Stabilization of an Aqueous Bio-Based Wax Nano-Emulsion through Encapsulation
Abstract
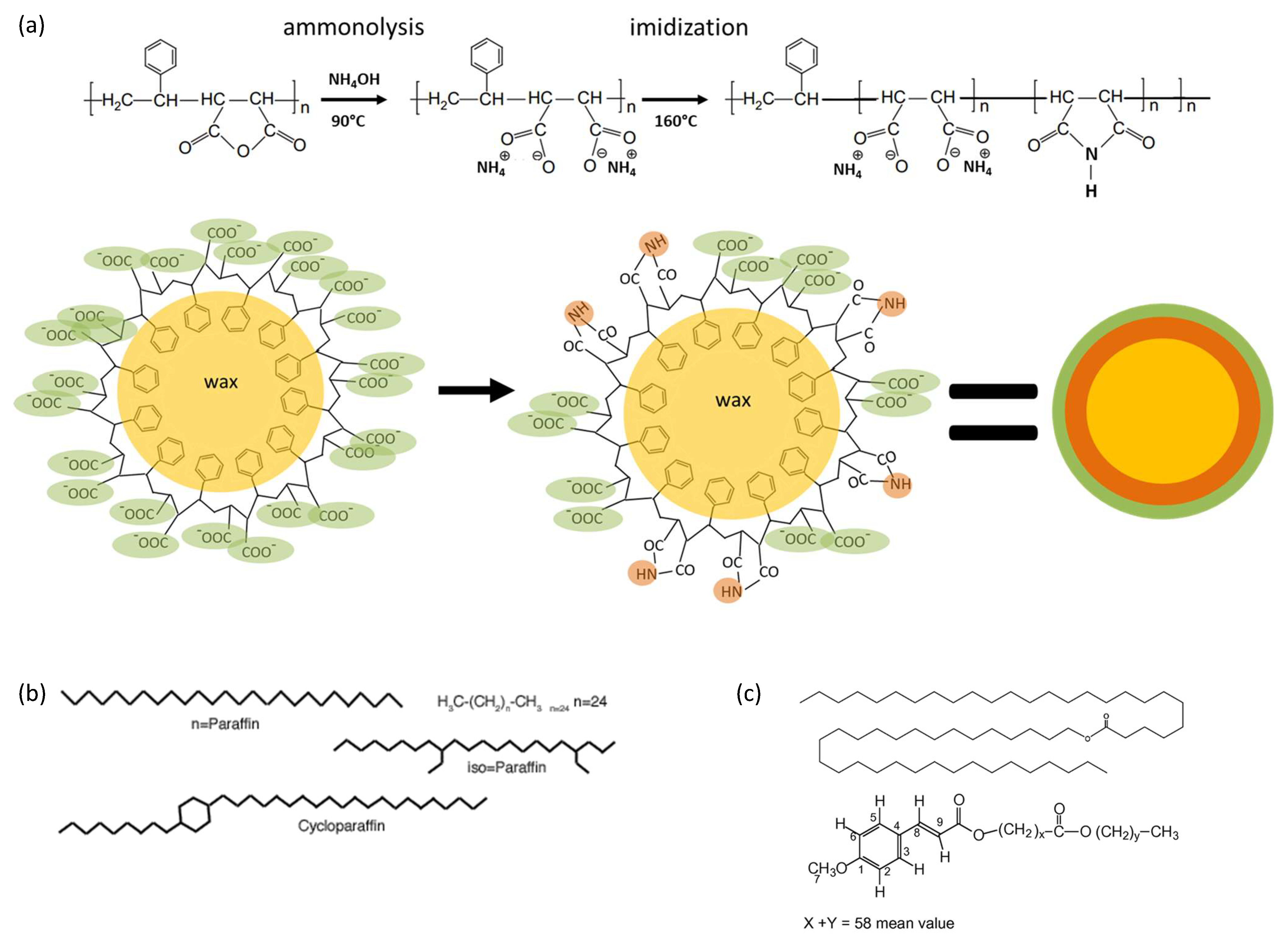
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
3. Results
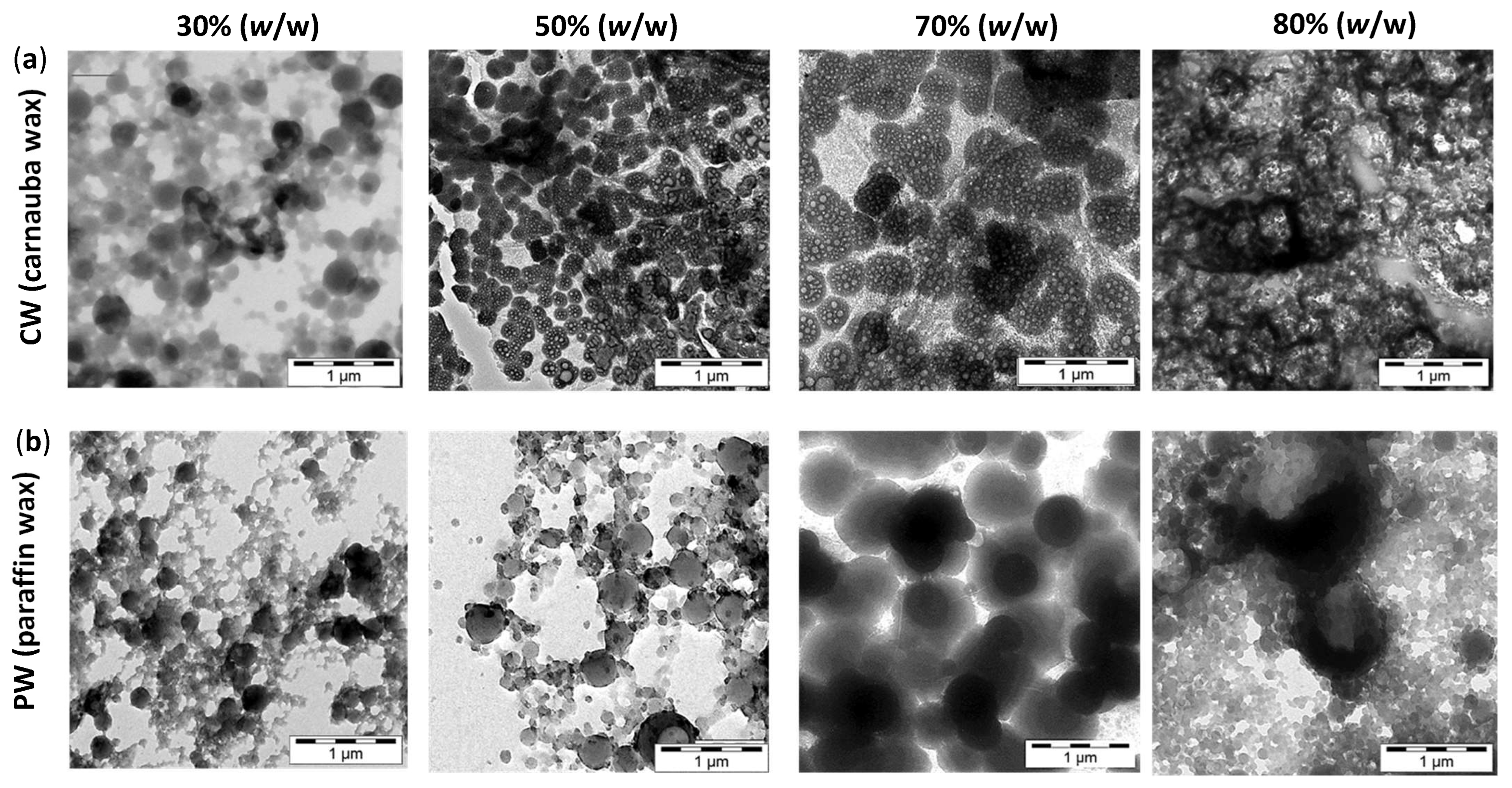
3.1. Dispersion Properties and Morphology
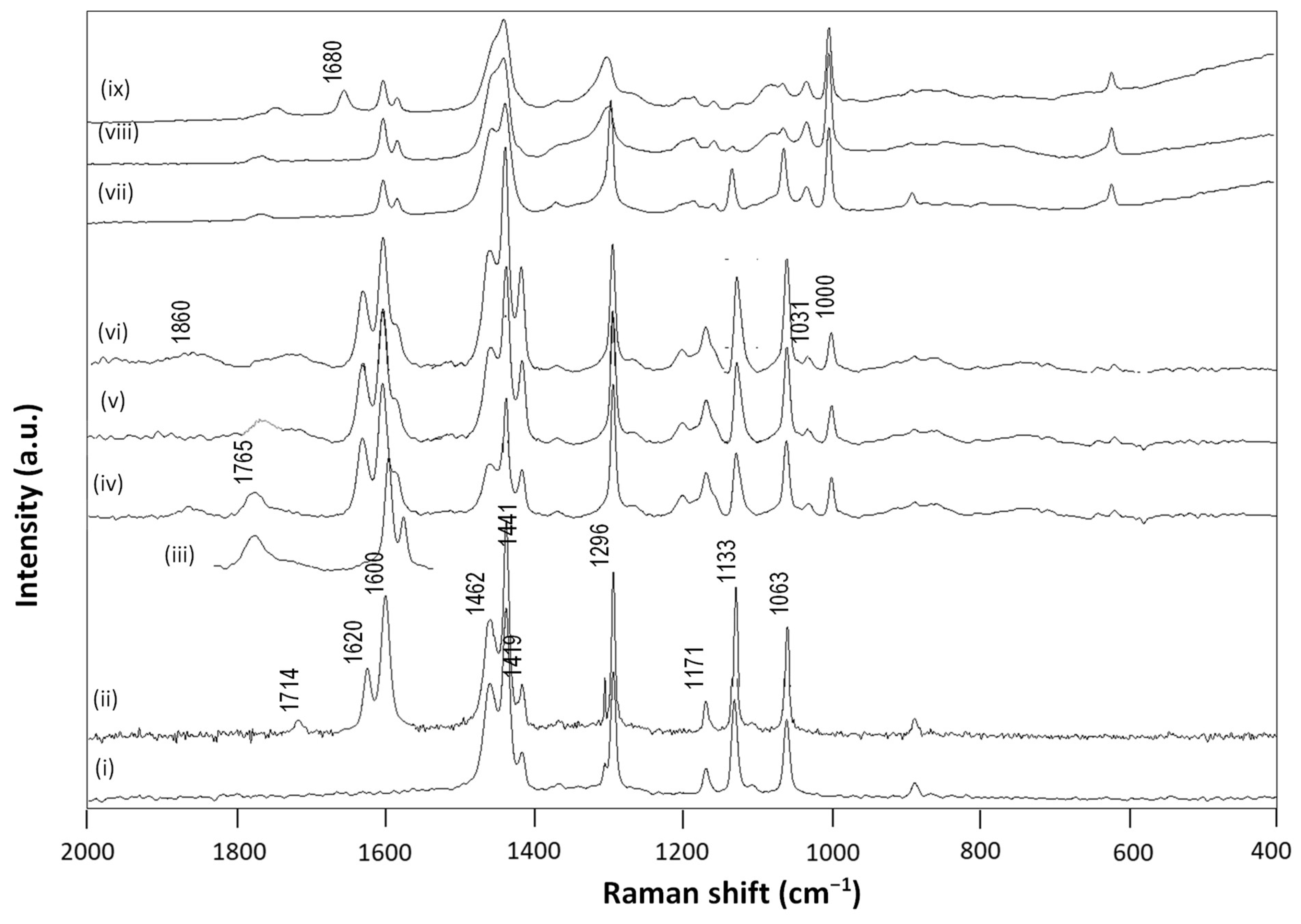
3.2. Chemical Properties
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fei, T.; Wang, T. A review of recent development of sustainable waxes derived from vegetable oils. Curr. Opin. Food Sci. 2017, 16, 7–14. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes, Ann. Plant Rev. 2018, 23, 145–181. [Google Scholar]
- Floros, M.C.; Raghunanan, L.; Narine, S.S. A toolbox for the characterization of biobased waxes, Eur. J. Lip. Sci. Technol. 2016, 119, 1600360. [Google Scholar] [CrossRef]
- Palou, A.; Cruz, J.; Blanco, M.; Larraz, R.; Frontela, J.; Bengoechea, C.M.; Gonzalez, J.M.; Alcala, M. Characterization of the composition of paraffin waxes on industrial applications. Energy Fuels 2014, 28, 956–963. [Google Scholar] [CrossRef]
- Turner, W.R.; Brown, D.S.; Harisson, D.V. Properties of paraffin waxes. Ind. Eng. Chem. 1955, 47, 1219–1226. [Google Scholar] [CrossRef]
- Krendlinger, E.J.; Wolfmeier, U.H. Fischer–Tropsch Synthesis (FTS) and Waxes. In Natural and Synthetic Waxes, 1st ed.; Krendlinger, E.J., Wolfmeier, U.H., Eds.; Wiley VCH: Weinheim, Germany, 2023; pp. 515–572. [Google Scholar]
- Le Roux, J.H. Fischer-Tropsch waxes. II. Crystallinity and physical properties. J. Appl. Chem. Lond. 1969, 19, 39. [Google Scholar] [CrossRef]
- Tulloch, A.P. The composition of beeswax and other waxes secreted by insects. Lipids 1970, 5, 247–258. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Post-Beittenmiller, Biochemistry and molecular biology of wax production in plants. Annu. Rev. Plant. Physiol. Mol. Biol. 1996, 47, 405–430. [CrossRef]
- Koch, K.; Barthlott, W. Plant epicuticular waxes: Chemistry, form, self-assembly and function. Nat. Prod. Commun. 2006, 1, 1067–1072. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef]
