Effect of Different Carbon Sources on Bacterial Nanocellulose Production and Structure Using the Low pH Resistant Strain Komagataeibacter Medellinensis
Abstract
:1. Introduction
2. Results
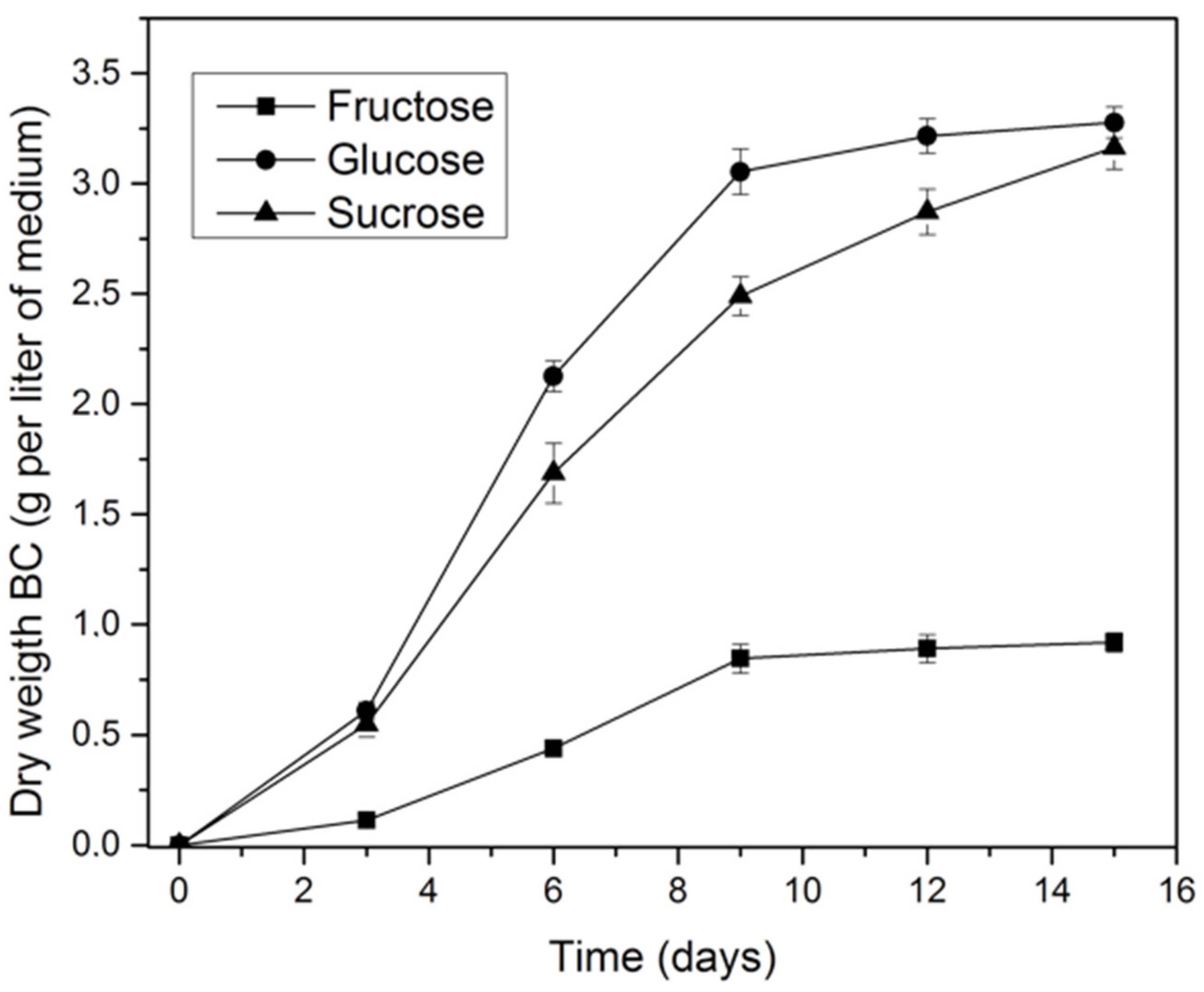
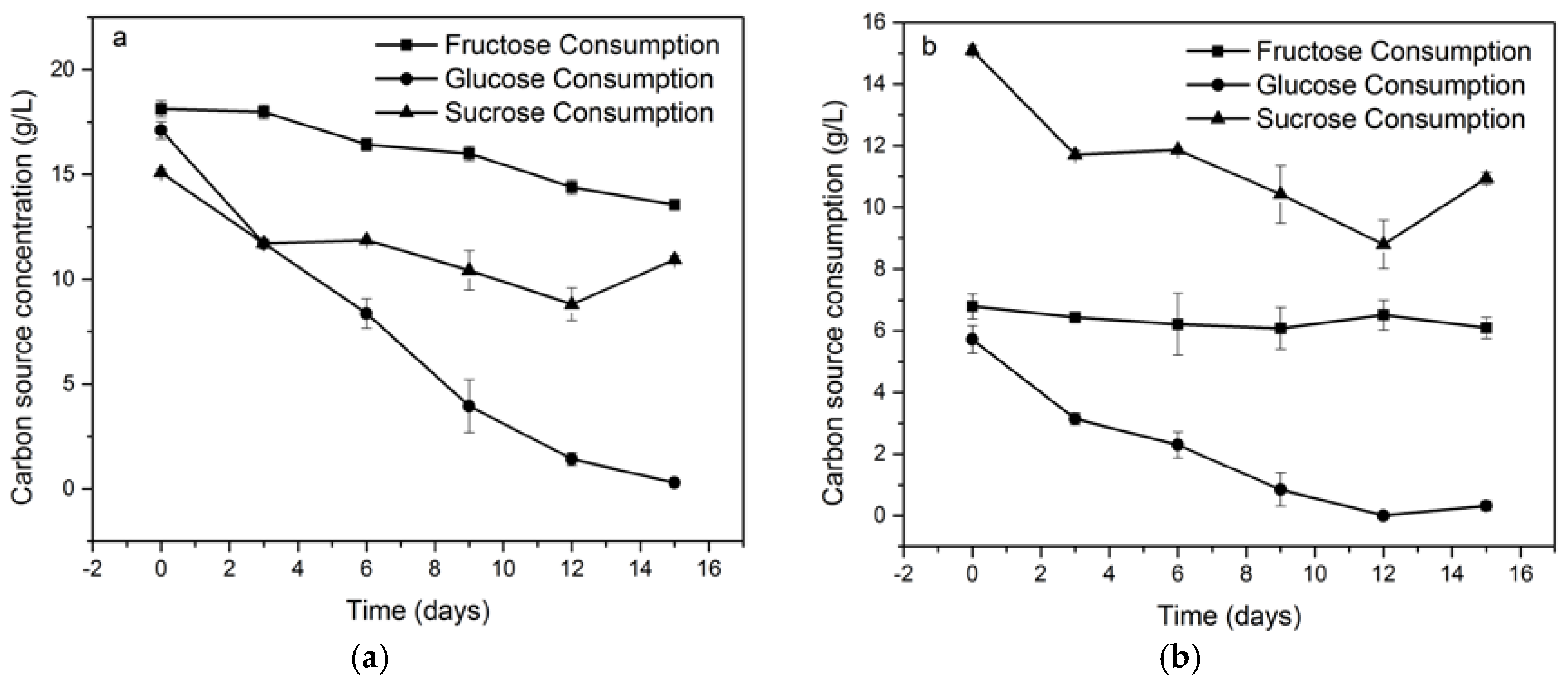
2.1. Effect of Carbon Source on Bacterial Cellulose Production
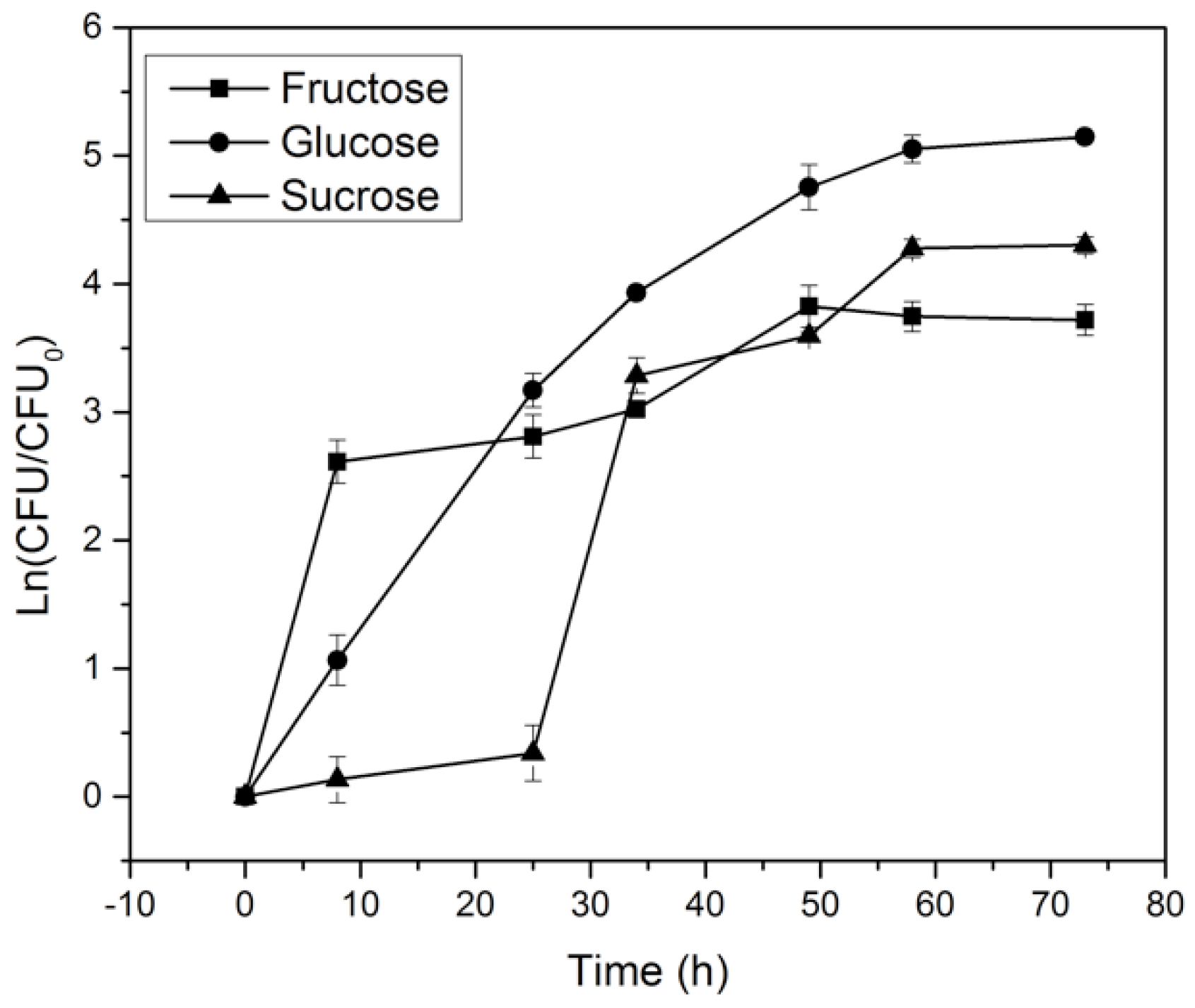
2.2. Effect of Carbon Source on K. medellinensis Growth
2.3. BC Film Characterization
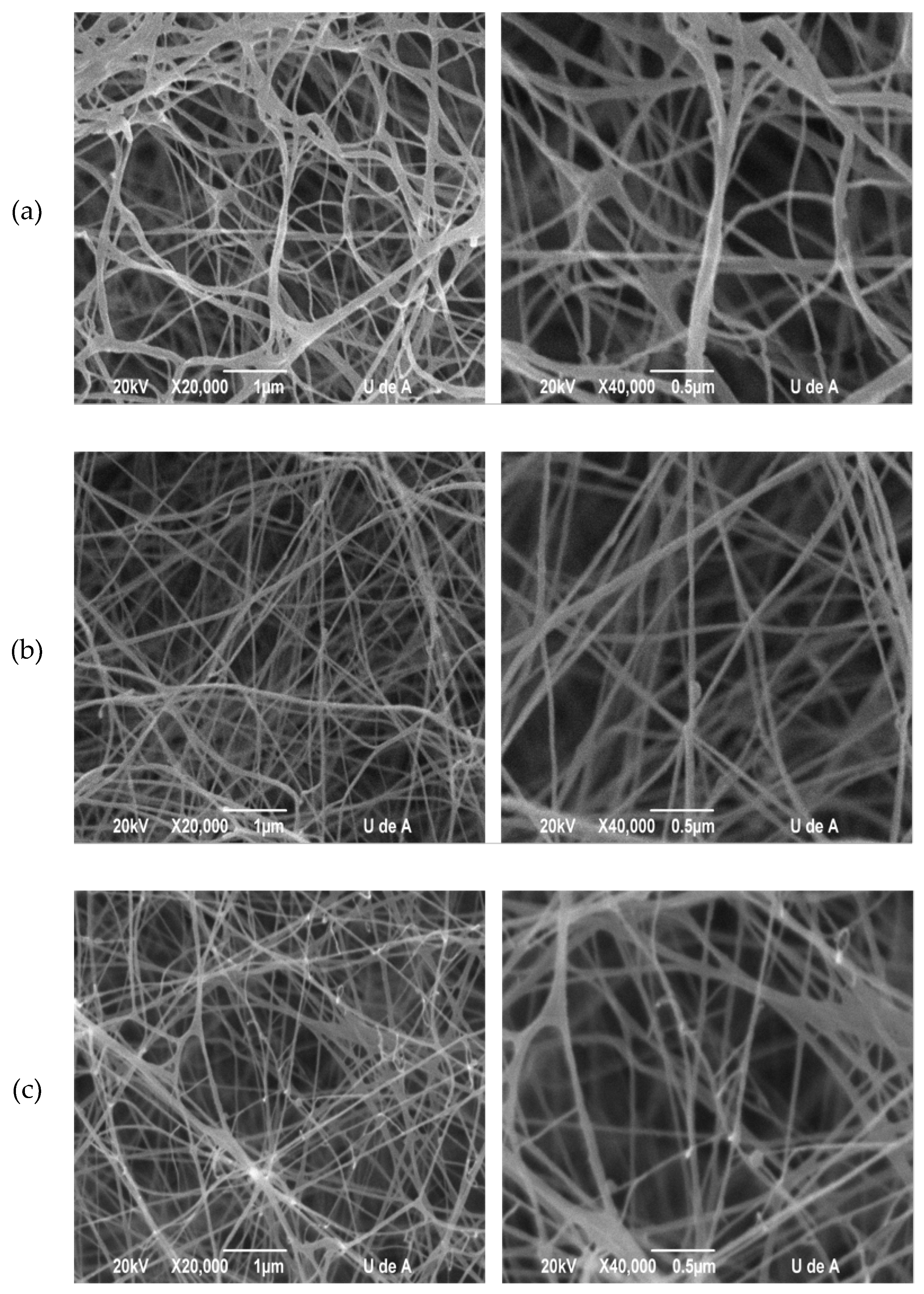
2.3.1. Scanning Electronic Microscopy (SEM)
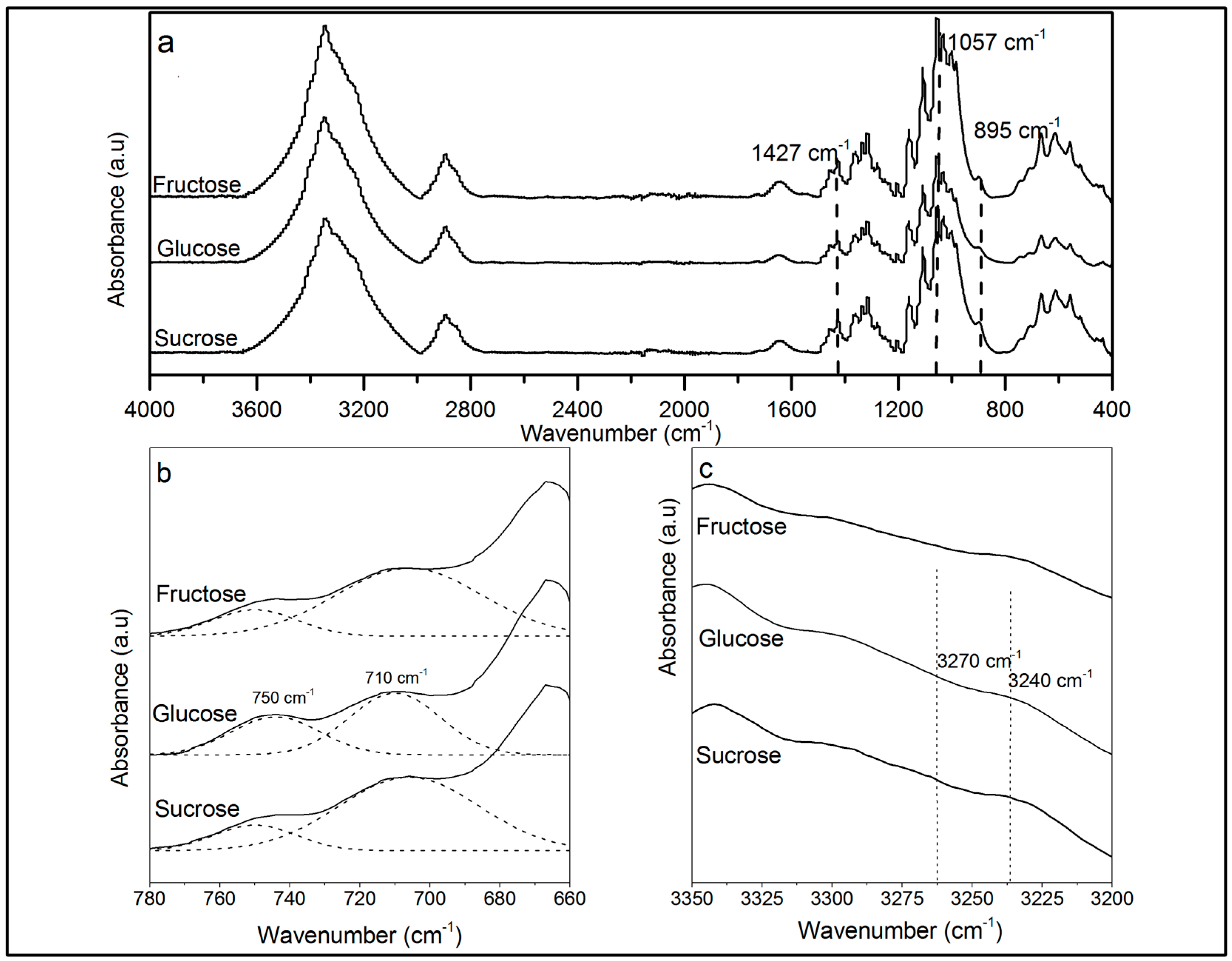
2.3.2. Attenuated Total Reflection Fourier Transformed Infrared Spectroscopy (ATR-FTIR)
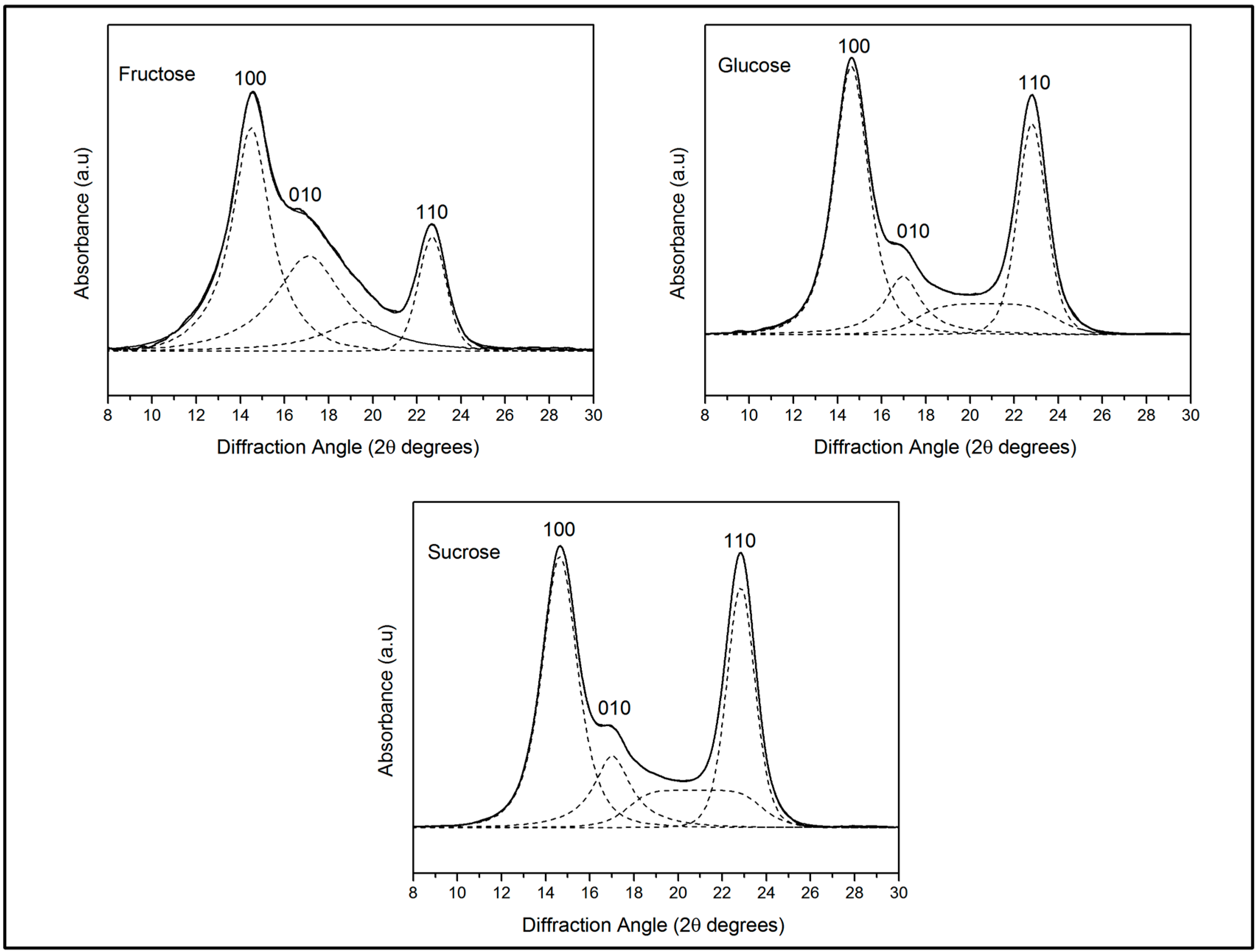
2.3.3. X-ray Diffraction
3. Discussion
4. Materials and Methods
4.1. Culture Media and Growth Conditions
4.2. Biomass Quantification
4.3. Consumption Dynamics of Carbon Sources
4.4. Bacterial Cellulose Characterization
4.5. Scanning Electronic Microscopy (SEM)
4.6. Attenuated Total Reflection Fourier Transformed Infrared Spectroscopy
