Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts
Abstract
1. Introduction
2. Results
2.1. Influence of Extraction Parameters on TPC and TFC Values
2.2. Influence of Extraction Parameters on Antioxidant Capacity
2.3. HPLC-MS Analysis of the Extracts
2.3.1. Analysis of Polyphenolic Compounds
2.3.2. Analysis of Flavonoid Compounds
2.3.3. Analysis of Sterolic Compounds
2.4. Determination of Antimicrobial Activity
2.4.1. Antimicrobial Activity—In Vitro Qualitative Study
2.4.2. Antimicrobial Activity—In Vitro Quantitative Evaluation
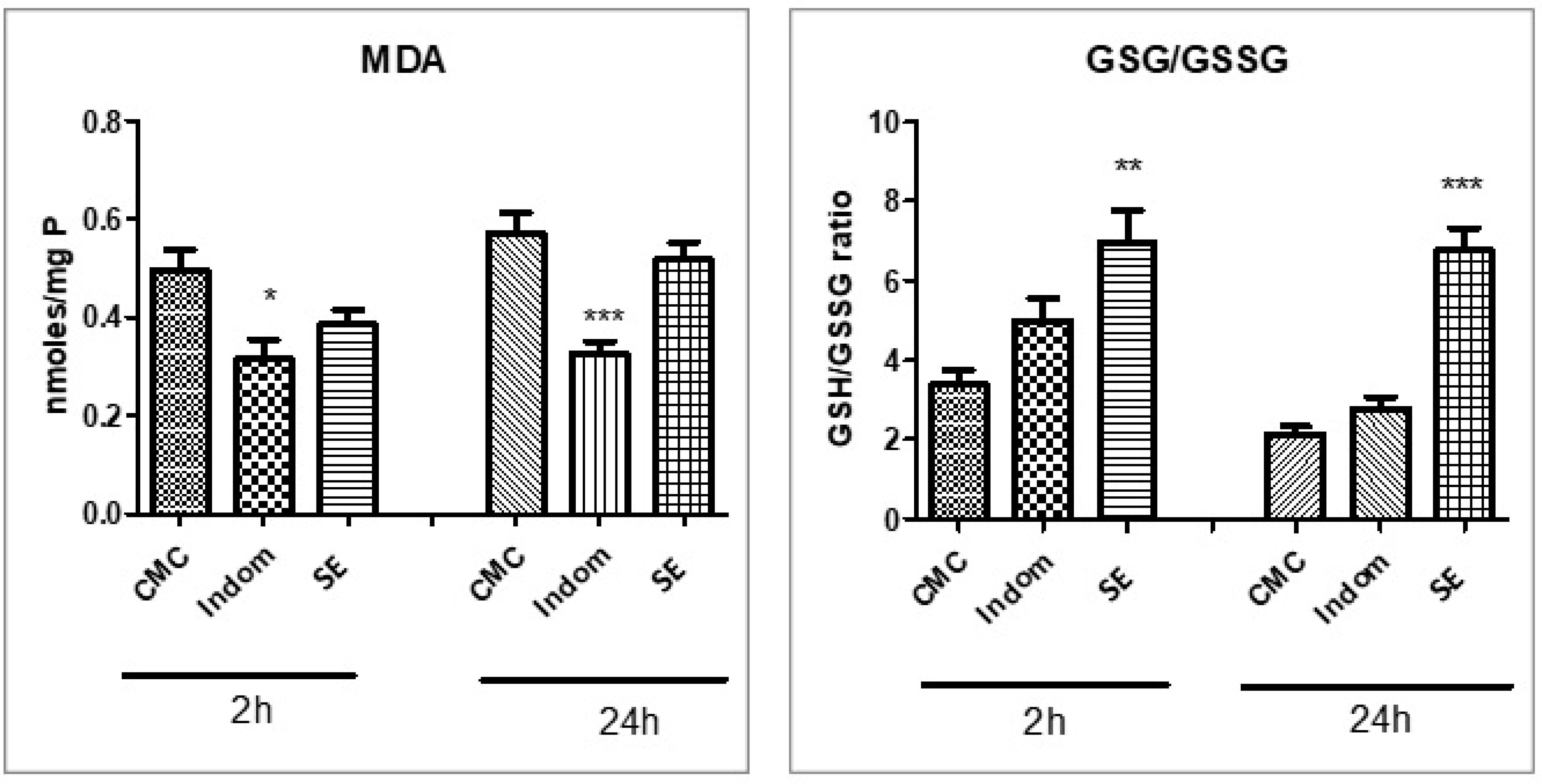
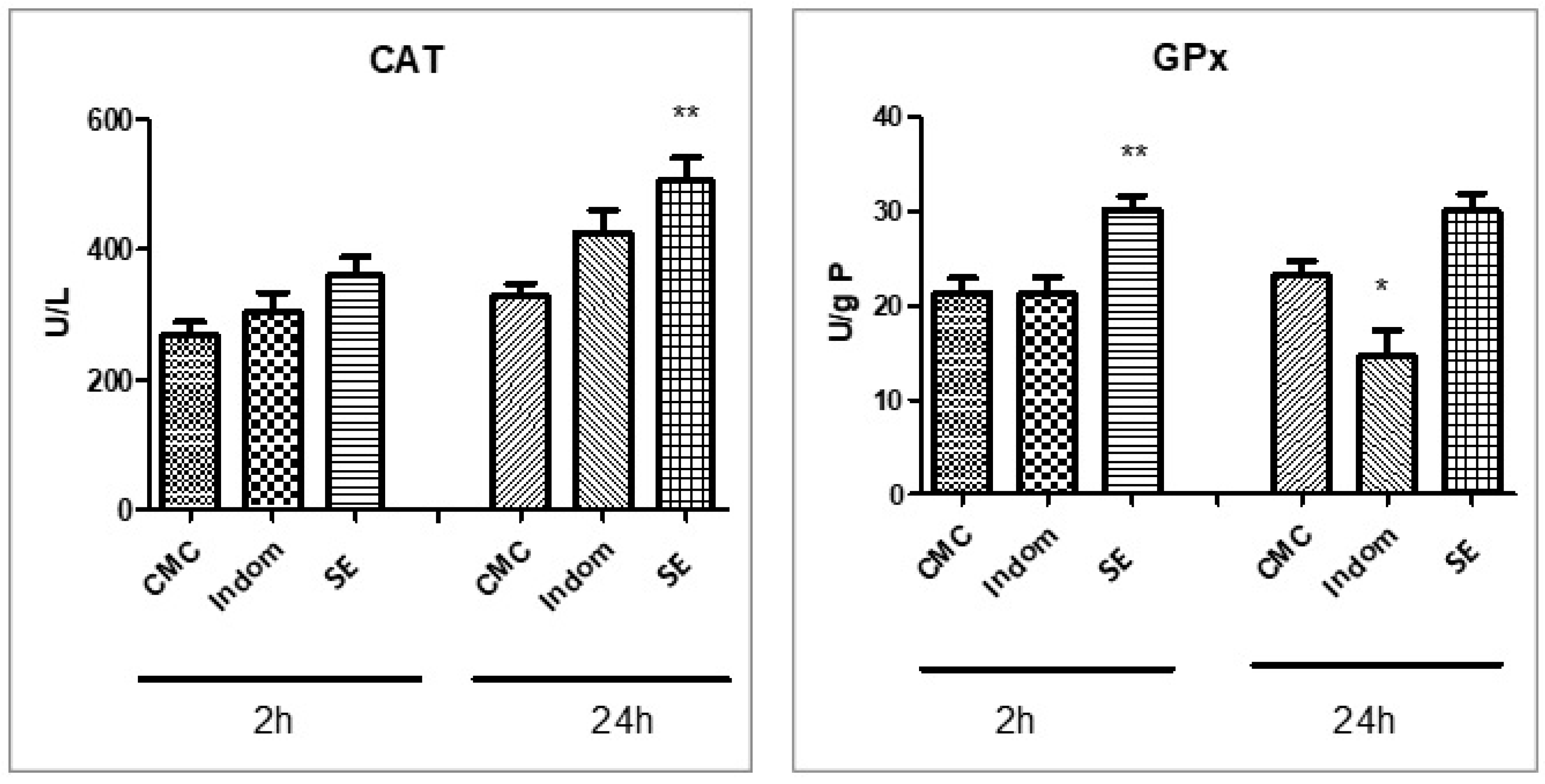
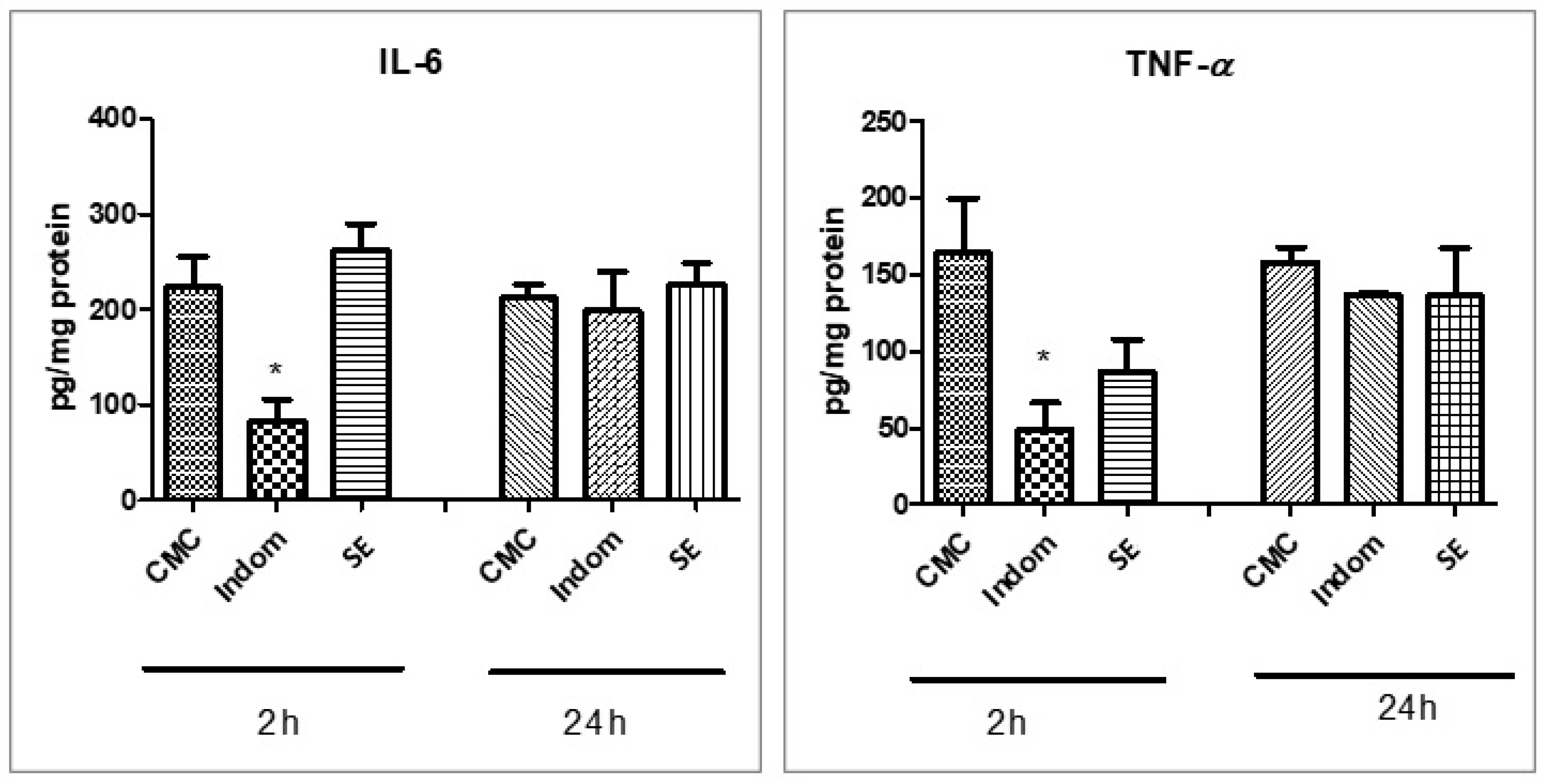
2.5. Assessment of Oxidative Stress and Proinflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Extraction Processes
4.3.1. Maceration
4.3.2. Soxhlet extraction (SE)
4.3.3. Turboextraction (TBE)
4.3.4. Ultrasound-Assisted Extraction (UAE)
4.3.5. Combination of UAE and TBE (UTE)
4.4. Determination of Total Phenolic Content (TPC)
4.5. Determination of Total Flavonoid Content (TFC)
4.6. Antioxidant Activity Analysis
4.6.1. DPPH Radical Scavenging Activity
4.6.2. ABTS+ Scavenging Activity
4.6.3. FRAP Assay
4.7. HPLC-MS Analysis
4.8. Determination of Antimicrobial Activity
4.8.1. Antimicrobial activity—In Vitro Qualitative Study
4.8.2. Antimicrobial Activity—In Vitro Quantitative Evaluation
4.9. Evaluation of Biological Activities
4.9.1. Carrageenan-Induced Inflammation Model in Rats
4.9.2. Oxidative Stress Assessment
4.9.3. Proinflammatory Cytokine Evaluation
4.9.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomasello, S. How many names for a beloved genus?–Coalescent-based species delimitation in Xanthium L. (Ambrosiinae, Asteraceae). Mol. Phylogenet. Evol. 2018, 127, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int. J. Green Pharm. 2010, 4, 129–139. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zheng, X.; Zhao, Y.; Liu, Y.; Zhou, S.; Wei, C.; Hu, Y.; Shao, H. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef]
- Bosco, A.; Golsteyn, R.M. Emerging anti-mitotic activities and other bioactivities of sesquiterpene compounds upon human cells. Molecules 2017, 22, 459. [Google Scholar] [CrossRef]