- Nasri, N.S.; Ahmed, M.; Neemah, M.N.; Mohammed, J.; Hamza, U.D.; Zain, H.M. Hydrophobicity characterization of bio-wax derived from taro leaf for surface coating applications. Adv. Mater. Res. 2014, 1043, 187–188. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.D.; Silva, G.D.C.; Oldoni, F.C.A.; Miranda, M.; Florencio, C.; Oliveira, R.M.D.D.; Gomes, M.D.P.; Ferreira, M.D. Edible coating based on carnauba wax nano-emulsion and cymbopogon martinii essential oil on papaya postharvest preservation. Coatings 2022, 12, 1700. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.D.; Albiero, B.R.; Calisto, I.H.; Bertolo, M.R.V.; Oldoni, F.C.A.; Egea, M.B.; Bogusz Junior, S.; de Azeredo, H.M.C.; Ferreira, M.D. Bio-nanocomposite edible coatings based on arrowroot starch/cellulose nanocrystals/carnauba wax nano-emulsion containing essential oils to preserve quality and improve shelf life of strawberry. Int. J. Biol. Macromol. 2022, 219, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Oliveria Filho, J.G.D.; Albiero, B.R.; Cipriano, L.; Oliveira Nobre Bezerra, C.C.; Oldoni, F.C.A.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. Arrowroot starch-based films incorporated with a carnauba wax nano-emulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511. [Google Scholar] [CrossRef]
- Gomes, A.V.R.; Goncalves, F.C.P.; da Silva, M.Q., Jr.; Leite, R.H.; dos Santos, F.K.G.; Aroucha, A.M.M. Effect of carnauba wax and coconut fiber contents on tensile properties of corn starch-based biocomposites. Mat. Res. 2019, 22, e20190053. [Google Scholar] [CrossRef]
- Sevault, A.; Naess, E. Active latent heat storage using biowax in a central heating system of a ZEB living lab. In Proceedings of the 4th Gustav Lorentzen Conference, Kyoto, Japan, 6–9 December 2020. [Google Scholar]
- Sivapalan, B.; Neelesh Chandran, M.; Manikandan, S.; Saranprabhu, M.K.; Pavithra, S.; Rajan, K.S. Paraffin wax–water nano-emulsion: A superior thermal energy storage medium providing higher rate of thermal energy storage per unit heat exchanger volume than water and paraffin wax. Energy Convers. Manag. 2018, 162, 109–117. [Google Scholar] [CrossRef]
