Deep-Level Emission Tailoring in ZnO Nanostructures Grown via Hydrothermal Synthesis
Abstract
:1. Introduction
2. Methods
2.1. Nanostructures’ Growth
2.2. Raman Spectroscopy
2.3. Grazing Incidence X-ray Diffraction (GIXRD) Analysis
2.4. Photoluminescence Spectroscopy
3. Results and Discussion
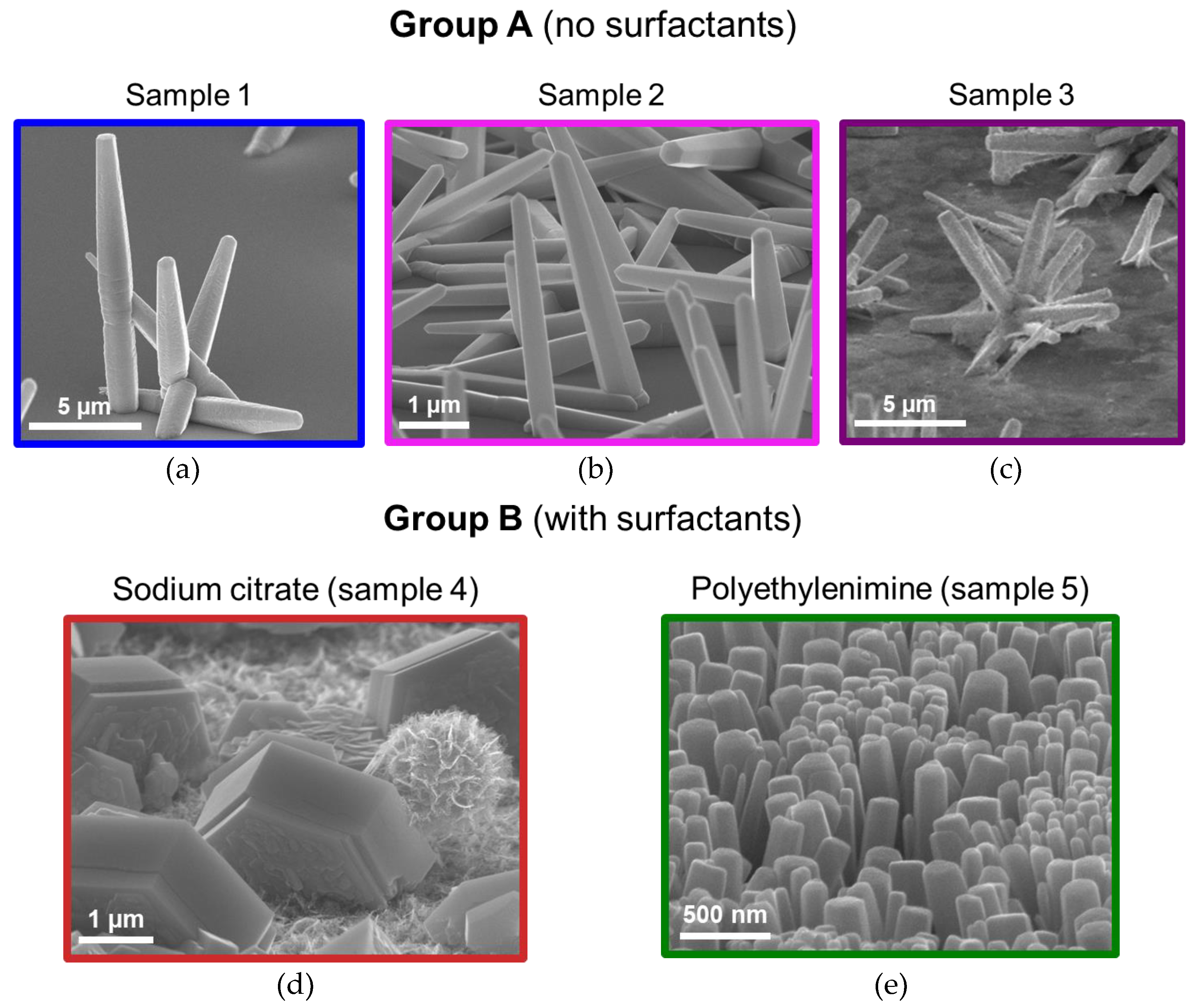
3.1. Nanostructures’ Morphology
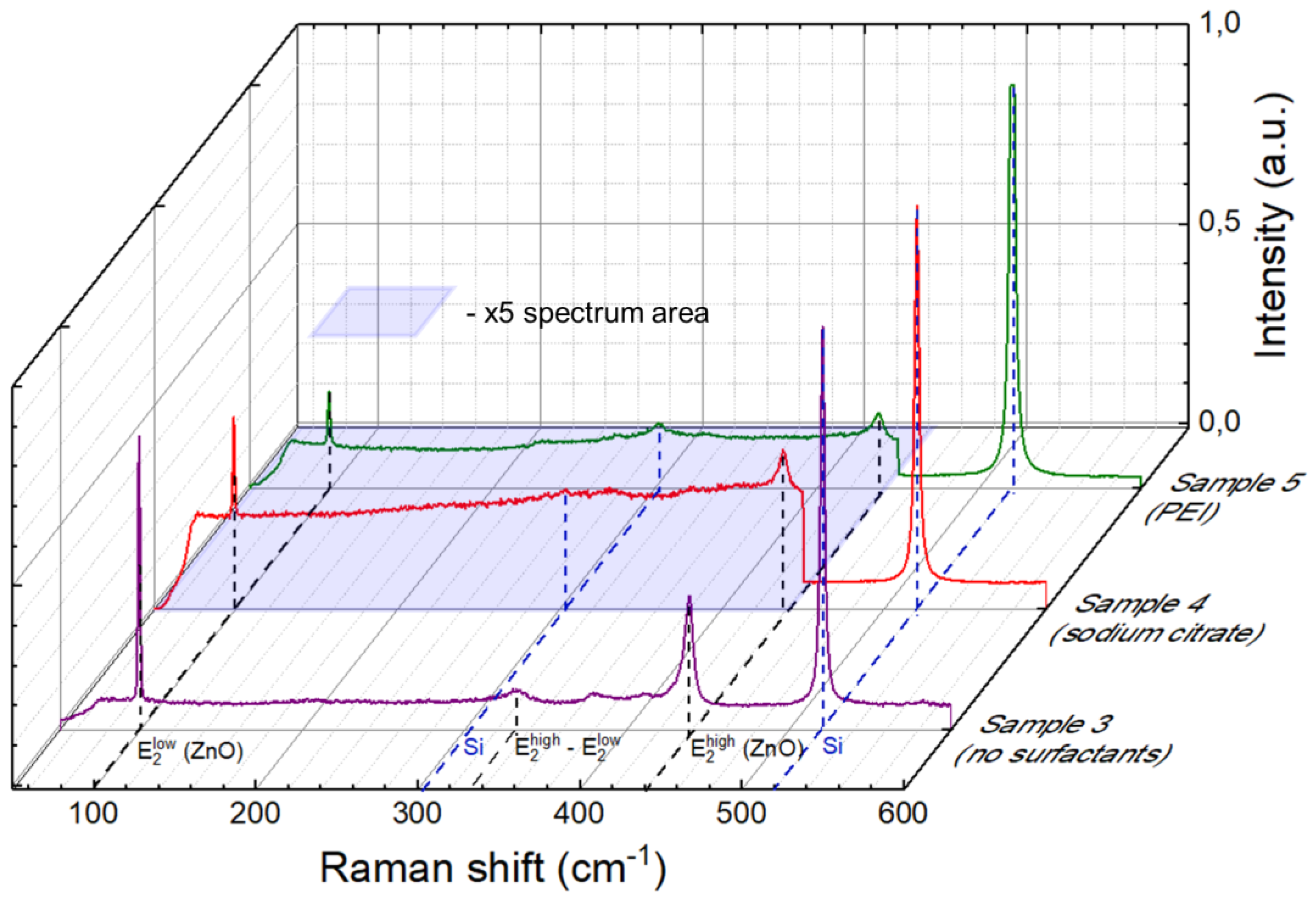
3.2. Raman Spectroscopy
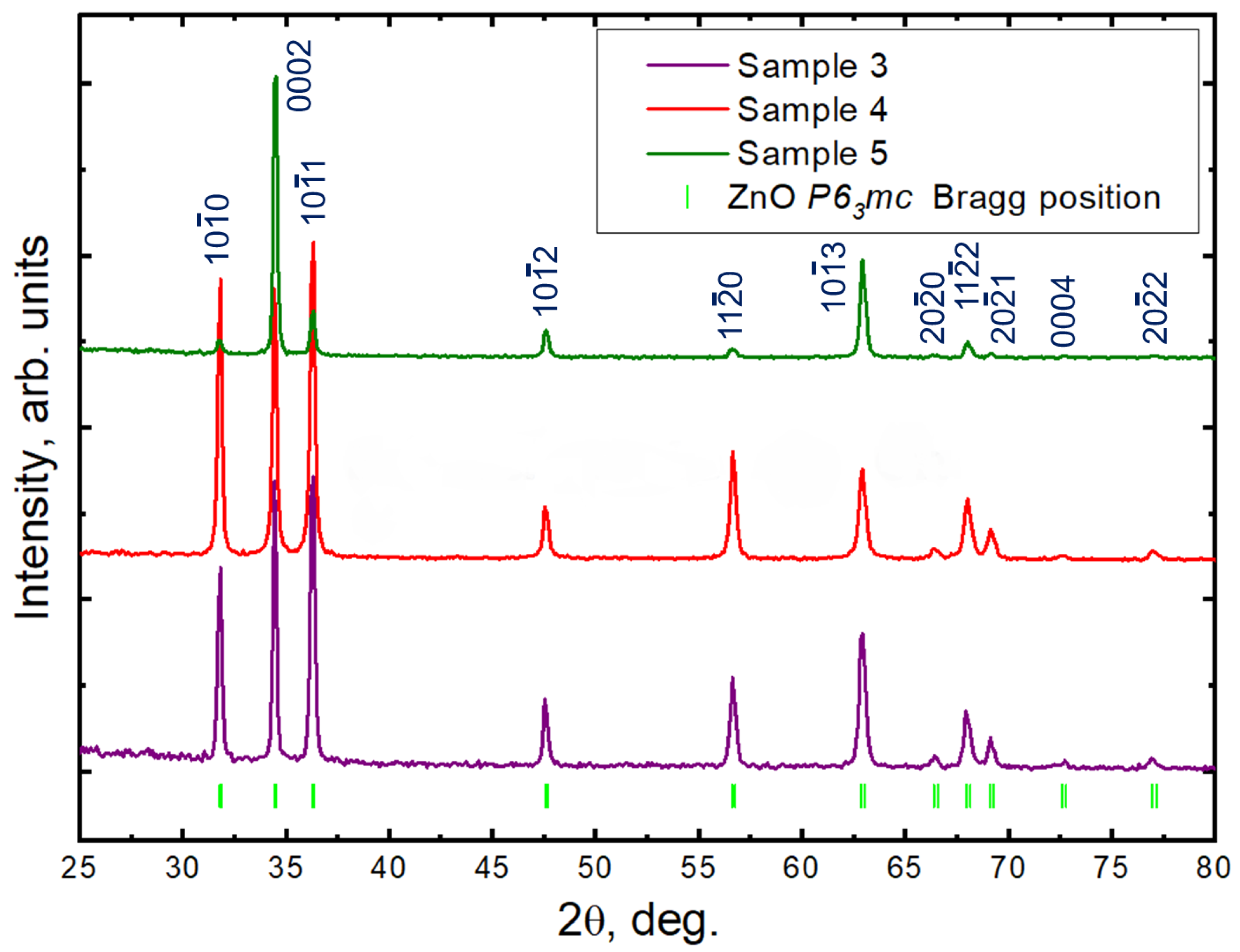
3.3. Grazing Incidence X-ray Diffraction (GIXRD) Analysis
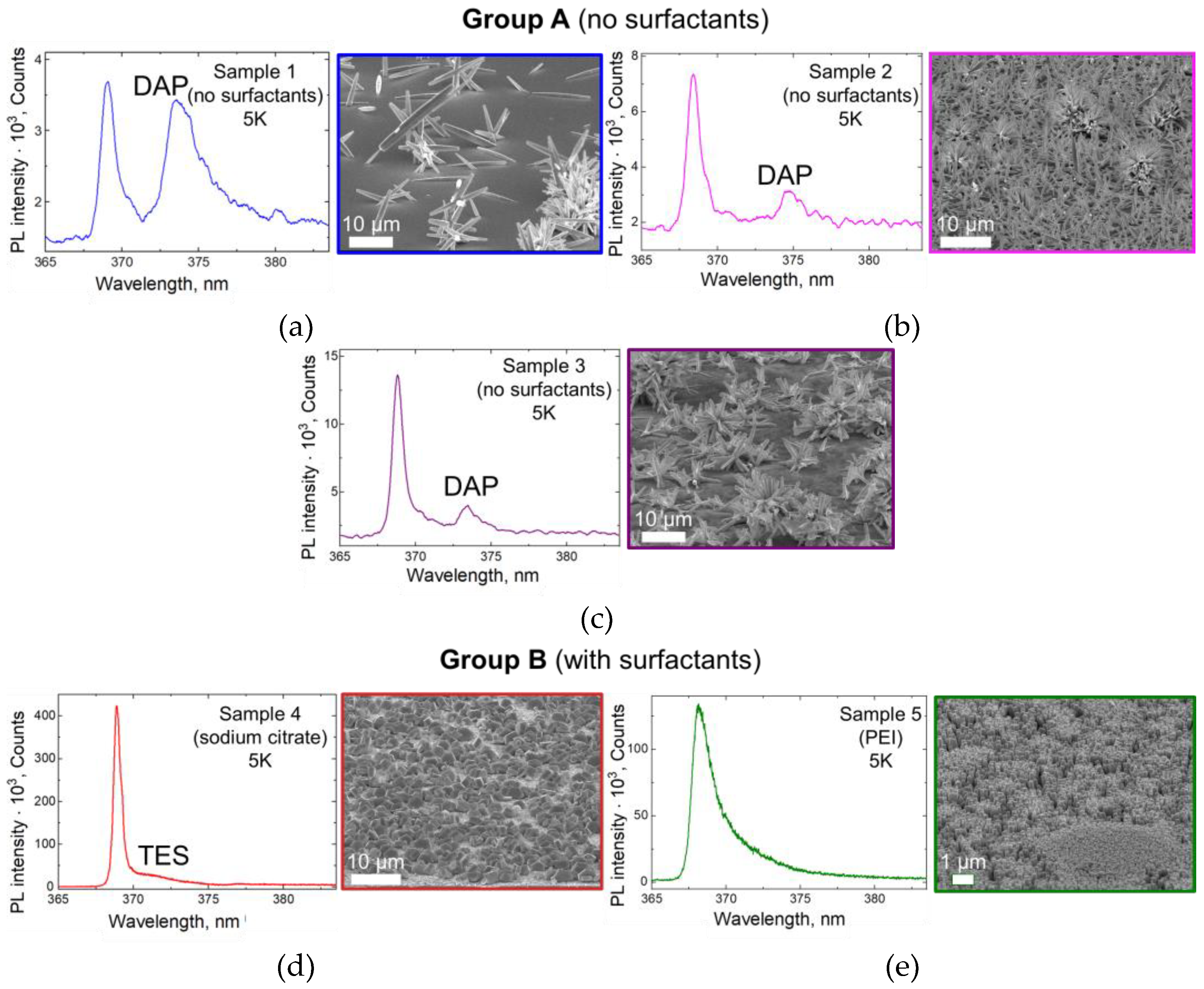
3.4. Photoluminescence Spectroscopy
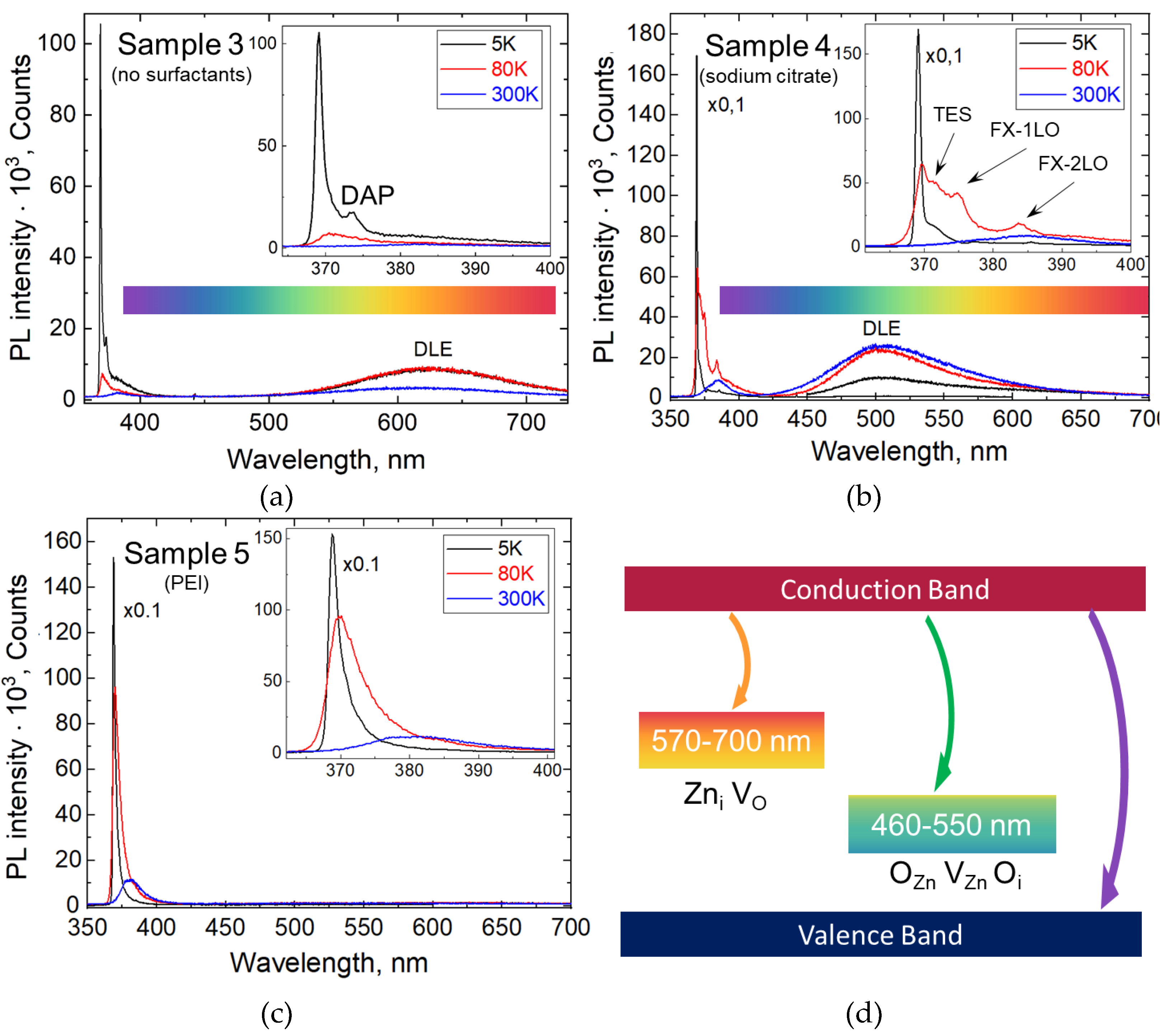
3.5. Deep-Level Emission and PL Temperature Dependence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Look, D.C.; Claflin, B.; Alivov, Y.I.; Park, S.J. The Future of ZnO Light Emitters. Phys. Status Solidi 2004, 201, 2203–2212. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Jang, J.-W.; Jung, S.-H.; Oh, E.; Lee, B.R.; Lee, K.-H. Large-Scale Fabrication of Sub-20-Nm-Diameter ZnO Nanorod Arrays at Room Temperature and Their Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 10452–10458. [Google Scholar] [CrossRef]
- Sapkota, A.; Anceno, A.J.; Baruah, S.; Shipin, O.V.; Dutta, J. Zinc Oxide Nanorod Mediated Visible Light Photoinactivation of Model Microbes in Water. Nanotechnology 2011, 22, 215703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Liu, P.; Wang, P.; Zhang, L. Photocatalytic Degradation of E.Coli Membrane Cell in the Presence of ZnO Nanowires. J. Wuhan Univ. Technol. Sci. Ed. 2011, 26, 222–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef] [Green Version]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Baji, Z.; Lábadi, Z.; Horváth, Z.E.; Molnár, G.; Volk, J.; Bársony, I.; Barna, P. Nucleation and Growth Modes of ALD ZnO. Cryst. Growth Des. 2012, 12, 5615–5620. [Google Scholar] [CrossRef]
- El-Shaer, A.; Mofor, A.C.; Bakin, A.; Kreye, M.; Waag, A. High-Quality ZnO Layers Grown by MBE on Sapphire. Superlattices Microstruct. 2005, 38, 265–271. [Google Scholar] [CrossRef]