- Kumar, P.V.; Kala, S.M.J.; Prakash, K.S. Green synthesis derived Pt-nanoparticles using Xanthium strumarium leaf extract and their biological studies. J. Environ. Chem. Eng. 2019, 7, 103146. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, R.X. Biosynthesis of gold nanoparticles using Caffeoylxanthiazonoside, chemical isolated from Xanthium strumarium L. fruit and their anti-allergic rhinitis effect-a traditional Chinese medicine. J. Photochem. Photobiol. B Biol. 2019, 192, 13–18. [Google Scholar] [CrossRef]
- Kumar, V.; Gundampati, R.K.; Singh, D.K.; Jagannadham, M.V.; Sundar, S.; Hasan, S.H. Photo-induced rapid biosynthesis of silver nanoparticle using aqueous extract of Xanthium strumarium and its antibacterial and antileishmanial activity. J. Ind. Eng. Chem. 2016, 37, 224–236. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. S. Afr. J. Chem. Eng. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Khamparia, S.; Jaspal, D.K. Xanthium strumarium L. seed hull as a zero cost alternative for Rhodamine B dye removal. J. Environ. Manag. 2017, 197, 498–506. [Google Scholar] [CrossRef]
- Danalache, F.; Mata, P.; Alves, V.D.; Moldao-Martins, M. Enzyme-assisted extraction of fruit juices. In Fruit Juices: Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 183–200. [Google Scholar]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Renard, C.M. Extraction of bioactives from fruit and vegetables: State of the art and perspectives. Lwt 2018, 93, 390–395. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wong-paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Ascacio-Valdés, J.A.; Cruz, K.; Reyes-Luna, C.; Rodríguez, R.; Aguilar, C.N. Extraction of Bioactive Phenolic Compounds by Alternative Technologies. In Ingredients Extraction by Physico-Chemical Methods in Food: Handbook of Food Bioengineering, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 229–252. [Google Scholar]
- Romero, M.; Zanuy, M.; Rosell, E.; Cascante, M.; Piulats, J.; Font-Bardia, M.; Balzarini, J.; De Clerq, E.; Pujol, M. Optimization of xanthatin extraction from Xanthium spinosum L. and its cytotoxic, anti-angiogenesis and antiviral properties. Eur. J. Med. Chem. 2015, 90, 491–496. [Google Scholar] [CrossRef]