- Gapsari, F.; Wijaya, H.; Septiari, R.; Andoko. Evaluation of bee wax propolis inhibitor for corrosion protection on stainless steel in various pH solution. Case Studies Chem. Environ. Eng. 2022, 6, 100227. [Google Scholar] [CrossRef]
- Liu, D.; Duan, Y.; Wang, S.; Gong, M.; Dai, H. Improvement of oil and water barrier properties of food packaging paper by coating with microcrystalline wax emulsion. Polymers 2022, 14, 1786. [Google Scholar] [CrossRef]
- Basjari, A.; Salehi, A.H.; Salamatipour, N. Bioinspired and green water repellent finishing of textiles using carnauba wax and layer-by-layer technique. J. Text. Inst. 2020, 111, 1148–1158. [Google Scholar] [CrossRef]
- Clermont-Gallerande, H.; Daquin, C.; Malvezin, C.; Lesbros, C.; Nagahiro, C.; Bertron, E.; Slaim, N.; Sanchez, M.A.; Pichoutou, O.; Guarillof, P. Substitution of synthetic waxes by plant-based waxes in lipsticks. Lipids Cosmet. 2022, 29, 19. [Google Scholar] [CrossRef]
- De Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Janesch, J.; Arminger, B.; Gindl-Altmutter, W.; Hansmann, C. Superhydrophobic coatings on wood made of plant oil and natural wax. Prog. Org. Coat. 2020, 148, 105891. [Google Scholar] [CrossRef]
- Domergue, F.; Miklaszewska, M. The production of wax esters in transgenic plants: Towards a sustainable source of bio-lubricants. J. Exp. Bot. 2022, 73, 2817–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, B.; Zhao, L. Fabrication of hydrophobic coating on filter paper from self-emulsifying carnauba wax-alcohol emulsions with nano-TiO2 particles for water/diesel separation. BioRes 2017, 12, 7774–7783. [Google Scholar]
- Woch, J.; Malachowska, E.; Korasiak, K.; Lipkiewicz, A.; Dubowik, M.; Chrobak, J.; Ilowska, J.; Przybysz, P. Barrier dispersion-based coatings containing natural and paraffin waxes. Molecules 2022, 27, 90. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Fei, T.; Metzger, K.C.; Wang, T. Coating performance and rheological characteristics of novel soybean oil-based wax emulsions. Ind. Crops Prod. 2019, 140, 111654. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.P.; Liao, K.J. The preparation of wax emulsions stabilized by C5 petroleum resin. Petrol. Sci. Technol. 2019, 31, 284–292. [Google Scholar] [CrossRef]