- Nakayama, T. Laser-Induced Sputtering of ZnO, TiO2, CdSe and GaP near Threshold Laser Fluence. Surf. Sci. 1983, 133, 101–113. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Cao, H.; Chang, R.P.H. Growth Mechanism and Properties of ZnO Nanorods Synthesized by Plasma-Enhanced Chemical Vapor Deposition. J. Appl. Phys. 2004, 95, 3141–3147. [Google Scholar] [CrossRef]
- Khuri-Yakub, B.T.; Kino, G.S.; Galle, P. Studies of the Optimum Conditions for Growth of Rf-sputtered ZnO Films. J. Appl. Phys. 1975, 46, 3266–3272. [Google Scholar] [CrossRef]
- Semiconductor-On-Insulator Materials for Nanoelectronics Applications; Nazarov, A.; Colinge, J.-P.; Balestra, F.; Raskin, J.-P.; Gamiz, F.; Lysenko, V.S. (Eds.) Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-15867-4. [Google Scholar]
- Van Meter, R.; Ladd, T.D.; Fowler, A.G.; Yamamoto, Y. Distributed quantum computation architecture using semiconductor nanophotonics. Int. J. Quantum Inf. 2010, 08, 295–323. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A Comparative Analysis of Deep Level Emission in ZnO Layers Deposited by Various Methods. J. Appl. Phys. 2009, 105, 013502. [Google Scholar] [CrossRef]
- Singh, S.; Thiyagarajan, P.; Mohan Kant, K.; Anita, D.; Thirupathiah, S.; Rama, N.; Tiwari, B.; Kottaisamy, M.; Ramachandra Rao, M.S. Structure, Microstructure and Physical Properties of ZnO Based Materials in Various Forms: Bulk, Thin Film and Nano. J. Phys. D. Appl. Phys. 2007, 40, 6312–6327. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Raifuku, I.; Ishikawa, Y.; Uraoka, Y.; Yanagi, H. Hierarchical Core–Shell Heterostructure of H2O-Oxidized ZnO Nanorod@Mg-Doped ZnO Nanoparticle for Solar Cell Applications. Mater. Adv. 2020, 1, 1253–1261. [Google Scholar] [CrossRef]
- Badgujar, A.C.; Yadav, B.S.; Jha, G.K.; Dhage, S.R. Room Temperature Sputtered Aluminum-Doped ZnO Thin Film Transparent Electrode for Application in Solar Cells and for Low-Band-Gap Optoelectronic Devices. ACS Omega 2022, 7, 14203–14210. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Yanagi, H. Effect of Rubrene:P3HT Bilayer on Photovoltaic Performance of Perovskite Solar Cells with Electrodeposited ZnO Nanorods. J. Energy Chem. 2018, 27, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal Synthesis of ZnO Nanostructures with Controllable Morphology Change. Cryst. Eng. Comm. 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Kim, J.; Park, M.; Im, S. Optimizing N-ZnO/p-Si Heterojunctions for Photodiode Applications. Thin Solid Films 2002, 403–404, 553–557. [Google Scholar] [CrossRef]