4.7. X-ray Diffraction
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Varshney, V.K.; Naithani, S. Chemical functionalization of cellulose derived from nonconventional sources. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–60. [Google Scholar]
- Shoda, M.; Sugano, Y. Recent advances in bacterial cellulose production. Biotechnol. Bioprocess. Eng. 2005, 10, 1–8. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [PubMed]
- Yamada, Y. Transfer of Gluconacetobacter kakiaceti, Gluconacetobacter medellinensis and Gluconacetobacter maltaceti to the genus Komagataeibacter as Komagataeibacter kakiaceti comb. nov., Komagataeibacter medellinensis comb. nov. and Komagataeibacter maltaceti comb. Int. J. Syst. Evol. Microbiol. 2014, 64, 1670–1672. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, S.; Krystynowicz, A.; Turkiewicz, M.; Kalinowska, H. Bacterial cellulose. In Biopolymers Online; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohydr. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- El-Saied, H.; Basta, A.H.; Gobran, R.H. Research Progress in Friendly Environmental Technology for the Production of Cellulose Products (Bacterial Cellulose and Its Application). Polym. Plast. Technol. Eng. 2004, 43, 797–820. [Google Scholar] [CrossRef]
- Jung, H.I.; Jeong, J.H.; Lee, O.M.; Park, G.T.; Kim, K.K.; Park, H.C.; Lee, S.M.; Kim, Y.G.; Son, H.J. Influence of glycerol on production and structural-physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour. Technol. 2010, 101, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.M.A.S.; Sameshima, K. Evaluation of different carbon sources for bacterial cellulose production. Afr. J. Biotechnol. 2005, 4, 478–482. [Google Scholar]
- Max, B.; Salgado, J.M.; Rodríguez, N.; Cortés, S.; Converti, A.; Domínguez, J.M. Biotechnological production of citric acid. Brazilian J. Microbiol. 2010, 41, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Barja, F.; Andrés-Barrao, C.; Ortega Pérez, R.; Cabello, E.M.; Chappuis, M.-L. Physiology of Komagataeibacter spp. During Acetic Acid Fermentation. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 201–221. [Google Scholar]