- Milanovic, J.; Levic, S.; Manojlovic, V.; Nedovic, V.; Bugarski, B. Carnauba wax microparticles produced by melt dispersion technique. Chem. Pap. 2011, 65, 2. [Google Scholar] [CrossRef]
- Lindner, M.; Bäumler, M.; Stäbler, A. Inter-correlation among the hydrophilic–lipophilic balance, surfactant system, viscosity, particle size, and stability of candelilla wax-based dispersions. Coatings 2018, 8, 469. [Google Scholar] [CrossRef]
- Ejeta, D.D.; Wang, C.F.; Kuo, S.W.; Chen, J.K.; Tsai, H.S.; Hung, W.S.; Hu, C.C.; Lai, J.Y. Preparation and characterization of bio-wax emulsion used for superhydrophobic on cotton fibrous materials. Chem. Eng. J. 2020, 402, 126289. [Google Scholar]
- Forgiarini, A.M.; Esquena, J.; Azon, C.G.; Solans, C. Formation of nano-emulsions by low-energy emulsification methods at constant temperature. Langmuir 2001, 17, 2076–2083. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qjan, L. Beeswax–chitosan emulsion coated paper with enhanced water vapor barrier efficiency. Appl. Surf. Sci. 2014, 46, 80–85. [Google Scholar] [CrossRef]
- Meleson, K.; Graves, S.; Mason, T.G. Formation of concentrated nano-emulsions by extreme shear. Soft Mater. 2004, 2–3, 109–123. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions in mixed nonionic surfactant systems. Adv. Coll. Interfac. Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Sainz, C.; Avena-Bustillos, J.; Wood, D.F.; Williams, T.G.; McHugh, T.H. Nanoemulsions prepared by a low-energy emulsification method applied to edible films. J. Agricul. Food Chem. 2010, 58, 11932–11938. [Google Scholar] [CrossRef] [PubMed]
