Energy 2019, 44, 4919–4929. [Google Scholar] [CrossRef]
- Tan, K.C.; He, T.; Chua, Y.S.; Chen, P. Recent Advances of Catalysis in the Hydrogenation and Dehydrogenation of N-Heterocycles for Hydrogen Storage. J. Phys. Chem. C 2021, 125, 18553–18566. [Google Scholar] [CrossRef]
- Kim, Y.; Song, Y.; Choi, Y.; Jeong, K.; Park, J.H.; Ko, K.C.; Na, K. Catalytic Consequences of Supported Pd Catalysts on Dehydrogenative H2 Evolution from 2-[(n-Methylcyclohexyl)methyl]piperidine as the Liquid Organic Hydrogen Carrier. ACS Sustain. Chem. Eng. 2021, 9, 809–821. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, C.; Li, W.; Li, W.; Qiu, M.; Chen, X.; Wang, H.; Sun, Y. Ultralow Rh Bimetallic Catalysts with High Catalytic Activity for the Hydrogenation of N-Ethylcarbazole. ACS Sustain. Chem. Eng. 2021, 9, 5260–5267. [Google Scholar] [CrossRef]
- Mollar-Cuni, A.; Ventura-Espinosa, D.; Martín, S.; García, H.; Mata, J.A. Reduced Graphene Oxides as Carbocatalysts in Acceptorless Dehydrogenation of N-Heterocycles. ACS Catal. 2021, 11, 14688–14693. [Google Scholar] [CrossRef]
- Mejuto, C.; Ibáñez-Ibáñez, L.; Guisado-Barrios, G.; Mata, J.A. Visible-Light-Promoted Iridium(III)-Catalyzed Acceptorless Dehydrogenation of N-Heterocycles at Room Temperature. ACS Catal. 2022, 12, 6238–6245. [Google Scholar] [CrossRef]
- Lim, S.; Song, Y.; Jeong, K.; Park, J.H.; Na, K. Enhanced Dehydrogenative H2 Release from N-Containing Amphicyclic LOHC Boosted by Pd-Supported Nanosheet MFI Zeolites Having Strong Acidity and Large Mesoporosity. ACS Sustain. Chem. Eng. 2022, 10, 3584–3594. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Dong, Y.; Mei, P.; Cheng, H. Hydrogen storage and release from a new promising Liquid Organic Hydrogen Storage Carrier (LOHC): 2-methylindole. Int. J. Hydrogen Energy 2016, 41, 16129–16134. [Google Scholar] [CrossRef]
- Muller, K.; Stark, K.; Mueller, B.; Arlt, W. Amine Borane Based Hydrogen Carriers: An Evaluation. Energy Fuel 2012, 26, 3691–3696. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Nafchi, F.M.; Baniasadi, E.; Afshari, E.; Javani, N. Performance assessment of a solar hydrogen and electricity production plant using high temperature PEM electrolyzer and energy storage. Int. J. Hydrogen Energy 2018, 43, 5820–5831. [Google Scholar] [CrossRef]
- Auer, F.; Blaumeiser, D.; Bauer, T.; Bösmann, A.; Szesni, N.; Libuda, J.; Wasserscheid, P. Boosting the activity of hydrogen release from liquid organic hydrogen carrier systems by sulfur-additives to Pt on alumina catalysts. Catal. Sci. Technol. 2019, 9, 3537–3547. [Google Scholar] [CrossRef]
- Wang, W.; Miao, L.; Wu, K.; Chen, G.; Huang, Y.; Yang, Y. Hydrogen evolution in the dehydrogenation of methylcyclohexane over Pt/CeMgAlO catalysts derived from their layered double hydroxides. Int. J. Hydrogen Energy 2019, 44, 2918–2925. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, M.; Chen, X.; Dong, Y.; Zhang, Z.; Cheng, H. A highly active bifunctional Ru–Pd catalyst for hydrogenation and dehydrogenation of liquid organic hydrogen carriers. J. Catal. 2019, 378, 382–391. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Vehkamaki, M.; Kemell, M.; Keskivali, L.; Simell, P.; Reinikainen, M.; Tapper, U.; Repo, T. Hydrogen release from liquid organic hydrogen carriers catalysed by platinum on rutileanatase structured titania. Chem. Commun. 2020, 56, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, Y.; Qi, S.; Smith, K.J.; Tan, X.; Yan, J.; Yi, C. Pt Catalysts Supported on H2 and O2 Plasma-Treated Al2O3 for Hydrogenation and Dehydrogenation of the Liquid Organic Hydrogen Carrier Pair Dibenzyltoluene and Perhydrodibenzyltoluene. ACS Catal. 2020, 10, 10661–10671. [Google Scholar] [CrossRef]
- Hamayun, M.H.; Maafa, I.M.; Hussain, M.; Aslam, R. Simulation study to investigate the efects of operational conditions on methylcyclohexane dehydrogenation for hydrogen production. Energies 2020, 13, 206. [Google Scholar] [CrossRef]
- Chen, X.; Gierlich, C.H.; Schötz, S.; Blaumeiser, D.; Bauer, T.; Libuda, J.; Palkovits, R. Hydrogen Production Based on Liquid Organic Hydrogen Carriers through Sulfur Doped Platinum Catalysts Supported on TiO2. ACS Sustain. Chem. Eng. 2021, 9, 6561–6573. [Google Scholar] [CrossRef]