- Alivov, Y.I.; Johnstone, D.; Özgür, U.; Avrutin, V.; Fan, Q.; Akarca-Biyikli, S.S.; Morkoç, H. Electrical and Optical Properties of N-ZnO/p-SiC Heterojunctions. Jpn. J. Appl. Phys. 2005, 44, 7281–7284. [Google Scholar] [CrossRef]
- Aziz, T.N.T.A.; Rosli, A.B.; Yusoff, M.M.; Herman, S.H.; Zulkifli, Z. Transparent Hybrid ZnO-Graphene Film for High Stability Switching Behavior of Memristor Device. Mater. Sci. Semicond. Process. 2019, 89, 68–76. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiu, F.; Jiang, T.; Xu, J.; Chen, J.; Liu, J.; Huang, W. Solution-Processable Zinc Oxide Nanorods and a Reduced Graphene Oxide Hybrid Nanostructure for Highly Flexible and Stable Memristor. J. Mater. Chem. C 2019, 7, 10764–10768. [Google Scholar] [CrossRef]
- RANI, S.; SURI, P.; SHISHODIA, P.; MEHRA, R. Synthesis of Nanocrystalline ZnO Powder via Sol–Gel Route for Dye-Sensitized Solar Cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1639–1645. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Synthesis and Growth of ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 5566–5572. [Google Scholar] [CrossRef]
- Bachari, E.; Baud, G.; Ben Amor, S.; Jacquet, M. Structural and Optical Properties of Sputtered ZnO Films. Thin Solid Films 1999, 348, 165–172. [Google Scholar] [CrossRef]
- Ito, K.; Nakazawa, T. Transparent and Highly Conductive Films of ZnO Prepared by RF Sputtering. Jpn. J. Appl. Phys. 1983, 22, L245–L247. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Litton, C.W.; Look, D.C.; Hoelscher, J.E.; Claflin, B.; Collins, T.C.; Nause, J.; Nemeth, B. High-Quality, Melt-Grown ZnO Single Crystals. J. Appl. Phys. 2004, 95, 4802–4805. [Google Scholar] [CrossRef]
- Barui, S.; Gerbaldo, R.; Garino, N.; Brescia, R.; Laviano, F.; Cauda, V. Facile Chemical Synthesis of Doped ZnO Nanocrystals Exploiting Oleic Acid. Nanomaterials 2020, 10, 1150. [Google Scholar] [CrossRef]