- Krupa, I.; Nogellova, Z.; Janigova, I.; Podgornik, B.B.; Sumiga, B.; Kleinova, A.; Karkri, M.; AlMa’adeed, M.A.A.; Spitalsky, Z. Phase change materials based on high-density polyethylene filled with microencapsulated paraffin wax. Eng. Convers. Man. 2014, 87, 400–409. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; Tsavalas, J.; Sundberg, D.; Sanchez, P.; Rodriguez, J.F. Synthesis and characterization of paraffin wax microcapsules with acrylic-based polymer shells. Ind. Eng. Chem. Res. 2010, 49, 12204–12211. [Google Scholar] [CrossRef]
- Mayya, K.S.; Bhattacharyya, A.; Argillier, J.F. Micro-encapsulation by complex coacervation: Influence of surfactant. Polym. Internat. 2003, 52, 644–647. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Liu, J.; Yang, Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer 2008, 49, 2903–2910. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, M.; Han, A.; Ding, Y. Preparation of nano-encapsulated polyethylene wax particles for color toner by in situ emulsion polymerization. J. Appl. Polym. Sci. 2017, 134, 44399. [Google Scholar]
- Zhang, N.; Yuan, Y. Synthesis and thermal properties of nanoencapsulation of paraffin as phase change material for latent heat thermal energy storage. Energy Built Environ. 2020, 1, 410–416. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Sun, R.; Ban, B.; Li, J.; Chen, J. Nanoencapsulations of paraffin wax by miniemulsion polymerization and their thermal properties as phase change materials. Mater. Chem. Phys. 2019, 231, 244–251. [Google Scholar] [CrossRef]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; Van den Abbeele, H. Synthesis and characterization of imidized poly(styrene-maleic anhydride) nanoparticles in stable aqueous dispersion. Polym. Adv. Technol. 2012, 23, 311–325. [Google Scholar] [CrossRef]
- Ishaka, A.; Imam, M.U.; Mahamud, R.; Zuki, A.B.Z.; Maznah, I. Characterization of rice bran wax policosanol and its nano-emulsion formulation. Int. J. Nanomed. 2014, 9, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Lozhechnikova, A.; Bellanger, H.; Michen, B.; Burgert, I.; Österberg, M. Surfactant-free carnauba wax dispersion and its use for layer-by-layer assembled protective surface coatings on wood. Appl. Sur. Sci. 2016, 396, 1273–1281. [Google Scholar] [CrossRef]