- Zahan, K.A.; Pa’e, N.; Muhamad, I.I. Monitoring the Effect of pH on Bacterial Cellulose Production and Acetobacter xylinum 0416 Growth in a Rotary Discs Reactor. Arab. J. Sci. Eng. 2015, 40, 1881–1885. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Caro, G.; Gañán, P. Proceso Para Producer Celulosa Bacateriana en Presencia de un Agente Químico de Entrecruzamiento. WO2015028968A1, 5 March 2015. [Google Scholar]
- Castro, C.; Cleenwerck, I.; Zuluaga, R.; Caro, G.; Putaux, J.-L.; Rojas, O.J.; Gañán, P. Production of Bacterial Cellulose: Use of a New Strain of Microorganism. In Handbook of Green Materials; WORLD SCIENTIFIC: Singapore, 2014; pp. 105–122. [Google Scholar]
- Castro, C.; Cleenwerck, I.; Trcek, J.; Zuluaga, R.; De Vos, P.; Caro, G.; Aguirre, R.; Putaux, J.L.; Ganan, P. Gluconacetobacter medellinensis sp. nov., cellulose- and non-cellulose-producing acetic acid bacteria isolated from vinegar. Int. J. Syst. Evol. Microbiol. 2013, 63, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- El-Saied, H.; El-Diwany, A.I.; Basta, A.H.; Atwa, N.A.; El-Ghwas, D.E. Production and characterization of economical bacterial cellulose. BioResources 2008, 3, 1196–1217. [Google Scholar]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Tomar, A.; Singh, L. Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World J. Microbiol. Biotechnol. 2000, 16, 245–248. [Google Scholar] [CrossRef]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr. Polym. 2012, 89, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, S.A.; León, R.V.; O’neill, H.M.; Evans, B.R. Statistical analysis of optimal culture conditions for Gluconacetobacter hansenii cellulose production. Lett. Appl. Microbiol. 2007, 44, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.N.; Wee, Y.J.; Park, D.H.; Ryu, H.W. Production of bacterial cellulose by Gluconacetobacter sp. RKY5 isolated from persimmon vinegar. Appl. Biochem. Biotechnol. 2006, 131, 705–715. [Google Scholar] [CrossRef]