- Mozharov, A.M.; Bolshakov, A.D.; Kudryashov, D.A.; Kryzhanovskaya, N.V.; Cirlin, G.E.; Mukhin, I.S.; Harmand, J.C.; Tchernysheva, M. Modeling, Synthesis and Study of Highly Efficient Solar Cells Based on III-Nitride Nanowire Arrays Grown on Si Substrates. J. Phys. Conf. Ser. 2015, 643, 012115. [Google Scholar] [CrossRef]
- Koval, O.Y.; Fedorov, V.V.; Bolshakov, A.D.; Fedina, S.V.; Kochetkov, F.M.; Neplokh, V.; Sapunov, G.A.; Dvoretckaia, L.N.; Kirilenko, D.A.; Shtrom, I.V.; et al. Structural and Optical Properties of Self-Catalyzed Axially Heterostructured GaPN/GaP Nanowires Embedded into a Flexible Silicone Membrane. Nanomaterials 2020, 10, 2110. [Google Scholar] [CrossRef]
- Bolshakov, A.D.; Mozharov, A.M.; Sapunov, G.A.; Fedorov, V.V.; Dvoretckaia, L.N.; Mukhin, I.S. Theoretical Modeling of the Self-Catalyzed Nanowire Growth: Nucleation- and Adsorption-Limited Regimes. Mater. Res. Express 2017, 4, 125027. [Google Scholar] [CrossRef]
- Cui, J. Zinc Oxide Nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Roy, P.; Bolshakov, A.D. Ga-GaP Nanowire Hybrid Optical System for Enhanced Coupling, Focusing and Steering of Light. J. Phys. D. Appl. Phys. 2020, 53, 295101. [Google Scholar] [CrossRef]
- Chu, S.; Wang, G.; Zhou, W.; Lin, Y.; Chernyak, L.; Zhao, J.; Kong, J.; Li, L.; Ren, J.; Liu, J. Electrically Pumped Waveguide Lasing from ZnO Nanowires. Nat. Nanotechnol. 2011, 6, 506–510. [Google Scholar] [CrossRef]
- Na, J.H.; Kitamura, M.; Arita, M.; Arakawa, Y. Hybrid P-n Junction Light-Emitting Diodes Based on Sputtered ZnO and Organic Semiconductors. Appl. Phys. Lett. 2009, 95, 253303. [Google Scholar] [CrossRef]
- Sudhagar, P.; Kumar, R.S.; Jung, J.H.; Cho, W.; Sathyamoorthy, R.; Won, J.; Kang, Y.S. Facile Synthesis of Highly Branched Jacks-like ZnO Nanorods and Their Applications in Dye-Sensitized Solar Cells. Mater. Res. Bull. 2011, 46, 1473–1479. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Yanagi, H. Efficient Solid-State Perovskite Solar Cells Based on Nanostructured Zinc Oxide Designed by Strategic Low Temperature Water Oxidation. J. Mater. Chem. C 2017, 5, 8059–8070. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.; Wang, Y.; Zang, S.; Wei, S.; Li, J.; Zhang, X. Defect Passivation of Low-Temperature Processed ZnO Electron Transport Layer with Polyethylenimine for PbS Quantum Dot Photovoltaics. ACS Appl. Energy Mater. 