- Bellows, S.M.; Chakraborty, S.; Gary, J.B.; Jones, W.D.; Cundari, T.R. An uncanny dehydrogenation mechanism: Polar bond control over stepwise or concerted transition states. Inorg. Chem. 2017, 56, 5519–5524. [Google Scholar] [CrossRef]
- Bernskoetter, W.H.; Hazari, N. Hydrogenation and dehydrogenation reactions catalyzed by iron pincer compounds. In Pincer Compounds, 1st ed.; Morales-Morales, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–131. [Google Scholar]
- Sisáková, K.; Podrojková, N.; Oriňaková, R.; Oriňak, A. Novel catalysts for dibenzyltoluene as a potential Liquid Organic Hydrogen Carrier use—A mini-review. Energy Fuels 2021, 35, 7608–7623. [Google Scholar] [CrossRef]
- Stenina, I.A.; Safronova, E.Y.; Levchenko, A.V.; Dobrovolsky, Y.A.; Yaroslavtsev, A.B. Low-temperature fuel cells: Outlook for application in energy storage systems and materials for their development. Therm. Eng. 2016, 63, 385–398. [Google Scholar] [CrossRef]
- Jeppesen, C.; Polverino, P.; Andreasen, S.J.; Araya, S.S.; Sahlin, S.L.; Pianese, C.; Kær, S.K. Impedance characterization of high temperature proton exchange membrane fuel cell stack under the influence of carbon monoxide and methanol vapor. Int. J. Hydrogen Energy 2017, 42, 21901–21912. [Google Scholar] [CrossRef]
- Sutharssan, T.; Montalvao, D.; Chen, Y.K.; Wang, W.C.; Pisac, C.; Elemara, H. A review on prognostics and health monitoring of proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2017, 75, 440–450. [Google Scholar] [CrossRef]
- Briceño, K.; Montanè, D.; Garcia-Valls, R.; Iulianelli, A.; Basile, A. Fabrication variables affecting the structure and properties of supported carbon molecular sieve membranes for hydrogen separation. J. Membr. Sci. 2012, 415, 288–297. [Google Scholar] [CrossRef]
- Sazali, N.; Mohamed, M.A.; Salleh, W.N.W. Membranes for hydrogen separation: A significant review. Int. J. Adv. Manuf. Technol. 2020, 107, 1859–1881. [Google Scholar] [CrossRef]
- Rezaee, P.; Naeij, H.R. A new approach to separate hydrogen from carbon dioxide using graphdiyne-like membrane. Sci. Rep. 2020, 10, 13549. [Google Scholar] [CrossRef]
- Cheng, H. Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review. Membranes 2022, 12, 647. [Google Scholar] [CrossRef]
- Arratibel, A.; Astobieta, U.; Pacheco Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. N2, He and CO2 diffusion mechanism through nanoporous YSZ/γ-Al2O3 layers and their use in a pore-filled membrane for hydrogen membrane reactors. Int. J. Hydrogen Energy 2016, 41, 8732–8744. [Google Scholar] [CrossRef]
- Kim, C.-H.; Han, J.-Y.; Kim, S.; Lee, B.; Lim, H.; Lee, K.-Y.; Ryi, S.-K. Hydrogen production by steam methane reforming in a membrane reactor equipped with a Pd composite membrane deposited on a porous stainless steel. Int. J. Hydrogen Energy 2018, 43, 7684–7692. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Medrano, J.; Coenen, K.; Arratibel, A.; Melendez, J.; de Nooijer, N.; Spallina, V.; Viviente, J.L.; Zuniga, J. Palladium based membranes and membrane reactors for hydrogen production and purification: An overview of research activities at Tecnalia and Tu/e. Int. J. Hydrogen Energy 2017, 42, 13763–13776. [Google Scholar] [CrossRef]
- Fernandez, E.; Medrano, J.A.; Melendez, J.; Parco, M.; Viviente, J.L.; Van Sint Annaland, M.; Gallucci, F.; Tanaka, P.D.A. Preparation and characterization of metallic supported thin Pd–Ag membranes for hydrogen separation. Chem. Eng. J. 2016, 305, 182–190. [Google Scholar] [CrossRef]
- Pati, S.; Jat, R.A.; Mukerjee, S.; Parida, S. X-ray diffraction study of thermal parameters of Pd, Pd–Ag and Pd–Ag–Cu alloys as hydrogen purification membrane materials. Physica B Cond. Matter 2016, 484, 42–47. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, A.; Upadhyay, R.K. Performance comparison of methanol steam reforming integrated to Pd-Ag membrane: Membrane reformer vs. membrane separator. Sep. Purif. Technol. 2017, 183, 194–203. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, B.; Jiang, J.; Xu, W. H2 purification process with double layer bcc-PdCu alloy membrane at ambient temperature. Int. J. Hydrogen Energy 2020, 45, 17540–17547. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Prizhimov, A.S.; Dontsov, A.I. Structure of the α–β Interface in a PdCu Solid Solution. Phys. Solid State 2020, 62, 59–64. [Google Scholar] [CrossRef]