- Ingawale, A.S.; Sadiq, M.B.; Nguyen, L.T.; Ngan, T.B. Optimization of extraction conditions and assessment of antioxidant, α-glucosidase inhibitory and antimicrobial activities of Xanthium strumarium L. fruits. Biocatal. Agric. Biotechnol. 2018, 14, 40–47. [Google Scholar] [CrossRef]
- Bader, A.; Giner, R.M.; Martini, F.; Schinella, G.R.; Ríos, J.L.; Braca, A.; Prieto, J.M. Modulation of COX, LOX and NFκB activities by Xanthium spinosum L. root extract and ziniolide. Fitoterapia 2013, 91, 284–289. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Zheng, F.; Yu, H.; Wei, K. Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation. Molecules 2022, 27, 4603. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Gupta, M.; Singh, N.; Gulati, M.; Gupta, R.; Sudhakar, K.; Kapoor, B. Herbal bioactives in treatment of inflammation: An overview. S. Afr. J. Bot. 2021, 143, 205–225. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Ghahari, S.; Alinezhad, H.; Nematzadeh, G.A.; Tajbakhsh, M.; Baharfar, R. Biochemical composition, antioxidant and biological activities of the essential oil and fruit extract of Xanthium strumarium Linn. from Northern Iran. J. Agric. Sci. Technol. 2017, 19, 1603–1616. [Google Scholar]
- Scherer, R.; Duarte, M.C.T.; Catharino, R.R.; Nachtigall, F.M.; Eberlin, M.N.; Teixeira Filho, J.; Godoy, H. Xanthium strumarium L. antimicrobial activity and carboxyatractyloside analysis through electrospray ionization mass spectrometry. Rev. Bras. Plantas Med. 2009, 11, 159–163. [Google Scholar] [CrossRef]
- Hossen, M.J.; Cho, J.Y.; Kim, D. PDK1 in NF-κB signaling is a target of Xanthium strumarium methanolic extract-mediated anti-inflammatory activities. J. Ethnopharmacol. 2016, 190, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Han, P.; Yu, L.; Chen, Y.; Ye, B.; Qin, L.; Xin, H.; Han, T. Anti-allergic rhinitis effects of caffeoylquinic acids from the fruits of Xanthium strumarium in rodent animals via alleviating allergic and inflammatory reactions. Rev. Bras. Farm. 2019, 29, 46–53. [Google Scholar] [CrossRef]