2019, 2, 1695–1701. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Chang, S.-J.; Chang, S.-P.; Lee, C.-T.; Kuo, C.-F.; Chang, H.-M.; Chiou, Y.-Z.; Hsu, C.-L.; Chen, I.-C. Ultraviolet Photodetectors with ZnO Nanowires Prepared on ZnO:Ga/Glass Templates. Appl. Phys. Lett. 2006, 89, 153101. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.-J. Controlled Growth of ZnO Nanowires and Their Optical Properties. Adv. Funct. Mater. 2002, 12, 323. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Kondratev, V.M.; Bolshakov, A.D.; Nalimova, S.S. Technologically Feasible ZnO Nanostructures for Carbon Monoxide Gas Sensing. In Proceedings of the 2021 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), St. Petersburg, Russia, 26 January 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1163–1166. [Google Scholar]
- Desai, M.A.; Sharma, V.; Prasad, M.; Jadkar, S.; Saratale, G.D.; Sartale, S.D. Seed-Layer-Free Deposition of Well-Oriented ZnO Nanorods Thin Films by SILAR and Their Photoelectrochemical Studies. Int. J. Hydrogen. Energy 2020, 45, 5783–5792. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Maier-Flaig, F.; Schneider, D.; Kalt, H. 65 Years of ZnO Research—Old and Very Recent Results. Phys. Status Solidi 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Labzowskaya, M.E.; Akopyan, I.K.; Novikov, B.V.; Serov, A.E.; Filosofov, N.G.; Basov, L.L.; Drozd, V.E.; Lisachenko, A.A. Exciton Photoluminescence of ZnO Thin Films Grown by ALD-Technique. Phys. Procedia 2015, 76, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Akopyan, I.K.; Labzovskaya, M.E.; Novikov, B.V.; Talalaev, V.G.; Tomm, J.W.; Schilling, J. Emission Kinetics of Surface (Bi)Excitons in ZnO Thin Films. Phys. Solid State 2019, 61, 402–407. [Google Scholar] [CrossRef]
- Vasilyev, N.; Borisov, E.N.; Novikov, B.V.; Akopyan, I.K.; Labzovskaya, M.E. Random Lasing in ZnO Self-Organized Nanoparticles Produced by Laser Induced Breakdown. J. Lumin. 2019, 215, 116668. [Google Scholar] [CrossRef]