- El Hawa, H.A.W.; Paglieri, S.; Craig, M.C.; Harale, A.; Way, D.J. Application of a Pd–Ru composite membrane to hydrogen production in a high temperature membrane reactor. Sep. Purif. Technol. 2015, 147, 388–397. [Google Scholar] [CrossRef]
- Lee, S.M.; Xu, N.; Kim, S.S.; Li, A.; Grace, J.R.; Lim, J.C.; Boyd, T.; Ryi, S.K.; Susdorf, A.; Schaadt, A. Palladium/ruthenium composite membrane for hydrogen separation from the off-gas of solar cell production via chemical vapor deposition. J. Membr. Sci. 2017, 541, 1–8. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Maksimenko, A.A.; Sitnikov, A.I.; Solntsev, K.A.; Chernyavskiy, A.S.; Dontsov, A.I. Composite metal-ceramic heterostructure for membranes of deep purification of hydrogen. Inorg. Mater. Appl. Res. 2016, 7, 586–589. [Google Scholar] [CrossRef]
- Plazaola, A.; Tanaka, D.A.P.; Van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef]
- Alimov, V.N.; Bobylev, I.V.; Busnyuk, A.O.; Kolgatin, S.N.; Kuzenov, S.R.; Peredistov, E.Y.; Livshits, A.I. Extraction of ultrapure hydrogen with V-alloy membranes: From laboratory studies to practical applications. Int. J. Hydrogen Energy 2018, 43, 13318–13327. [Google Scholar] [CrossRef]
- Helmi, A.; Fernandez, E.; Melendez, J.; Tanaka, D.A.P.; Gallucci, F.; van Sint Annaland, M. Fluidized Bed Membrane Reactors for Ultra Pure H2 Production—A Step forward towards Commercialization. Molecules 2016, 21, 376. [Google Scholar] [CrossRef] [PubMed]
- Iulianelli, A.; Ghasemzadeh, K.; Basile, A. Progress in Methanol Steam Reforming Modelling via Membrane Reactors Technology. Membranes 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen Production via Steam Reforming: A Critical Analysis of MR and RMM Technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef]
- Liguori, S.; Iulianelli, A.; Dalena, F.; Piemonte, V.; Huang, Y.; Basile, A. Methanol steam reforming in an Al2O3 supported thin pd-layer membrane reactor over Cu/ZnO/Al2O3 catalyst. Int. J. Hydrogen Energy 2014, 39, 18702–18710. [Google Scholar] [CrossRef]
- Piskin, F.; Öztürk, T. Combinatorial screening of Pd-Ag-Ni membranes for hydrogen separation. J. Memb. Sci. 2017, 524, 631–636. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Dontsov, A.I.; Morozova, N.B.; Gorbunov, S.V.; Ievlev, V.M.; Yaroslavtsev, A.B. Lamp Processing of the Surface of PdCu Membrane Foil: Hydrogen Permeability and Membrane Catalysis. Inorg. Mater. 2021, 57, 781–789. [Google Scholar] [CrossRef]
- Fernandez, E.; Sanchez-Garcia, J.A.; Melendez, J.; Spallina, V.; van Sint Annaland, M.; Gallucci, F.; Tanaka, D.P.; Prema, R. Development of highly permeable ultra-thin Pd-based supported membranes. Chem. Eng. J. 2016, 305, 149–155. [Google Scholar] [CrossRef]
- Pati, S.; Jat, R.A.; Anand, N.; Derose, D.J.; Karn, K.; Mukerjee, S.; Parida, S. Pd-Ag-Cu dense metallic membrane for hydrogen isotope purification and recovery at low pressures. J. Memb. Sci. 2017, 522, 151–158. [Google Scholar] [CrossRef]
- Incelli, M.; Santucci, A.; Tosti, S.; Sansovini, M.; Carlini, M. Heavy water decontamination tests through a pd-ag membrane reactor: Water gas shift and isotopic swamping performances. Fusion Eng. Des. 2017, 124, 692–695. [Google Scholar] [CrossRef]
- Pati, S.; Jangam, A.; Wang, Z.; Dewangan, N.; Wai, M.H.; Kawi, S. Catalytic Pd0.77Ag0.23 alloy membrane reactor for high temperature water-gas shift reaction: Methane suppression. Chem. Eng. J. 2019, 362, 116–125. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd–Ag membrane applied in a microchannel module configuration. Int. J. Hydrogen Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A.; Tosti, S.; Iulianelli, A.; Drioli, E. Membrane catalysis in the dehydrogenation and hydrogen production processes. Int. J. Hydrogen Energy 2007, 32, 1201. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Harasi, J.; Amiri, T.; Basile, A.; Iulianelli, A. Methanol steam reforming for hydrogen generation: A comparative modeling study between silica and Pd-based membrane reactors by cfd method. Fuel Process. Technol. 2020, 199, 106273. [Google Scholar] [CrossRef]