- Huang, M.-H.; Wang, B.-S.; Chiu, C.-S.; Amagaya, S.; Hsieh, W.-T.; Huang, S.-S.; Shie, P.-H.; Huang, G.-J. Antioxidant, antinociceptive, and anti-inflammatory activities of Xanthii fructus extract. J. Ethnopharmacol. 2011, 135, 545–552. [Google Scholar] [CrossRef]
- Amin, S.; Barkatullah Khan, H. Pharmacology of Xanthium species. A review. J. Phytopharm. 2016, 5, 126–127. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 1–17. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Benedec, D.; Hanganu, D.; Filip, L.; Oniga, I.; Tiperciuc, B.; Olah, N.K.; Gheldiu, A.-M.; Raita, O.; Vlase, L. Chemical, antioxidant and antibacterial studies of Romanian Heracleum sphondylium. Farmacia 2017, 65, 252–256. [Google Scholar]
- Toiu, A.; Vlase, L.; Gheldiu, A.M.; Vodnar, D.; Oniga, I. Evaluation of the antioxidant and antibacterial potential of bioactive compounds from Ajuga reptans extracts. Farmacia 2017, 65, 351–355. [Google Scholar]
- Toiu, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.M.; Oniga, I. Solidago graminifolia L. Salisb. (Asteraceae) as a valuable source of bioactive polyphenols: HPLC profile, in vitro antioxidant and antimicrobial potential. Molecules 2019, 24, 2666. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizesan, I.; Pop, A.; Mocan, A.; Gheldiu, A.M.; Babota, M.; Vodnar, D.C.; Jurj, A.; Berindan-Neagoe, I.; Vlase, L.; et al. Walnut (Juglans regia L.) septum: Assessment of bioactive molecules and in vitro biological effects. Molecules 2020, 25, 2187. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced recovery of phenolics and tocopherolic compounds from walnut (Juglans regia L.) male flowers based on process optimization of ultrasonic assisted-extraction: Phytochemical profile and biological activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.M.; Mocan, A.; Cirșan, G.; Vlase, L.; Loghin, F.; Popa, D.