- Akopyan, I.K.; Labzovskaya, M.E.; Novikov, B.V.; Serov, A.Y. On the Nature of Stimulated Emission in ZnO in a Wide Temperature Range. Phys. Solid State 2022, 64, 1–5. [Google Scholar] [CrossRef]
- Xu, C.; Dai, J.; Zhu, G.; Zhu, G.; Lin, Y.; Li, J.; Shi, Z. Whispering-Gallery Mode Lasing in ZnO Microcavities. Laser Photon. Rev. 2014, 8, 469–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, H.; Wang, R.; Chen, C.; Luo, X.; Yu, D.; Lee, C. Low-Temperature Growth and Raman Scattering Study of Vertically Aligned ZnO Nanowires on Si Substrate. Appl. Phys. Lett. 2003, 83, 4631–4633. [Google Scholar] [CrossRef] [Green Version]
- Calleja, J.M.; Cardona, M. Resonant Raman Scattering in ZnO. Phys. Rev. B 1977, 16, 3753–3761. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Montenegro, D.N.; Hortelano, V.; Martínez, O.; Martínez-Tomas, M.C.; Sallet, V.; Muñoz-Sanjosé, V.; Jiménez, J. Non-Radiative Recombination Centres in Catalyst-Free ZnO Nanorods Grown by Atmospheric-Metal Organic Chemical Vapour Deposition. J. Phys. D. Appl. Phys. 2013, 46, 235302. [Google Scholar] [CrossRef]
- Sood, S.; Kumar, A.; Sharma, N. Photocatalytic and Antibacterial Activity Studies of ZnO Nanoparticles Synthesized by Thermal Decomposition of Mechanochemically Processed Oxalate Precursor. ChemistrySelect 2016, 1, 6925–6932. [Google Scholar] [CrossRef]
- Arefi, M.R.; Rezaei-Zarchi, S. Synthesis of Zinc Oxide Nanoparticles and Their Effect on the Compressive Strength and Setting Time of Self-Compacted Concrete Paste as Cementitious Composites. Int. J. Mol. Sci. 2012, 13, 4340–4350. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gahlaut, U.P.S.; Kumar, V.; Goswami, Y.C. Enhanced Photocatalytic Activity of Low Cost Synthesized Al Doped Amorphous ZnO/ ZnS Heterostructures. Phys. E Low-Dimens. Syst. Nanostr. 2020, 117, 113792. [Google Scholar] [CrossRef]
- Nesakumar, N.; Rayappan, J.B.B.; Jeyaprakas, B.G.; Krishnan, U.M. Influence of PH on Structural Morphology of ZnO Nanoparticle. J. Appl. Sci. 2012, 12, 1758–1761. [Google Scholar] [CrossRef] [Green Version]
- Rietveld, H.M. Line Profiles of Neutron Powder-Diffraction Peaks for Structure Refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic Dye Degradation and Antimicrobial Activities of Pure and Ag-Doped ZnO Using Cannabis Sativa Leaf Extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef] [PubMed]