- Borgognoni, F.; Tosti, S.; Vadrucci, M.; Santucci, A. Combined methane and ethanol reforming for pure hydrogen production through pd-based membranes. Int. J. Hydrogen Energy 2013, 38, 1430–1438. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, J.; Yu, J.; Yang, X.; Sheng, X.; Xu, H.; Sun, C.; Shen, W.; Goldbach, A. Efficient H2 production via membrane-assisted ethanol steam reforming over Ir/CeO2 catalyst. Int. J. Hydrogen Energy 2019, 44, 24733–24745. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Mironova, E.Y.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. Ru-containing catalysts for methanol and ethanol steam reforming in conventional and membrane reactors. Inorg. Mater. 2019, 55, 547–555. [Google Scholar] [CrossRef]
- Jia, H.; Xu, H.; Sheng, X.; Yang, X.; Shen, W.; Goldbach, A. Hightemperature ethanol steam reforming in PdCu membrane reactor. J. Memb. Sci. 2020, 605, 118083. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Ban, X.; Li, N.; Chen, C.; Zhan, Z. Membrane-based catalytic partial oxidation of ethanol coupled with steam reforming for solid oxide fuel cells. J. Membr. Sci. 2021, 622, 119032. [Google Scholar] [CrossRef]
- Eremeev, N.; Krasnov, A.; Bespalko, Y.; Bobrova, L.; Smorygo, O.; Sadykov, V. An Experimental Performance Study of a Catalytic Membrane Reactor for Ethanol Steam Reforming over a Metal Honeycomb Catalyst. Membranes 2021, 11, 790. [Google Scholar] [CrossRef]
- Viviente, J.L.; Meléndez, J.; Tanaka, D.A.P.; Gallucci, F.; Spallina, V.; Manzolini, G.; Foresti, S.; Palma, V.; Ruocco, C.; Roses, L. Advanced m-chp fuel cell system based on a novel bio-ethanol fluidized bed membrane reformer. Int. J. Hydrogen Energy 2017, 42, 13970–13987. [Google Scholar] [CrossRef]
- Iulianelli, A.; Palma, V.; Bagnato, G.; Ruocco, C.; Huang, Y.; Veziroğlu, N.T.; Basile, A. From bioethanol exploitation to high grade hydrogen generation: Steam reforming promoted by a co-pt catalyst in a pd-based membrane reactor. Renew. Energy 2018, 119, 834–843. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Basile, A. CO-free hydrogen production by steam reforming of acetic acid carried out in a pd–ag membrane reactor: The effect of co-current and counter-current mode. Int. J. Hydrogen Energy 2008, 33, 4091–4096. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen production tchnologies overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Kayfeci, M.; Kecebas, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production: Processes, Systems and Technologies, 1st ed.; Calise, F., D’Accadia, M.D., Santarelli, M., Lanzini, A., Ferrero, D., Eds.; Academic Press: London, UK, 2019; pp. 45–83. [Google Scholar]
- Skúlason, E.; Karlberg, G.S.; Rossmeisl, J.; Bligaard, T.; Greeley, J.; Jónsson, H.; Nørskov, J.K. Density functional theory calculations for the hydrogen evolution reaction in an electrochemical double layer on the Pt(111) electrode. Phys. Chem. Chem. Phys. 2007, 9, 3241–3250. [Google Scholar] [CrossRef]
- Markovića, N.M.; Sarraf, S.T.; Gasteiger, H.A.; Ross, P.N. Hydrogen electrochemistry on platinum low-index single-crystal surfaces in alkaline solution. J. Chem. Soc. Faraday Trans. 1996, 92, 3719–3725. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H.; et al. Ultrahigh Pt-Mass-Activity Hydrogen Evolution Catalyst Electrodeposited from Bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Cavaliere, P.D.; Perrone, A.; Silvello, A. Water Electrolysis for the Production of Hydrogen to Be Employed in the Ironmaking and Steelmaking Industry. Metals 2021, 11, 1816. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Nanomaterials for lithium-ion batteries and hydrogen energy. Pure Appl. Chem. 2017, 89, 1185–1194. [Google Scholar] [CrossRef]
- Immerz, C.; Paidar, M.; Papakonstantinou, G.; Bensmann, B.; Bystron, T.; Vidakovic-Koch, T.; Bouzek, K.; Sundmacher, K.; Hanke-Rauschenbach, R. Effect of the MEA design on the performance of PEMWE single cells with different sizes. J. Appl. Electrochem. 2018, 48, 701–711. [Google Scholar] [CrossRef]