-S.; Tomuta, I. Enhanced recovery of antioxidant compounds from hazelnut (Corylus avellana L.) involucre based on extraction optimization: Phytochemical profile and biological activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.M.; Moldovan, C.; Oniga, I. Comparative phytochemical profile, antioxidant, antimicrobial and in vivo anti-inflammatory activity of different extracts of traditionally used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Patil, C.R.; Gadekar, A.R.; Patel, P.N.; Rambhade, A.; Surana, S.J.; Gaushal, M.H. Dual effect of Toxicodendron pubescens on Carrageenan induced paw edema in rats. Homeopathy 2009, 98, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Morand, P.; Levillain, P.; Lemonnier, A. Improved fluorometric determination of malonaldehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [PubMed]
| Extraction Method | Studied Extraction Parameters | Sample Name | |||
|---|---|---|---|---|---|
| Maceration | * | M | |||
| Soxhlet extraction (SE) | Time (min) | 20 | S20 | ||
| 40 | S40 | ||||
| 60 | S60 | ||||
| Turboextraction (TBE) | 10 min (2 cycles of 5 min) | Rotation speed (rpm) | 4000 6000 | T24 | |
| T26 | |||||
| 8000 | T28 | ||||
| 20 min (4 cycles of 5 min) | 4000 | T44 | |||
| 6000 | T46 | ||||
| 8000 | T48 | ||||
| Ultrasound-assisted extraction (UAE) | 10 | Temperature (°C) | 30 | U13 | |
| 40 | U14 | ||||
| 50 | U15 | ||||
| 20 | 30 | U23 | |||
| 40 | U24 | ||||
| 50 | U25 | ||||
| 30 | 30 | U33 | |||
| 40 | U34 | ||||
| 50 | U35 | ||||
| Combination of UAE and TBE (UTE) | ** | UT | |||
| Sample | TPC (GAE mg/mL) * | TFC (QE mM) * |
|---|---|---|
| M | 0.296 ± 0.016 | 0.526 ± 0.033 |
| S20 | 0.533 ± 0.003 | 0.406 ± 0.014 |
| S40 | 0.440 ± 0.010 | 0.304 ± 0.022 |
| S60 | 0.564 ± 0.003 | 0.597 ± 0.004 |
| T24 | 0.316 ± 0.022 | 0.195 ± 0.006 |
| T26 | 0.344 ± 0.006 | 0.214 ± 0.000 |
| T28 | 0.280 ± 0.013 | 0.077 ± 0.025 |
| T44 | 0.306 ± 0.009 | 0.136 ± 0.004 |
| T46 | 0.306 ± 0.004 | 0.227 ± 0.014 |
| T48 | 0.153 ± 0.002 | 0.070 ± 0.055 |
| U13 | 0.067 ± 0.002 | 0.298 ± 0.012 |
| U14 | 0.104 ± 0.004 | 0.205 ± 0.009 |
| U15 | 0.191 ± 0.002 | 0.176 ± 0.004 |
| U23 | 0.147 ± 0.007 | 0.300 ± 0.010 |
| U24 | 0.372 ± 0.006 | 0.455 ± 0.028 |
| U25 | 0.254 ± 0.004 | 0.488 ± 0.007 |
| U33 | 0.141 ± 0.009 | 0.332 ± 0.010 |
| U34 | 0.279 ± 0.010 | 0.697 ± 0.013 |
| U35 | 0.331 ± 0.011 | 0.217 ± 0.010 |