- Elakrmi, E.; Chaâbane, R.B.; Ouada, H. Ben Structure and Electrical Properties of Nanostructured Zinc Oxide Films Prepared for Optoelectronic Applications. Akademeia 2012, 2, 1–9. [Google Scholar]
- Moharram, A.H.; Mansour, S.A.; Hussein, M.A.; Rashad, M. Direct Precipitation and Characterization of ZnO Nanoparticles. J. Nanomater. 2014, 2014, 716210. [Google Scholar] [CrossRef] [Green Version]
- Perillo, P.M.; Atia, M.N. High Degradation Efficiency of Organic Dyes under Sunlight Irradiation for ZnO Nanorods. Chem. Technol. Ind. J. 2016, 11, 101. [Google Scholar]
- Travnikov, V.V.; Freiberg, A.; Savikhin, S.F. Surface Excitons in ZnO Crystals. J. Lumin. 1990, 47, 107–112. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; He, W.; Park, S.-J.; Kim, H.-K.; Hwang, Y.-H.; Lee, J.-H.; Kim, Y.-D. The Growth Mechanism and Optical Properties of Ultralong ZnO Nanorod Arrays with a High Aspect Ratio by a Preheating Hydrothermal Method. Nanotechnology 2009, 20, 155603. [Google Scholar] [CrossRef]
- Teke, A.; Özgür, Ü.; Doğan, S.; Gu, X.; Morkoç, H.; Nemeth, B.; Nause, J.; Everitt, H.O. Excitonic Fine Structure and Recombination Dynamics in Single-Crystalline ZnO. Phys. Rev. B 2004, 70, 195207. [Google Scholar] [CrossRef] [Green Version]
- Shan, W.; Walukiewicz, W.; Ager, J.W.; Yu, K.M.; Yuan, H.B.; Xin, H.P.; Cantwell, G.; Song, J.J. Nature of Room-Temperature Photoluminescence in ZnO. Appl. Phys. Lett. 2005, 86, 191911. [Google Scholar] [CrossRef]
- Lima, S.A.; Sigoli, F.; Jafelicci, M., Jr.; Davolos, M. Luminescent Properties and Lattice Defects Correlation on Zinc Oxide. Int. J. Inorg. Mater. 2001, 3, 749–754. [Google Scholar] [CrossRef]
- Lin, B.; Fu, Z.; Jia, Y. Green Luminescent Center in Undoped Zinc Oxide Films Deposited on Silicon Substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Ding, L.; Ge, W.K.; Chen, H.Y.; Gwo, S. Green, Yellow, and Orange Defect Emission from ZnO Nanostructures: Influence of Excitation Wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]