- Giancola, S.; Zatoń, M.; Reyes-Carmona, Á.; Dupont, M.; Donnadio, A.; Cavaliere, S.; Rozière, J.; Jones, D.J. Composite short side chain PFSA membranes for PEM water electrolysis. J. Memb. Sci. 2019, 570–571, 69–76. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Srinivasulu Reddy, D.; Bhagawan, D.; Himabindu, V. Synthesis of Polysulfone and zirconium oxide coated asbestos composite separators for alkaline water electrolysis. Int. J. Chem. Eng. Process Technol. 2017, 3, 1035–1041. [Google Scholar]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Source 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef]
- Kadier, A.; Sahaid Kalil, M.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Farhana Azman, N.; Logroño, W.; Simayi, Y.; Abdul Hamid, A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Dotan, H.; Landman, A.; Sheehan, S.W.; Malviya, K.D.; Shter, G.E.; Grave, D.A.; Arzi, Z.; Yehudai, N.; Halabi, M.; Gal, N.; et al. Decoupled hydrogen and oxygen evolution by a two-step electrochemical–chemical cycle for efficient overall water splitting. Nat. Energy 2019, 4, 786–795. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Rama Devi, B.; Himabindu, V. Phosphorus doped carbon nanoparticles supported palladium electrocatalyst for the hydrogen evolution reaction (HER) in PEM water electrolysis. Ionics 2018, 24, 3113–3121. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Pd Based Electrocatalysts for Hydrogen Evolution Reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Martin, S.; Garcia-Ybarra, P.; Castillo, J. Ten-fold reduction from the state-of-the-art platinum loading of electrodes prepared by electrospraying for high temperature proton exchange membrane fuel cells. Electrochem. Commun. 2018, 93, 57–61. [Google Scholar] [CrossRef]
- Lee, B.-S.; Park, H.-Y.; Choi, I.; Cho, M.K.; Kim, H.-J.; Yoo, S.J.; Henkensmeier, D.; Kim, J.Y.; Nam, S.W.; Park, S.; et al. Polarization characteristics of a low catalyst loading PEM water electrolyzer operating at elevated temperature. J. Power Source 2016, 309, 127–134. [Google Scholar] [CrossRef]
- Ramakrishna, S.U.B.; Srinivasulu Reddy, D.; Shiva Kumar, S.; Himabindu, V. Nitrogen doped CNTs supported Palladium electrocatalyst for hydrogen evolution reaction in PEM water electrolyser. Int. J. Hydrogen Energy 2016, 41, 20447–20454. [Google Scholar] [CrossRef]
- Li, T.-J.; Yeh, M.-H.; Chiang, W.-H.; Li, Y.-S.; Chen, G.-L.; Leu, Y.-A.; Tien, T.-C.; Lo, S.-C.; Lin, L.-Y.; Lin, J.-J.; et al. Boron-doped carbon nanotubes as metal-free electrocatalyst for dyesensitized solar cells: Heteroatom doping level effect on tri-iodide reduction reaction. J. Power Source 2018, 375, 29–36. [Google Scholar]
- Xie, L.; Liu, Q.; Shi, X.; Asiri, A.M.; Luo, Y.; Sun, X. Superior alkaline hydrogen evolution electrocatalysis enabled by an ultrafine PtNi nanoparticle-decorated Ni nanoarray with ultralow Pt loading. Inorg. Chem. Front. 2018, 5, 1365–1369. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi-O Nanostructure with Record-High Performance for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, X.; Luo, Y.; Wang, L.; Cui, G.; Xie, F.; Wang, H.; Xie, Y.; Sun, X. An ultrafine platinum–cobalt alloy decorated cobalt nanowire array with superb activity toward alkaline hydrogen evolution. Nanoscale 2018, 10, 12302–12307. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Wang, A.J.; Zhang, L.; Fang, K.-M.; Wu, L.-J.; Feng, J.J. Melamine-assisted solvothermal synthesis of PtNi nanodentrites as highly efficient and durable electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2018, 531, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cai, J.; Wu, Y.; Zang, Y.; Zheng, X.; Ye, J.; Cui, P.; Niu, S.; Liu, Y.; Zhu, J. Boosting Water Dissociation Kinetics on Pt–Ni Nanowires by N-Induced Orbital Tuning. Adv. Mater. 2019, 31, 1807780. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef]
- Jimenez-Orozco, C.; Florez, E.; Moreno, A.; Rodriguez, J.A. Platinum vs transition metal carbide surfaces as catalysts for olefin and alkyne conversion: Binding and hydrogenation of ethylidyne. J. Phys. Conf. Ser. 2019, 1247, 012003. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Gao, W.; Shi, Y.; Zhang, Y.; Zuo, L.; Lu, H.; Huang, Y.; Fan, W.; Liu, T. Molybdenum carbide anchored on graphene nanoribbons as highly efficient all-pH hydrogen evolution reaction electrocatalyst. ACS Sustain. Chem. Eng. 2016, 4, 6313–6321. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Yu, S.; Wen, T.; Zhu, X.; Yang, F.; Sun, X.; Wang, X.; Hu, W. Ternary NiCo2Px Nanowires as pH-Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2017, 29, 1605502. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, X.; Liu, S.; Chiang, C.-L.; Kuai, X.; Peng, C.-K.; Lin, Y.-C.; Meng, X.; Zhao, J.; Choi, J.; et al. Structural and Electronic Optimization of MoS2 Edges for Hydrogen Evolution. J. Am. Chem. Soc. 2019, 141, 18578–18584. [Google Scholar] [CrossRef]