- Vrabie, V.; Huez, R.; Gobinet, C.; Piot, O.; Tfayli, A.; Manfait, M. On the modelling of paraffin through Raman spectroscopy. IFAC Proc. Vol. 2006, 39, 201–206. [Google Scholar] [CrossRef]
- Bergamonti, L.; Cirlini, M.; Graiff, C.; Loittici, P.P.; Palla, G.; Casoli, A. Characterization of waxes in the Roman wall paintings of the Herculaneum Site (Italy). Appl. Sci. 2022, 12, 11264. [Google Scholar] [CrossRef]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Davila, J.; Anguebes-Franceshi, F. Characterization of beeswax, candelilla wax and paraffin wax for coating cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-based microemulsion for the removal of hydrophobic materials from works of art: Saxs characterization and application. Materials 2018, 11, 1144. [Google Scholar] [CrossRef]
- Ge, C.; Xu, X.; Ma, F.; Zhou, J.; Du, C. Biomimetic modification of waterborne polymer coating with carnauba wax for controlled release of urea. Int. J. Molec. Sci. 2022, 23, 7422. [Google Scholar] [CrossRef]
- Soliman, E.A.; Elkatory, M.R.; Hashem, A.I.; Ibrahim, H.S. Synthesis and performance of maleic anhydride copolymers with alkyl linoleate or tetra-esters as pour point depressants for waxy crude oil. Fuel 2018, 211, 535–547. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Zhang, S.; Cao, J.; Wang, W. Forming textured hydrophobic surface coatings via mixed wax emulsion impregnation and drying of poplar wood. Wood Sci. Technol. 2020, 54, 421–439. [Google Scholar] [CrossRef]
- Galvao, J.G.; Santos, R.L.; Lira, A.A.; Kaminski, R.C.; Sarmento, V.H.; Severino, P.; Dolabella, S.; Scher, R.; Souto, E.B.; Nunes, R.S. Stearic acid, beeswax and carnauba wax as green raw materials for the loading of carvacrol into nanostructured lipid carriers. Appl. Sci. 2020, 10, 6267. [Google Scholar] [CrossRef]
- Craig, R.G.; Powers, J.M.; Peyton, F.A. Thermogravimetric analysis of waxes. J. Dental Res. 1971, 50, 450–454. [Google Scholar] [CrossRef]