| UT | 0.248 ± 0.012 | 0.478 ± 0.035 |
| Sample | DPPH (TE mg/mL) * | FRAP (TE mM) * | ABTS+ (TE mM) * |
|---|---|---|---|
| M | 0.923 ± 0.284 | 8.741 ± 0.119 | 1.732 ± 0.191 |
| S20 | 1.317 ± 0.184 | 3.540 ± 0.118 | 2.641 ± 0.191 |
| S40 | 1.379 ± 0.128 | 3.213 ± 0.052 | 1.985 ± 0.262 |
| S60 | 1.518 ± 0.066 | 4.611 ± 0.005 | 2.843 ± 0.431 |
| T24 | 1.058 ± 0.411 | 3.428 ± 0.027 | 1.152 ± 0.473 |
| T26 | 1.035 ± 0.144 | 3.760 ± 0.152 | 1.581 ± 0.191 |
| T28 | 0.939 ± 0.052 | 3.966 ± 0.082 | 1.354 ± 0.493 |
| T44 | 1.653 ± 0.032 | 4.488 ± 0.171 | 1.581 ± 0.306 |
| T46 | 1.013 ± 0.135 | 5.484 ± 0.095 | 1.581 ± 0.374 |
| T48 | 0.536 ± 0.204 | 5.974 ± 0.239 | 0.823 ± 0.287 |
| U13 | 0.729 ± 0.029 | 3.871 ± 0.000 | 1.354 ± 0.191 |
| U14 | 0.643 ± 0.008 | 4.424 ± 0.027 | 6.732 ± 0.342 |
| U15 | 1.310 ± 0.031 | 4.867 ± 0.000 | 1.833 ± 0.152 |
| U23 | 0.698 ± 0.017 | 3.008 ± 0.109 | 1.960 ± 0.087 |
| U24 | 1.423 ± 0.008 | 4.915 ± 0.126 | 2.465 ± 0.158 |
| U25 | 1.124 ± 0.022 | 7.587 ± 0.137 | 4.207 ± 0.558 |
| U33 | 0.709 ± 0.029 | 3.885 ± 0.071 | 2.111 ± 0.116 |
| U34 | 1.371 ± 0.025 | 4.820 ± 0.082 | 2.717 ± 0.087 |
| U35 | 0.925 ± 0.037 | 4.361 ± 0.072 | 2.742 ± 0.000 |
| UT | 0.964 ± 0.008 | 4.361 ± 0.119 | 2.439 ± 0.076 |
| Sample | Protocatechuic Acid (µg/mL Extract) * | Vanillic Acid (µg/mL Extract) * | Chlorogenic Acid (µg/mL Extract) * | p-Coumaric Acid (µg/mL Extract) * | Caftaric Acid (µg/mL Extract) * |
|---|---|---|---|---|---|
| M | 0.31 ± 0.037 | 0.25 ± 0.752 | 13.54 ± 0.542 | <LOQ | <LOQ |
| S60 | 0.29 ± 0.023 | 0.31 ± 0.028 | 28.49 ± 1.424 | 1.58 ± 0.111 | <LOQ |
| T26 | 0.08 ± 0.007 | 0.20 ± 0.016 | 16.33 ± 0.490 | <LOQ | <LOQ |
| T44 | 0.11 ± 0.014 | 0.14 ± 0.020 | 16.56 ± 0.993 | <LOQ | <LOQ |
| T46 | 0.11 ± 0.011 | 0.18 ± 0.021 | 14.14 ± 1.131 | <LOQ | <LOQ |
| T48 | 0.04 ± 0.005 | 0.10 ± 0.004 | 9.01 ± 0.631 | <LOQ | <LOQ |
| U14 | 0.08 ± 0.010 | 0.18 ± 0.021 | 23.50 ± 3.525 | <LOQ | <LOQ |
| U24 | 0.10 ± 0.014 | 0.19 ± 0.027 | 28.33 ± 0.850 | <LOQ | 1.82 ± 0.055 |
| U25 | 0.07 ± 0.004 | 0.30 ± 0.039 | 30.68 ± 4.601 | <LOQ | <LOQ |
| U34 | 0.08 ± 0.009 | 0.18 ± 0.013 | 37.47 ± 2.623 | <LOQ | <LOQ |