- Han, D.; Gao, N.; Ge, J.; Liu, C.; Xing, W. Activating MoS2 via electronic structure modulation and phase engineering for hydrogen evolution reaction. Catal. Commun. 2022, 164, 106427. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E. High hydrogen production rate on RuS2@MoS2 hybrid nanocatalyst by PEM electrolysis. Int. J. Hydrogen Energy 2019, 44, 4398–4405. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Du, Y.; Xi, S.; Dou, S.; Nsanzimana, J.M.V.; Wang, C.; Xu, Z.J.; Wang, X. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 2019, 4, 329–338. [Google Scholar] [CrossRef]
- Zhu, Y.; Tahini, H.A.; Hu, Z.; Chen, Z.G.; Zhou, W.; Komarek, A.C.; Lin, Q.; Lin, H.J.; Chen, C.T.; Zhong, Y.; et al. Boosting Oxygen Evolution Reaction by Creating Both Metal Ion and Lattice-Oxygen Active Sites in a Complex Oxide. Adv. Mater. 2020, 32, 1905025. [Google Scholar] [CrossRef]
- Shan, S.; Li, J.; Maswadeh, Y.; O’Brien, C.; Kareem, H.; Tran, D.T.; Lee, I.C.; Wu, Z.P.; Wang, S.; Yan, S.; et al. Surface Oxygenation of Multicomponent Nanoparticles toward Active and Stable Oxidation Catalysts. Nat. Commun. 2020, 11, 4201. [Google Scholar] [CrossRef]
- Mefford, J.T.; Rong, X.; Abakumov, A.M.; Hardin, W.G.; Dai, S.; Kolpak, A.M.; Johnston, K.P.; Stevenson, K.J. Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat. Commun. 2016, 7, 11053. [Google Scholar] [CrossRef]
- Su, J.; Ge, R.; Jiang, K.; Dong, Y.; Hao, F.; Tian, Z.; Chen, G.; Chen, L. Assembling Ultrasmall Copper-Doped Ruthenium Oxide Nanocrystals into Hollow Porous Polyhedra: Highly Robust Electrocatalysts for Oxygen Evolution in Acidic Media. Adv. Mater. 2018, 30, 1801351. [Google Scholar] [CrossRef]
- Lim, J.; Park, D.; Jeon, S.S.; Roh, C.W.; Choi, J.; Yoon, D.; Park, M.; Jung, H.; Lee, H. Ultrathin IrO2 Nanoneedles for Electrochemical Water Oxidation. Adv. Funct. Mater. 2018, 28, 1704796. [Google Scholar] [CrossRef]
- Sun, X.; Xu, K.; Fleischer, C.; Liu, X.; Grandcolas, M.; Strandbakke, R.; Bjørheim, T.S.; Norby, T.; Chatzitakis, A. Earth-Abundant Electrocatalysts in Proton Exchange Membrane Electrolyzers. Catalysts 2018, 8, 657. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Chen, Y. Surface determination and electrochemical behavior of IrO2-RuO2-SiO2 ternary oxide coatings in oxygen evolution reaction application. Electrochim. Acta 2018, 264, 350–357. [Google Scholar] [CrossRef]
- Cherevko, S. Stability and dissolution of electrocatalysts: Building the bridge between model and “real world” systems. Curr. Opin. Electrochem. 2018, 8, 118–125. [Google Scholar] [CrossRef]
- Chuanpu, H.; Hong, L.; Cangen, M.; Song, Y.; Ma, J. Investigation of Mesoporous Niobium-Doped TiO2 as an Oxygen Evolution Catalyst Support in an SPE Water Electrolyzer. ACS Sustain. Chem. Eng. 2016, 4, 746–756. [Google Scholar]
- Ju, H.K.; Giddey, S.; Badwal, S.P.S. The role of nanosized SnO2 in Pt-based electrocatalysts for hydrogen production in methanol assisted water electrolysis. Electrochim. Acta 2017, 229, 39–47. [Google Scholar] [CrossRef]
- Yu, J.-W.; Jung, G.-B.; Su, Y.-J.; Yeh, C.-C.; Kan, M.-Y.; Lee, C.-Y.; Lai, C.-J. Proton exchange membrane water electrolysis system-membrane electrode assembly with additive. Int. J. Hydrogen Energy 2019, 44, 15721–15726. [Google Scholar] [CrossRef]
- Shrinath Dattatray, G.; Prasad Patel, P.; Datta, M.K.; Velikokhatnyi, O.I.; Pavithra Shanthi, M.; Prashant, N.K. First report of vertically aligned (Sn,Ir)O2:F solid solution nanotubes: Highly efficient and robust oxygen evolution electrocatalysts for proton exchange membrane based water electrolysis. J. Power Source 2018, 392, 139–149. [Google Scholar]
- Wang, Y.; Zhang, L.; Yin, K.; Zhang, J.; Gao, H.; Liu, N.; Peng, Z.; Zhang, Z. Nanoporous Iridium-Based Alloy Nanowires as Highly Efficient Electrocatalysts Toward Acidic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 39728–39736. [Google Scholar] [CrossRef]