- Kennedy, O.W.; White, E.R.; Howkins, A.; Williams, C.K.; Boyd, I.W.; Warburton, P.A.; Shaffer, M.S.P. Mapping the Origins of Luminescence in ZnO Nanowires by STEM-CL. J. Phys. Chem. Lett. 2019, 10, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, Z.; Yan, J.; Zhao, W.; Zhai, C.; Liu, J. Growth Mechanism and Photoluminescence Property of Hydrothermal Oriented ZnO Nanostructures Evolving from Nanorods to Nanoplates. J. Alloys Compd. 2017, 718, 161–169. [Google Scholar] [CrossRef]
- Hou, D.; Voss, T.; Ronning, C.; Menzel, A.; Zacharias, M. Deep-Level Emission in ZnO Nanowires and Bulk Crystals: Excitation-Intensity Dependence versus Crystalline Quality. J. Appl. Phys. 2014, 115, 233516. [Google Scholar] [CrossRef]
- Sree, V.G.; Bathula, C.; Kadam, A.N.; Ravindra, M.; Mahadevan, K.; Sohn, J.I.; Kim, H.-S.; Im, H. Halogen Free Solvent Processed Light-Emitting Diodes Achieving EQE Nearly 25% for Imidazole-Based Host Materials Synthesized by Ball Milling. Nano Energy 2022, 92, 106753. [Google Scholar] [CrossRef]
- Sree, V.G.; Naik, S.; Jana, A.; Kadam, A.; Sekar, S.; Im, H.; Kim, H.-S.; Sohn, J.I.; Bathula, C. Mechanochemically Interlocked Cubane Copper Complex Interface for WOLED. J. Colloid Interface Sci. 2023, 633, 589–597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadinskaya, S.A.; Kondratev, V.M.; Kindyushov, I.K.; Koval, O.Y.; Yakubovsky, D.I.; Kusnetsov, A.; Lihachev, A.I.; Nashchekin, A.V.; Akopyan, I.K.; Serov, A.Y.; et al. Deep-Level Emission Tailoring in ZnO Nanostructures Grown via Hydrothermal Synthesis. Nanomaterials 2023, 13, 58. https://doi.org/10.3390/nano13010058
Kadinskaya SA, Kondratev VM, Kindyushov IK, Koval OY, Yakubovsky DI, Kusnetsov A, Lihachev AI, Nashchekin AV, Akopyan IK, Serov AY, et al. Deep-Level Emission Tailoring in ZnO Nanostructures Grown via Hydrothermal Synthesis. Nanomaterials. 2023; 13(1):58. https://doi.org/10.3390/nano13010058
Chicago/Turabian StyleKadinskaya, Svetlana A., Valeriy M. Kondratev, Ivan K. Kindyushov, Olga Yu. Koval, Dmitry I. Yakubovsky, Alexey Kusnetsov, Alexey I. Lihachev, Alexey V. Nashchekin, Irina Kh. Akopyan, Alexey Yu. Serov, and et al. 2023. "Deep-Level Emission Tailoring in ZnO Nanostructures Grown via Hydrothermal Synthesis" Nanomaterials 13, no. 1: 58. https://doi.org/10.3390/nano13010058