| UT | 0.06 ± 0.009 | 0.21 ± 0.030 | 31.81 ± 3.817 | <LOQ | <LOQ |
| Sample | Kaempferol (µg/mL Extract) * | Isoquercitrin (µg/mL Extract) * | Quercitrin (µg/mL Extract) * | Rutin (µg/mL Extract) * | Hyperoside (µg/mL Extract) * |
|---|---|---|---|---|---|
| M | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| S60 | 0.35 ± 0.011 | 2.05 ± 0.061 | 17.19 ± 1.547 | <LOQ | <LOQ |
| T26 | <LOQ | 1.58 ± 0.047 | 7.47 ± 0.672 | <LOQ | <LOQ |
| T44 | <LOQ | 1.28 ± 0.128 | 7.66 ± 1.072 | <LOQ | <LOQ |
| T46 | <LOQ | 0.97 ± 0.087 | 7.66 ± 0.230 | <LOQ | <LOQ |
| T48 | <LOQ | 0.66 ± 0.066 | 4.85 ± 0.146 | <LOQ | <LOQ |
| U14 | 0.28 ± 0.014 | 2.35 ± 0.330 | 10.09 ± 1.513 | <LOQ | <LOQ |
| U24 | 0.28 ± 0.020 | 3.59 ± 0.323 | 12.33 ± 0.740 | 15.55 ± 1.711 | <LOQ |
| U25 | 0.42 ± 0.012 | 3.28 ± 0.492 | 15.14 ± 0.454 | <LOQ | <LOQ |
| U34 | 0.55 ± 0.082 | 4.05 ± 0.445 | 20.56 ± 0.617 | <LOQ | <LOQ |
| UT | 0.42 ± 0.058 | 3.28 ± 0.361 | 15.14 ± 0.605 | <LOQ | 1.86 ± 0.167 |
| Sample | Stigmasterol (ng/mL Extract) * | β-Sitosterol (ng/mL Extract) * | Campesterol (ng/mL Extract) * |
|---|---|---|---|
| M | 6116.07 ± 733.929 | 38,089.10 ± 4570.692 | 616.59 ± 18.498 |
| S60 | 5467.96 ± 820.193 | 32,740.34 ± 3928.840 | 663.46 ± 39.808 |
| T26 | 2926.73 ± 263.406 | 10,905.65 ± 327.170 | 425.56 ± 29.790 |
| T44 | 4544.47 ± 181.779 | 20,648.84 ± 1651.907 | 438.09 ± 52.571 |
| T46 | 3525.19 ± 423.023 | 18,160.25 ± 2179.230 | 291.00 ± 37.830 |
| T48 | 567.38 ± 62.412 | 1947.76 ± 194.776 | <LOQ |
| U14 | 5248.96 ± 157.469 | 25,350.01 ± 2535.001 | 440.67 ± 61.693 |
| U24 | 5874.92 ± 469.994 | 27,462.53 ± 3844.754 | 475.70 ± 57.084 |
| U25 | 7920.66 ± 792.066 | 38,442.64 ± 3075.411 | 521.30 ± 36.491 |
| U34 | 8296.93 ± 580.785 | 42,135.04 ± 5477.555 | 533.12 ± 69.305 |
| UT | 6816.47 ± 681.647 | 35,124.95 ± 2458.747 | 454.43 ± 27.266 |
| U34 | S60 | Amoxicillin | Ketoconazole | |
|---|---|---|---|---|
| Staphylococcus aureus ATCC 6538P | 6.7 | 8.26 | 24.38 | - |
| Enterococcus faecalis ATCC 29212 | 6.67 | 7.79 | 16.8 | - |
| Listeria monocytogenes ATCC 13932 | 6.36 | 6.39 | 18.96 | - |
| Bacillus cereus ATCC 11778 | 7.82 | 8.94 | 8.83 | - |