- Lu, A.; Peng, D.-L.; Chang, F.; Skeete, Z.; Shan, S.; Sharma, A.; Luo, J.; Zhong, C.J. Composition- and Structure-Tunable Gold–Cobalt Nanoparticles and Electrocatalytic Synergy for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 20082–20091. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.; Zhao, G.; Lin, Y.; Yu, Z.; Xu, X.; Wang, X.; Liu, H.K.; Sun, W.; Dou, S.X. Active-Site-Enriched Iron-Doped Nickel/Cobalt Hydroxide Nanosheets for Enhanced Oxygen Evolution Reaction. ACS Catal. 2018, 8, 5382–5390. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Bidin, N.; Azni, S.R. The effect of magnetic and optic field in water electrolysis. Int. J. Hydrogen Energy 2017, 42, 16325–16332. [Google Scholar] [CrossRef]
- Purnami; Hamidi, N.; Sasongko, M.N.; Widhiyanuriyawan, D.; Wardana, I.N.G. Strengthening external magnetic fields with activated carbon graphene for increasing hydrogen production in water electrolysis. Int. J. Hydrogen Energy 2020, 45, 19370–19380. [Google Scholar] [CrossRef]
- Kaya, M.F.; Demir, N.; Rees, N.V.; El-Kharouf, A. Improving PEM water electrolyser’s performance by magnetic field application. Appl. Energy 2020, 264, 114721. [Google Scholar] [CrossRef]
- Costa, C.M.; Merazzo, K.J.; Goncalves, R. Magnetically active lithium-ion batteries towards battery performance improvement. Iscience 2021, 24, 102691. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Fan, L.; Li, X.G. Effect of Alternating Magnetic Field on Electrochemical Behavior of 316L and TA2 in Simulated Seawater. J. Mater. Eng. Perform. 2021, 30, 9377–9389. [Google Scholar] [CrossRef]
- Jing, S.; Hu, H.; Wang, S. Design of pulsed power supply for repetitive pulsed high magnetic field for water electrolysis. Rev. Sci. Instrum. 2021, 92, 114708. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Zheng, X.; Liu, C.; Chen, Q. Hydrogen Production byWater Electrolysis with Low Power and High Efficiency Based on Pre-Magnetic Polarization. Energies 2022, 15, 1878. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Progr. Energy Comb. Sci. 2020, 80, 100859. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Bessarabov, D.G.; Glukhov, A.S. On the contamination of membrane–electrode assembles of water electrolyzers with solid polymer electrolyte by the elements of titanium alloys. Russ. J. Electrochem. 2017, 53, 808–817. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Leslie, T.; El-Hassan, Z.; Thomposon, J.; Olabi, A.G. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113, 109286. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Low- and intermediate-temperature proton-conducting electrolytes. Inorg. Mater. 2017, 53, 253–262. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Bessarabov, D.G.; Fateev, V.N. Degradation mechanisms of MEA characteristics during water electrolysis in solid polymer electrolyte cells. Russ. J. Electrochem. 2017, 53, 318–323. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research Progress of Proton Exchange Membrane Failure and Mitigation Strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409. [Google Scholar] [CrossRef]
- Yurova, P.A.; Malakhova, V.R.; Gerasimova, E.V.; Stenina, I.A.; Yaroslavtsev, A.B. Nafion/Surface Modified Ceria Hybrid Membranes for Proton Exchange Fuel Cell Application. Polymers 2021, 13, 2513. [Google Scholar] [CrossRef]
- Karpenko-Jereb, L.; Sternig, C.; Fink, C.; Tatschl, R. Membrane degradation model for 3D CFD analysis of fuel cell performance as a function of time. Int. J. Hydrogen Energy 2016, 41, 13644–13656. [Google Scholar] [CrossRef]
- Papakonstantinou, G.; Algara-Siller, G.; Teschner, D.; Vidaković-Koch, T.; Schlögl, R.; Sundmacher, K. Degradation study of a proton exchange membrane water electrolyzer under dynamic operation conditions. Appl. Energy 2020, 280, 115911. [Google Scholar] [CrossRef]