- Su, W.; Li, Y.; Zhou, T.; Darkwa, J.; Kokogiannakis, G.; Li, Z. Microencapsulation of paraffin wax with poly(uree methacrylate) shell for solar water heater. Energies 2019, 12, 3406. [Google Scholar] [CrossRef]
- Kim, S.; Moon, H.; Kim, J. Thermal characterization of the paraffin wax/low density polyethylene blends as a solid fuel. Thermochim. Acta 2015, 613, 9–16. [Google Scholar] [CrossRef]
- Gill, Y.Q.; Saeed, F.; Shoukat, M.H.; Irfan, M.S.; Abid, U. A study on the dewaxing behavior of carbon-black-modified LDPE–paraffin wax composites for investment casting applications. Arab. J. Sci. Eng. 2021, 46, 6715–6725. [Google Scholar] [CrossRef]
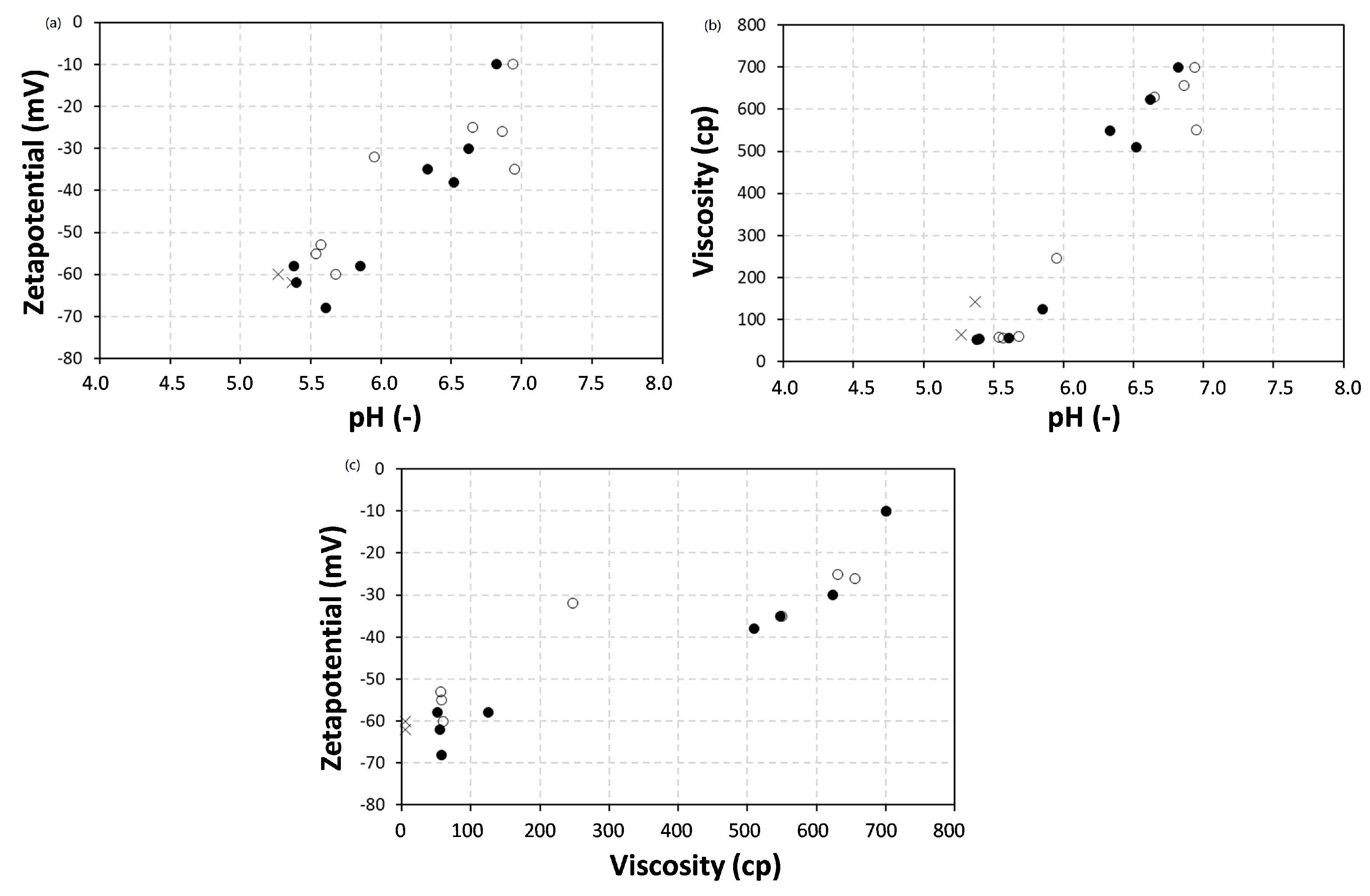
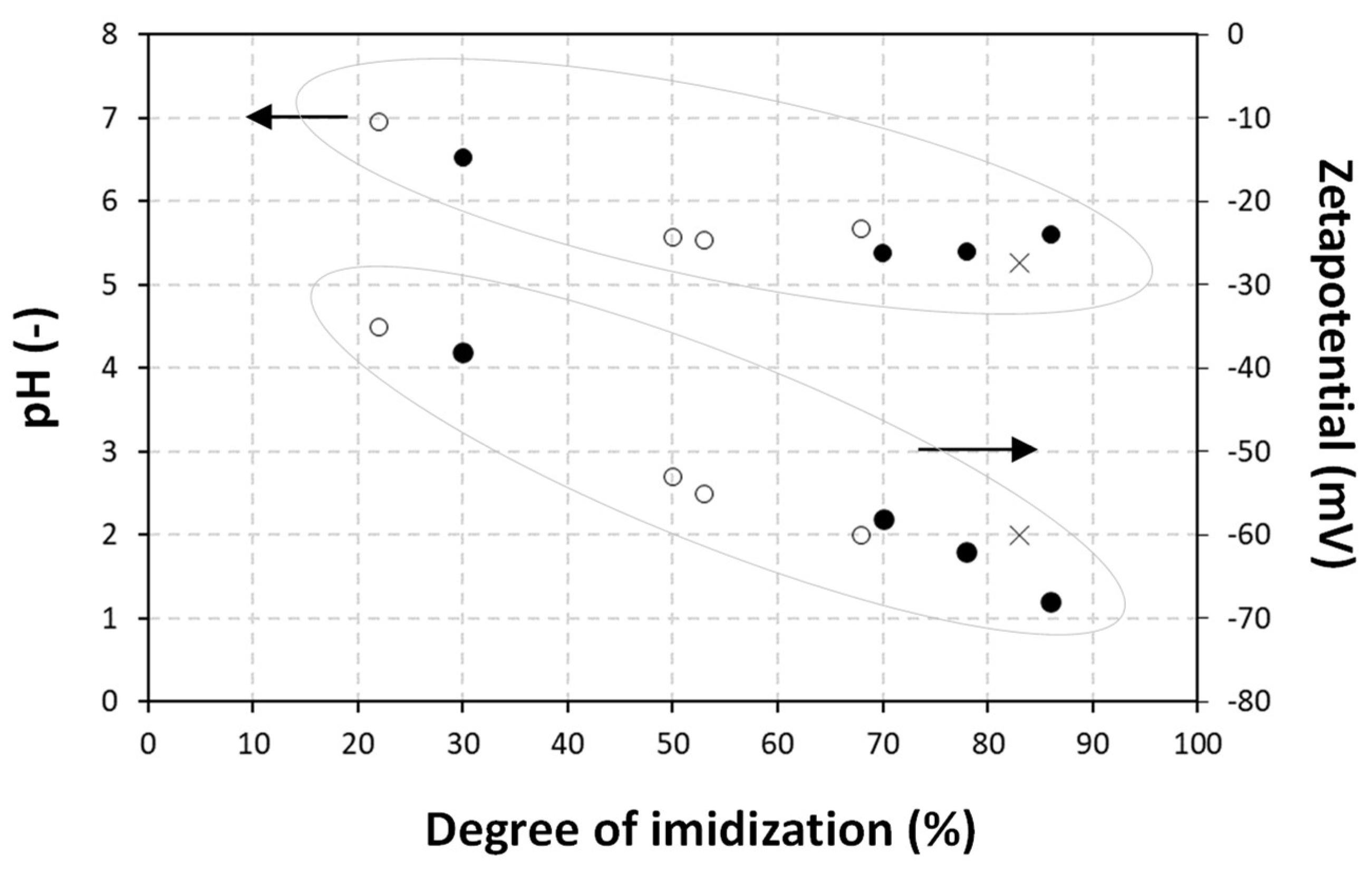


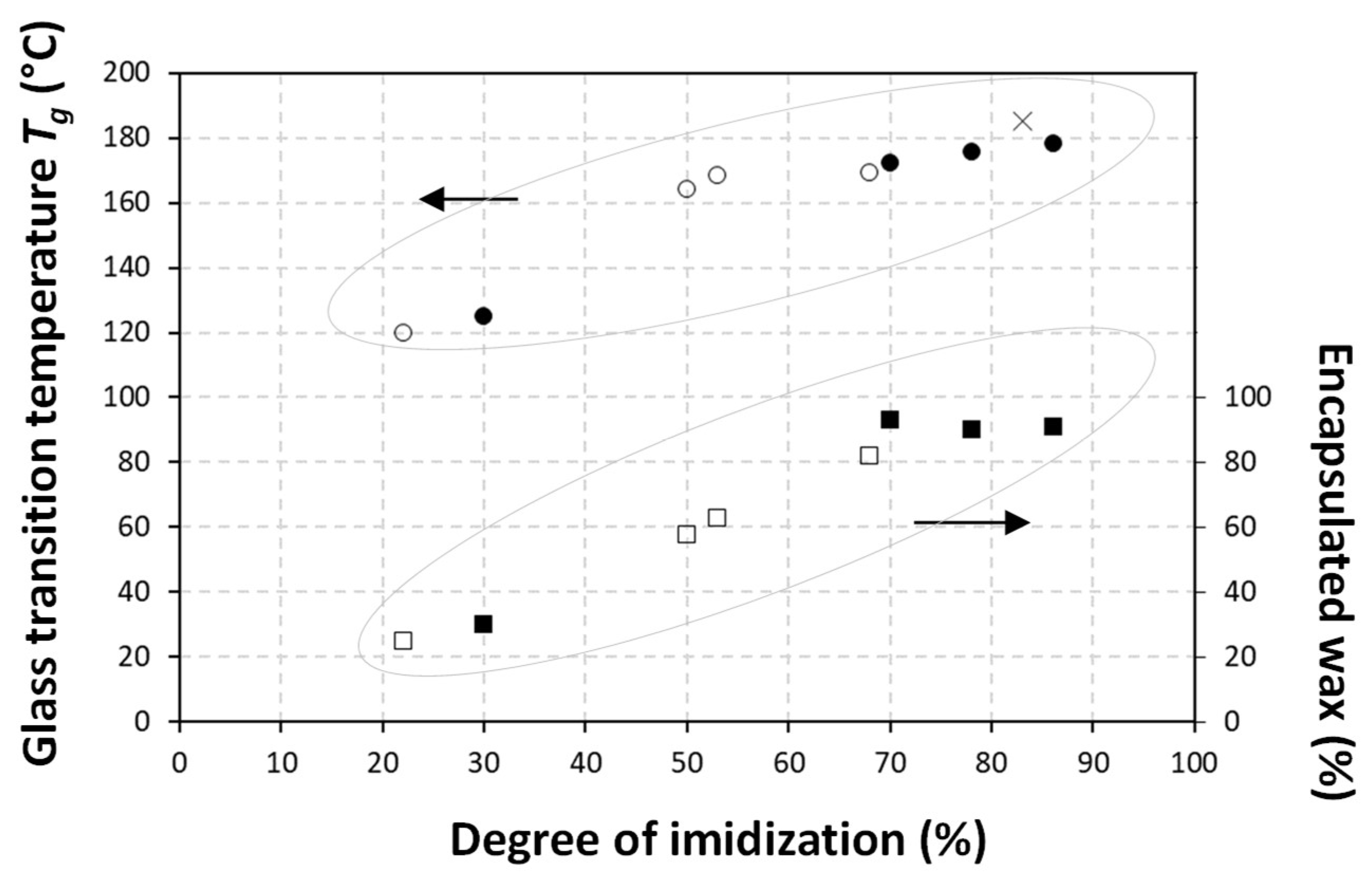


| Sample * Ratio wax/SMA | Solid Content (S.C.) (% w/w) | pH | Viscosity (cp) | Z-Average Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| No wax | |||||
| SMI | 50 | 5.27 | 64 | 98 | −60 |
| SMI | 60 | 5.37 | 143 | 90 | −62 |
| Carnauba wax | |||||
| CW50-30/70 | 50 | 5.61 | 57 | 206 | −68 |
| CW50-50/50 | 50 | 5.40 | 55 | 218 | −62 |
| CW50-70/30 | 50 | 5.38 | 50 | 250 | −58 |
| CW50-80/20 | 50 | 6.52 | 510 | N/A | −38 |
| CW60-30/70 | 60 | 5.85 | 125 | 180 | −58 |
| CW60-50/50 | 60 | 6.33 | 548 | 189 | −35 |
| CW60-70/30 | 60 | 6.62 | 623 | 196 | −30 |
| CW60-80/20 | 60 | 6.82 | >700 | N/A | >−10 |
| Paraffin wax | |||||
| PW50-30/70 | 50 | 5.68 | 60 | 200 | −60 |
| PW50-50/50 | 50 | 5.54 | 58 | 230 | −55 |
| PW50-70/30 | 50 | 5.57 | 54 | 258 | −53 |
| PW50-80/20 | 50 | 6.95 | 550 | N/A | −35 |
| PW60-30/70 | 60 | 5.95 | 247 | 206 | −32 |
| PW60-50/50 | 60 | 6.65 | 630 | 220 | −25 |
| PW60-70/30 | 60 | 6.86 | 656 | 360 | −26 |
| PW60-80/20 | 60 | 6.94 | >700 | N/A | >−10 |
| Sample | Encapsulated Wax (%) | Degree of Imidization (%) | |
|---|---|---|---|
| No wax | SMI | - | 83 |
| Carnauaba wax | CW50-30/70 | 91 | 86 |
| CW50-50/50 | 90 | 78 | |
| CW50-70/30 | 93 | 70 | |
| CW50-80/20 | 30 | 30 | |
| Paraffin wax | PW50-30/70 | 82 | 68 |
| PW50-50/50 | 63 | 53 | |
| PW50-70/30 | 58 | 50 | |
| PW50-80/20 | 25 | 22 |