| E. coli ATCC 10536 | 14.43 | 13.04 | 13.72 | - |
| Salmonella enteritidis ATCC 13076 | 13.47 | 11.29 | 18.43 | - |
| Pseudomonas aeruginosa ATCC 27853 | 12.53 | 11.77 | R | - |
| Candida albicans 10231 | 9.54 | 10.21 | - | 23.74 |
| U34 | S60 | Amoxicilin | Ketoconazole | |||||
|---|---|---|---|---|---|---|---|---|
| MIC 100 | MIC 50 | MIC 100 | MIC 50 | MIC 100 | MIC 50 | MIC 100 | MIC 50 | |
| Staphylococcus aureus ATCC 6538P | 1/16 | 1/32 | 1/32 | 1/32 | 4 * | 2 * | - | |
| Enterococcus faecalis ATCC 29212 | 1/16 | 1/32 | 1/16 | 1/16 | 8 * | 4 * | - | |
| Listeria monocytogenes ATCC 13932 | 1/16 | 1/32 | 1/32 | 1/32 | 1 * | 0.5 * | - | |
| Bacillus cereus ATCC 11778 | 1/16 | 1/32 | 1/32 | 1/64 | 16 * | 8 * | - | |
| E. coli ATCC 10536 | 1/16 | 1/32 | 1/16 | 1/32 | 8 * | 4 * | - | |
| Salmonella enteritidis ATCC 13076 | 1/8 | 1/16 | 1/8 | 1/16 | 4 * | 4 * | - | |
| Pseudomonas aeruginosa ATCC 27853 | 1/16 | 1/32 | 1/32 | 1/32 | R * | R * | - | |
| Candida albicans 10231 | 1/32 | 1/32 | 1/32 | 1/64 | - | 8 * | 4 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Filip, G.A.; Vlase, L.; Crișan, G. Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts. Plants 2023, 12, 96. https://doi.org/10.3390/plants12010096
Gligor O, Clichici S, Moldovan R, Muntean D, Vlase A-M, Nadăș GC, Filip GA, Vlase L, Crișan G. Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts. Plants. 2023; 12(1):96. https://doi.org/10.3390/plants12010096
Chicago/Turabian StyleGligor, Octavia, Simona Clichici, Remus Moldovan, Dana Muntean, Ana-Maria Vlase, George Cosmin Nadăș, Gabriela Adriana Filip, Laurian Vlase, and Gianina Crișan. 2023. "Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts" Plants 12, no. 1: 96. https://doi.org/10.3390/plants12010096
APA StyleGligor, O., Clichici, S., Moldovan, R., Muntean, D., Vlase, A.-M., Nadăș, G. C., Filip, G. A., Vlase, L., & Crișan, G. (2023). Influences of Different Extraction Techniques and Their Respective Parameters on the Phytochemical Profile and Biological Activities of Xanthium spinosum L. Extracts. Plants, 12(1), 96. https://doi.org/10.3390/plants12010096