- Li, D.; Motz, A.R.; Bae, C.; Fujimoto, C.; Yang, G.; Zhang, F.-Y.; Ayers, K.E.; Kim, Y.S. Durability of anion exchange membrane water electrolyzers. Energy Environ. Sci. 2021, 14, 3393–3419. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustain-ability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Kang, X.; Chaperman, L.; Galeckas, A.; Ammar, S.; Mammeri, F.; Norby, T.; Chatzitakis, A. Water Vapor Photoelectrolysis in a Solid-State Photoelectrochemical Cell with TiO2 Nanotubes Loaded with CdS and CdSe Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 46875–46885. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Franzitta, V.; Curto, D.; Rao, D.; Viola, A. Hydrogen Production from Sea Wave for Alternative Energy Vehicles for Public Transport in Trapani (Italy). Energies 2016, 9, 850. [Google Scholar] [CrossRef]
- Meda, U.S.; Rakesh, S.S.N.; Raj, M.A.L.A. Bio-hydrogen production in microbial electrolysis cell using waste water from sugar industry. Int. J. Eng. Sci. Res. Technol. 2015, 4, 452–458. [Google Scholar]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basseguy, R.; Delia, M.-L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; SahaidKalil, M. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Engin. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- He, Y.; Hamann, T.; Wang, D. Thin film photoelectrodes for solar water splitting. Chem. Soc. Rev. 2019, 48, 2182–2215. [Google Scholar] [CrossRef]
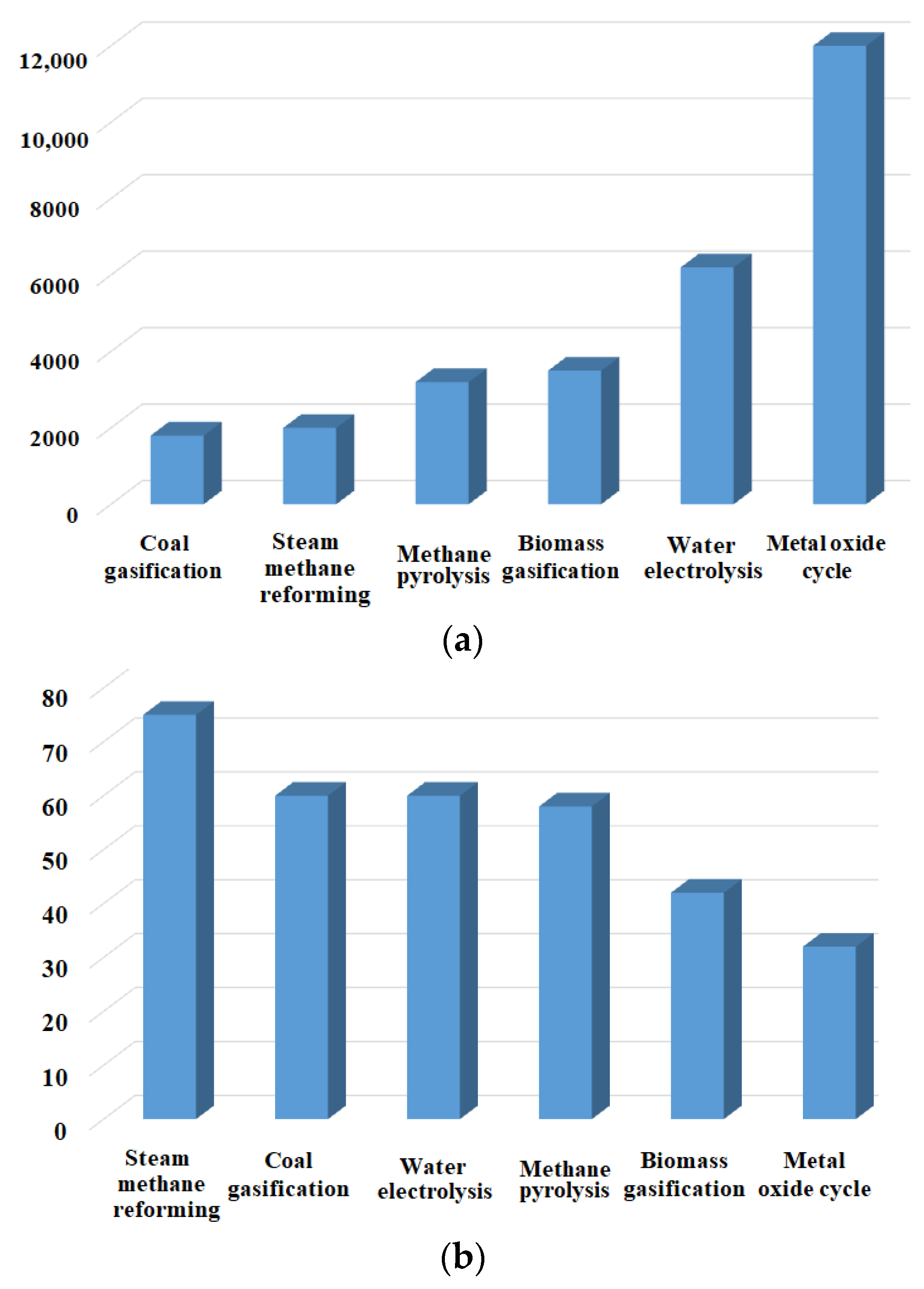
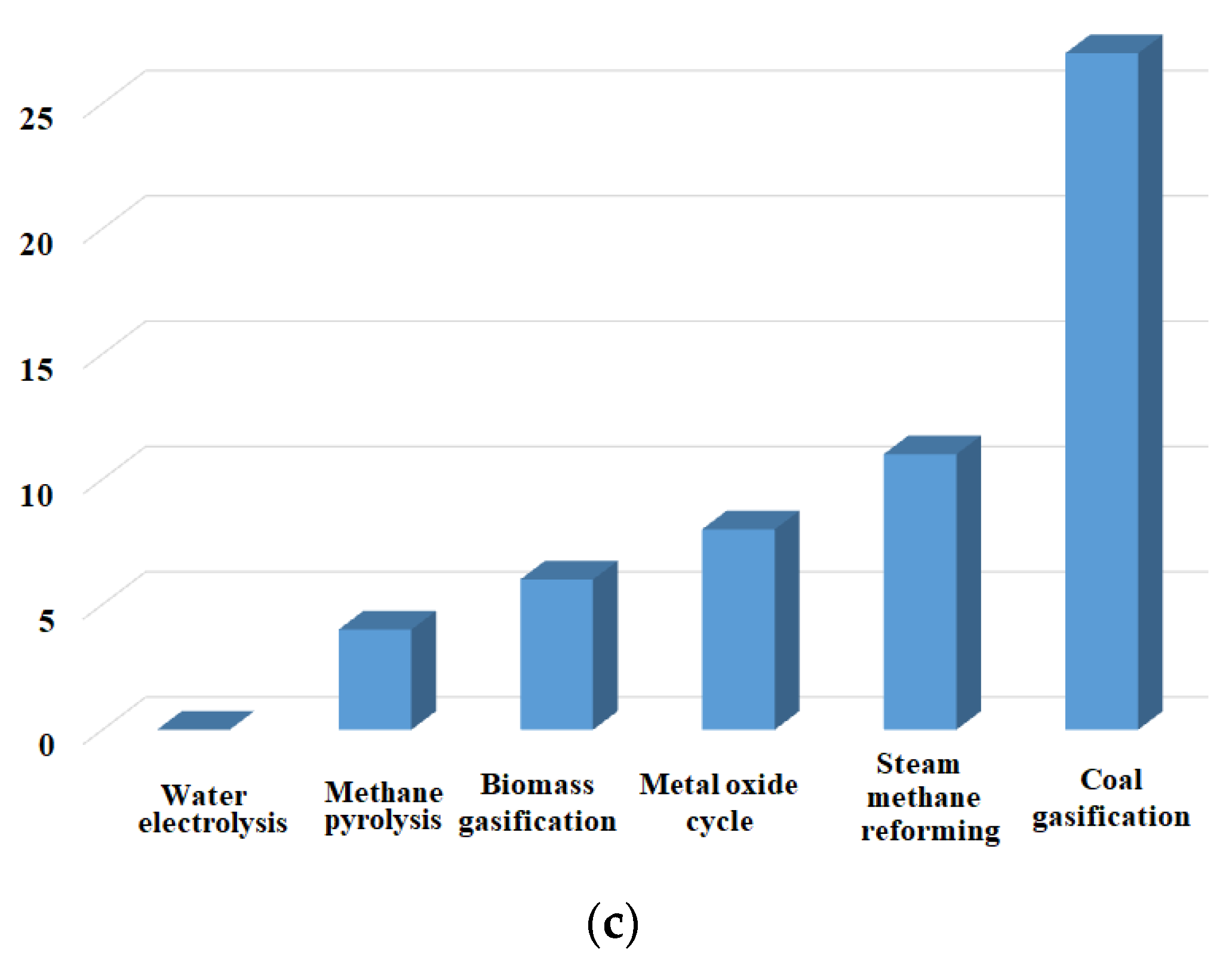


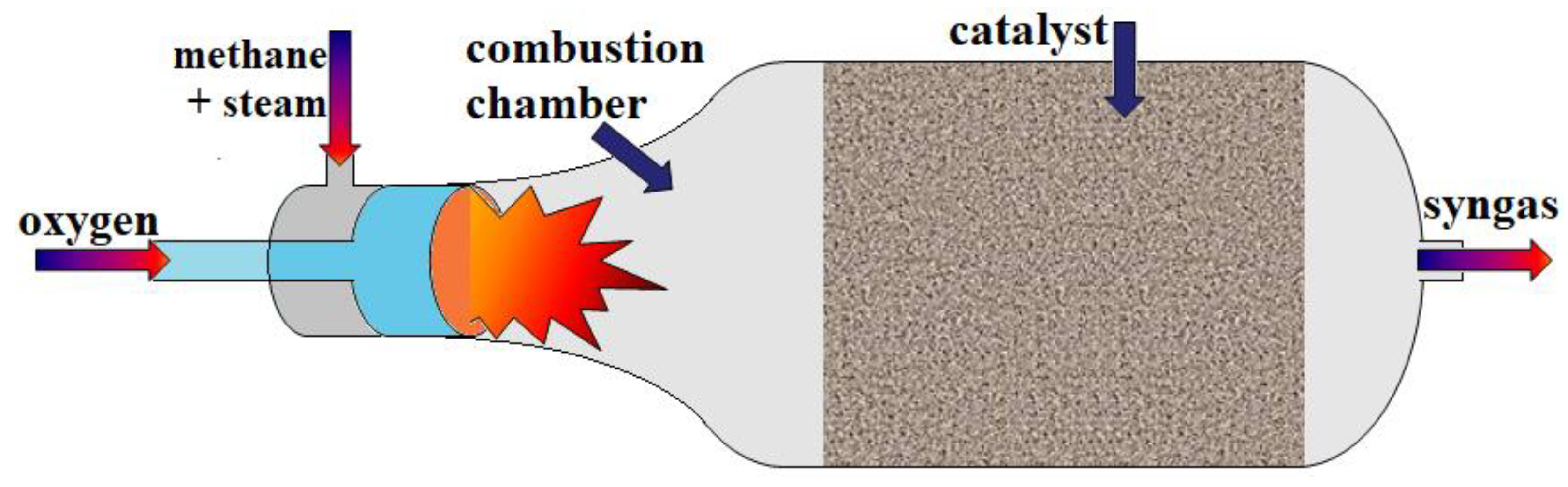
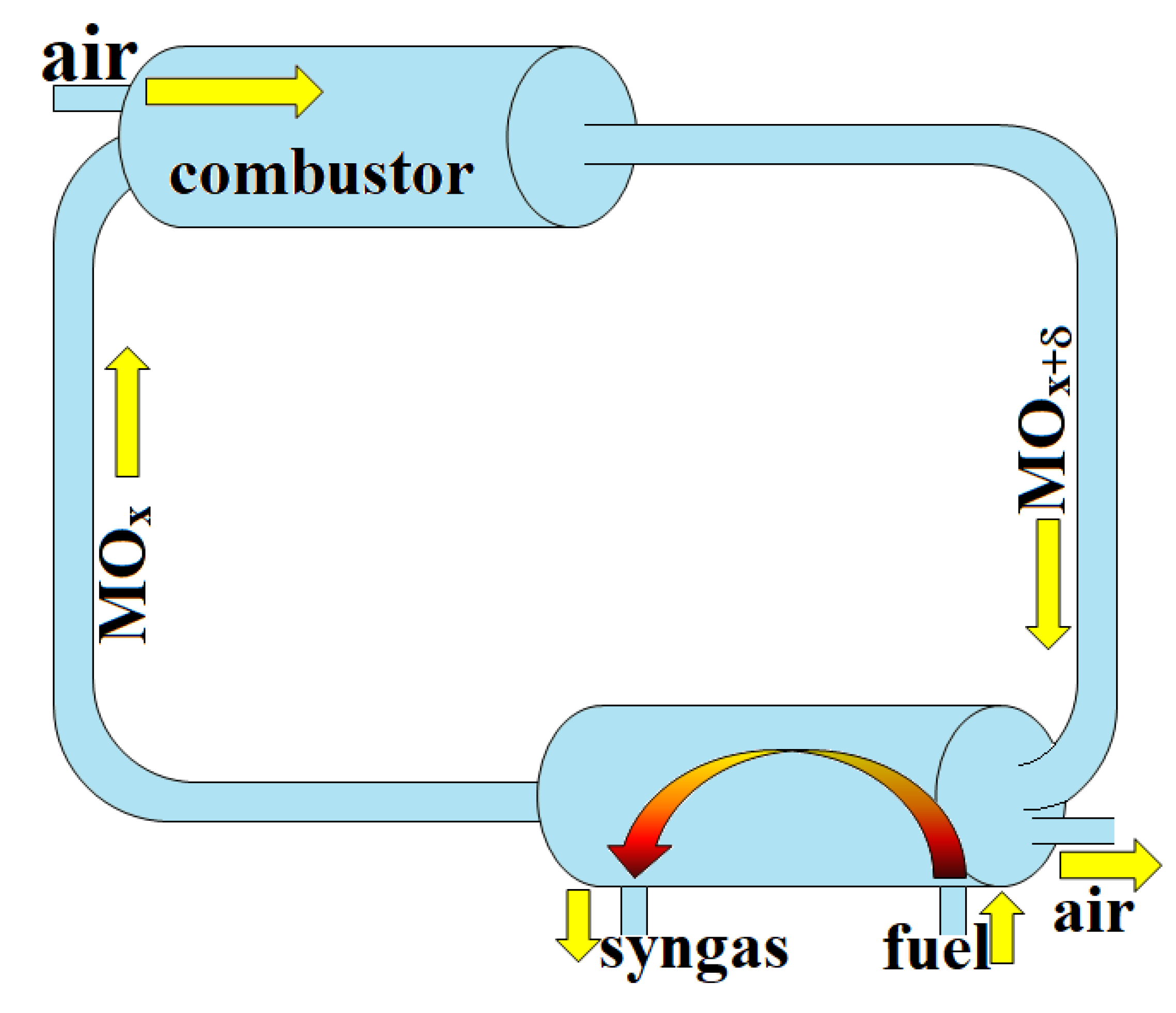
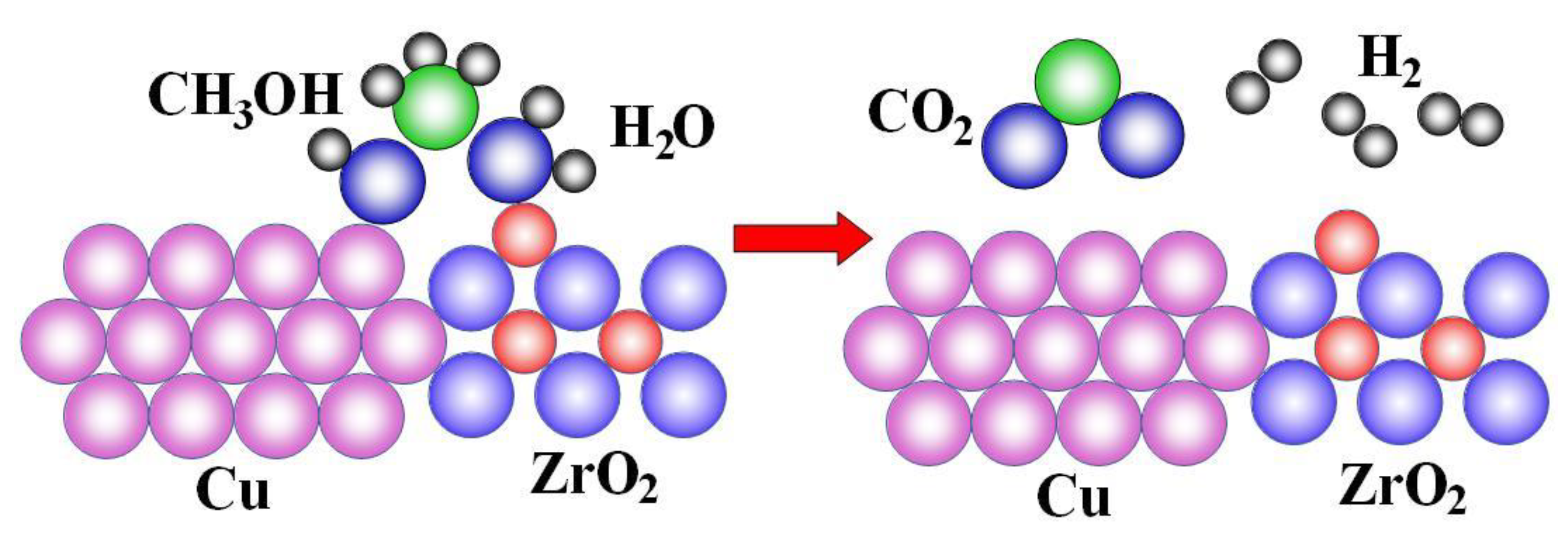
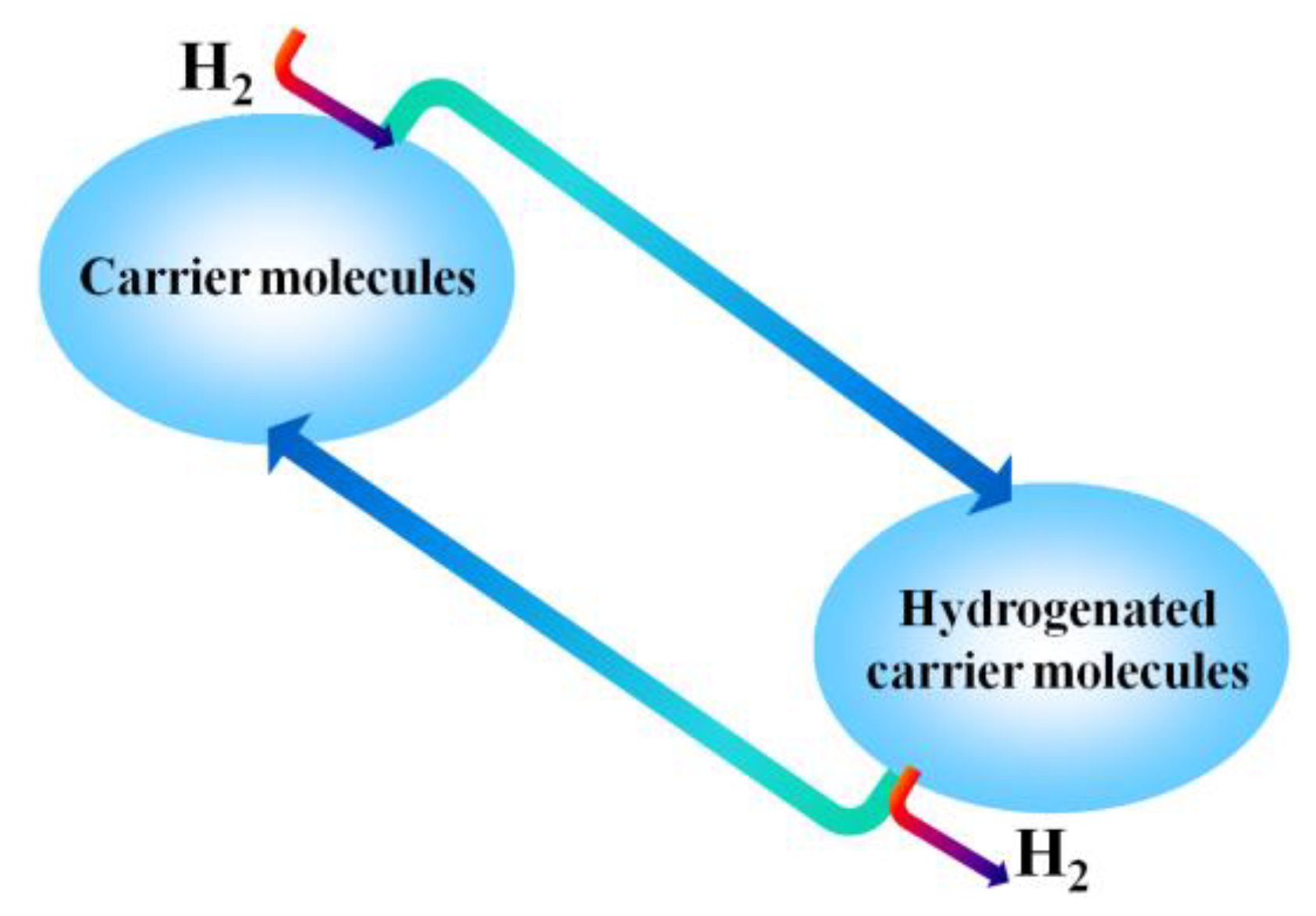
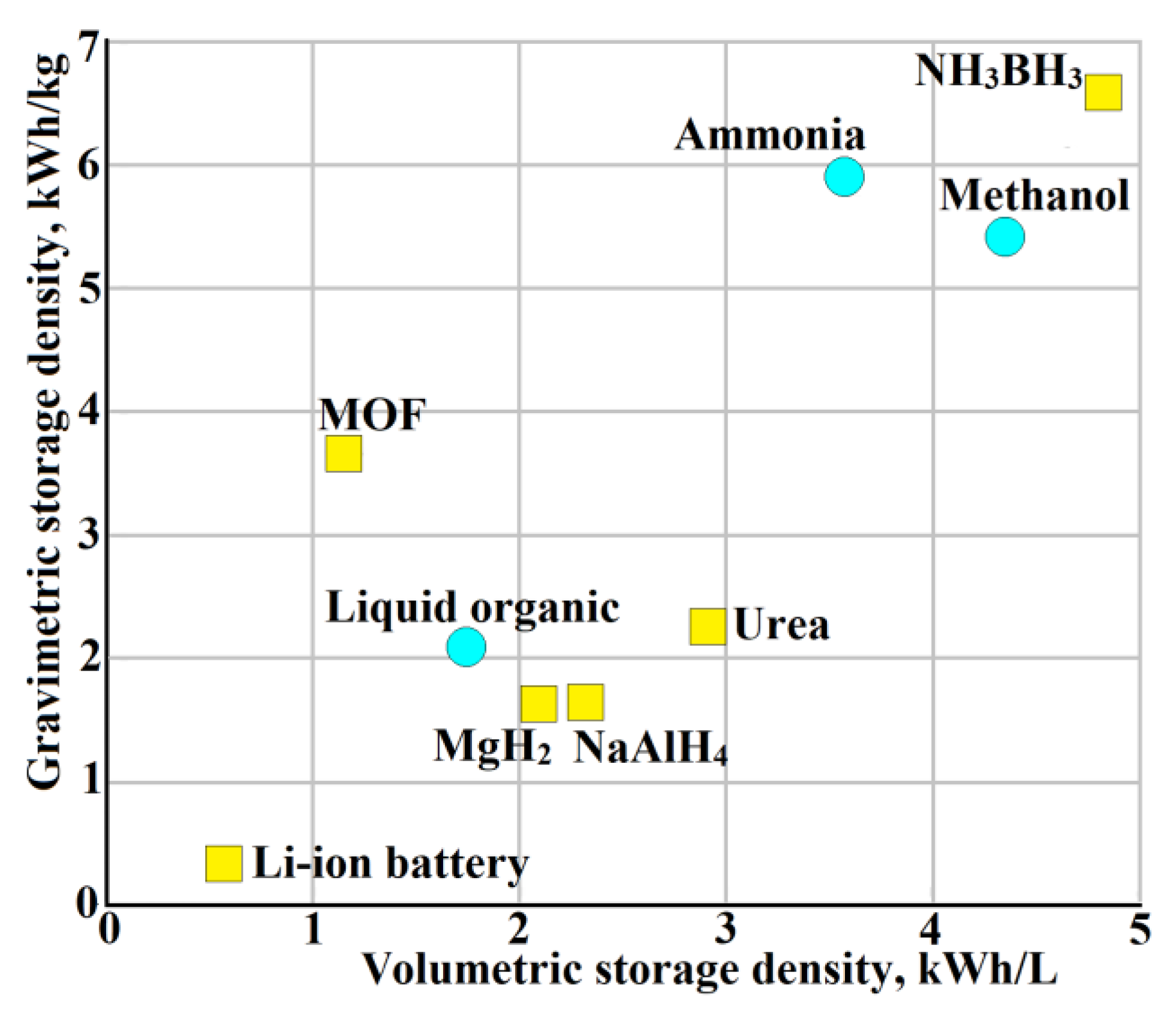


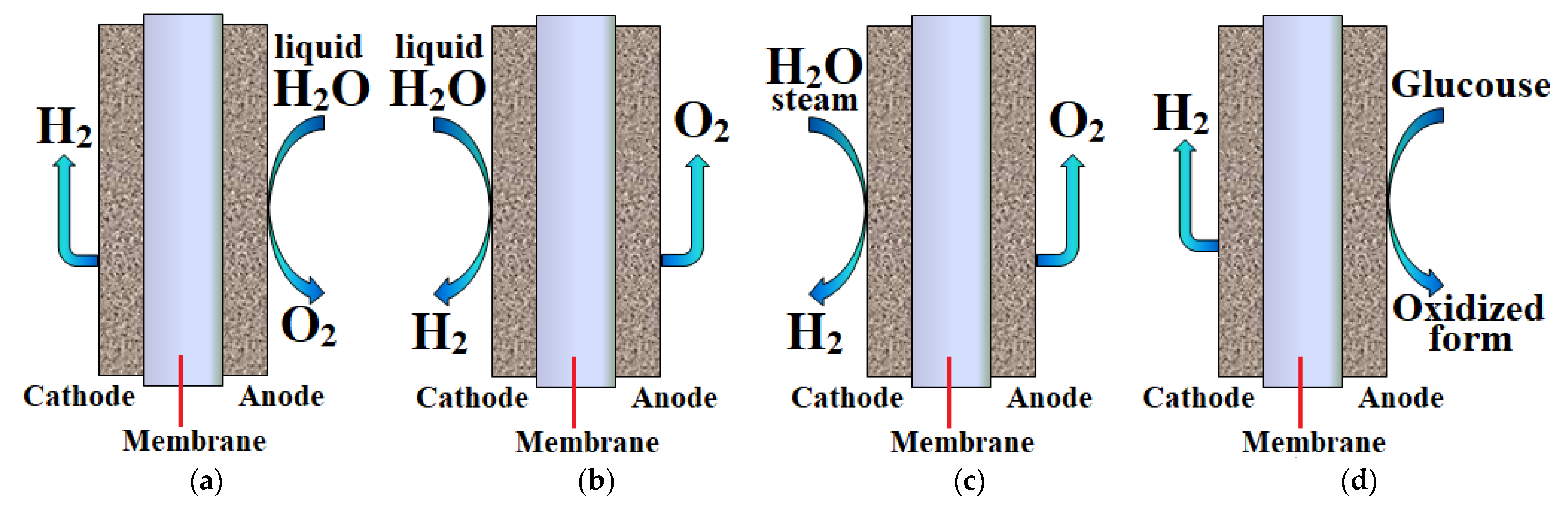
| Catalyst | T (°C) | Conversion (%) | Time on Stream (min) | Reference | ||
|---|---|---|---|---|---|---|
| Metal | Support | CH4 | CO2 | |||
| Ni | La2O3 | 650 | 62 | 67 | 3000 | [121] |
| Ni | SiO2/TiO2 | 650 | 65 | 54 | 1440 | [122] |
| Ni | Activated carbon | 900 | 80 | 98 | 500 | [123] |
| Ni | MgO/Ce0.8Zr0.2O2 | 800 | 95 | 96 | 12,000 | [124] |
| Ni | Al2O3 | 800 | 63 | 82 | 1200 | [125] |
| Ni | CeO2–Al2O3 | 850 | 100 | 100 | 600 | [126] |
| Ni | SBA-15 | 800 | 88 | 89 | 300 | [127] |
| Ni-Co | SBA-15 | 25 | 29 | 19 | 600 | [128] |
| Ni-La | SBA-15 | 750 | 88 | 96 | 660 | [129] |
| Co | Sr/La2O3 | 800 | 94 | 99 | 1800 | [130] |
| Storage Method | Advantages | Disadvantages |
|---|---|---|
| Compressed H2 |
|
|
| Liquid H2 |
|
|
| Cryo-compressed H2 |
|
|
| Solid carrier |
|
|
| Ammonia |
|
|
| Liquid organic hydrogen carrier |
|
|
| Electrolysis Method | Advantages | Disadvantages |
|---|---|---|
| Alkaline electrolysis (Commercialized) |
|
|
| Proton-exchange membrane water electrolysis (Good prospects for commercialization) |
|
|
| High-temperature electrolysis with solid oxide membranes (Laboratory scale) |
|
|
| Process | Advantages | Disadvantages |
|---|---|---|
| Steam reforming | Existing infrastructure, low-cost technology | CO and CO2 emission, high temperatures required, catalyst regeneration required |
| Partial oxidation | Existing infrastructure, low desulfurization requirement | CO and CO2 emission, high temperatures required, formation of heavy oils and coke along with H2, catalyst regeneration required |
| Auto thermal reforming | Existing infrastructure, developed technology | CO and CO2 emission, high-purity O2 required |
| Biomass gasification | Cheap feedstock, recycling of industrial waste, neutral CO2 emission, high biomass conversion efficiency | H2 yield variation due to different biomass compositions, high operating temperatures, seasonal availability |
| Pyrolysis | Low CO2 emission | High energy consumption, large carbon amounts, poor fuel efficiency, hydrogen requires deep purification |
| Electrolysis | CO2 zero emissions, O2 is a byproduct, existing infrastructure, high-purity H2 | Expensive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenina, I.; Yaroslavtsev, A. Modern Technologies of Hydrogen Production. Processes 2023, 11, 56. https://doi.org/10.3390/pr11010056
Stenina I, Yaroslavtsev A. Modern Technologies of Hydrogen Production. Processes. 2023; 11(1):56. https://doi.org/10.3390/pr11010056
Chicago/Turabian StyleStenina, Irina, and Andrey Yaroslavtsev. 2023. "Modern Technologies of Hydrogen Production" Processes 11, no. 1: 56. https://doi.org/10.3390/pr11010056
APA StyleStenina, I., & Yaroslavtsev, A. (2023). Modern Technologies of Hydrogen Production. Processes, 11(1), 56. https://doi.org/10.3390/pr11010056