| Sample | First DSC Heating Cycle | Second DSC Heating Cycle | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tm1 (°C) | Tm2 (°C) | Tm3 (°C) | ΔH J/g | Tg (°C) | Δcp J/(g°C) | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) | ΔH J/g | Tg (°C) | Δcp J/(g°C) | |
| No wax | ||||||||||||
| SMI | - | - | - | - | 182.5 | 0.279 | - | - | - | - | 185.3 | 0.701 |
| Pure Carnauaba wax (CW) | 61.4 | 78.3 | 83.4 | 188.7 | - | - | 61.5 | 78.5 | 84.1 | 185.2 | - | - |
| CW50-30/70 | - | 52.8 | 74.8 | 48.2 | 174.3 | 0.11 | - | 53.2 | 74.1 | 35.2 | 178.3 | 0.15 |
| CW50-50/50 | - | 52.4 | 74.0 | 50.3 | 175.3 | 0.09 | - | 54.8 | 74.2 | 47.2 | 175.9 | 0.07 |
| CW50-70/30 | 40.1 | 49.8 | 72.8 | 54.2 | 172.2 | 0.10 | 43.2 | 55.3 | 72.5 | 82.1 | 172.3 | 0.05 |
| CW50-80/20 | 39.4 | 48.1 | 7.1 | 80.4 | unclear | unclear | 39.6 | 53.3 | 73.3 | 118.1 | 125 | 0.368 |
| Pure Paraffin wax (PW) | - | - | 55.8 | 49.8 | - | - | - | - | 55.4 | 49.6 | - | - |
| PW50-30/70 | - | - | 53.8 | 12.3 | 171.8 | 0.123 | - | - | 53.5 | 18.4 | 169.3 | 0.25 |
| PW50-50/50 | - | - | 53.1 | 12.8 | 171.2 | 0.117 | - | - | 52.9 | 23.7 | 168.7 | 0.19 |
| PW50-70/30 | - | 41.1 | 53.8 | 13.1 | 166.3 | 0.111 | - | 41.3 | 53.5 | 35.2 | 164.2 | 0.18 |
| PW50-80/20 | - | 40.8 | 53.4 | 28.4 | unclear | unclear | - | 40.5 | 53.2 | 45.7 | 120.2 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samyn, P.; Rastogi, V.K. Stabilization of an Aqueous Bio-Based Wax Nano-Emulsion through Encapsulation. Nanomaterials 2022, 12, 4329. https://doi.org/10.3390/nano12234329
Samyn P, Rastogi VK. Stabilization of an Aqueous Bio-Based Wax Nano-Emulsion through Encapsulation. Nanomaterials. 2022; 12(23):4329. https://doi.org/10.3390/nano12234329
Chicago/Turabian StyleSamyn, Pieter, and Vibhore K. Rastogi. 2022. "Stabilization of an Aqueous Bio-Based Wax Nano-Emulsion through Encapsulation" Nanomaterials 12, no. 23: 4329. https://doi.org/10.3390/nano12234329
APA StyleSamyn, P., & Rastogi, V. K. (2022). Stabilization of an Aqueous Bio-Based Wax Nano-Emulsion through Encapsulation. Nanomaterials, 12(23), 4329. https://doi.org/10.3390/nano12234329