- Masaoka, S.; Ohe, T.; Sakota, N. Production of cellulose from glucose by Acetobacter xylinum. J. Ferment. Bioeng. 1993, 75, 18–22. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Appaiah, A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Ann. Microbiol. 2011, 61, 781–787. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R. Malcolm Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial Cellulose: Fermentative Production and Applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural Features and Properties of Bacterial Cellulose Produced in Agitated Culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Zuluaga, R.; Alvarez, C.; Putaux, J.L.; Caro, G.; Rojas, O.J.; Mondragon, I.; Ganan, P. Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydr. Polym. 2012, 89, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E.; Marks, J.S.; BeMiller, J.N. Bacterial cellulose. II. Optimization of cellulose production by Acetobacter xylinum through response surface methodology. Food Hydrocoll. 1994, 8, 419–430. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Forziati, F.H.; Rowen, J.W. Effect of changes in crystalline structure on absorption spectrum of cellulose. J. Res. Natl. Bur. Stand. 1951, 46, 38–42. [Google Scholar] [CrossRef]
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. II. Native celluloses in the region from 640 to 1700 cm−1. J. Polym. Sci. 1959, 39, 269–278. [Google Scholar] [CrossRef]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined infrared and electron diffraction study of the polymorphism of native celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Yousefi, A. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran. J. Biotechnol. 2011, 9, 94–101. [Google Scholar]
- Goelzer, F.D.E.; Faria-Tischer, P.C.S.; Vitorino, J.C.; Sierakowski, M.R.; Tischer, C.A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater. Sci. Eng. C. 2009, 29, 546–551. [Google Scholar] [CrossRef]
- Römling, U. Molecular biology of cellulose production in bacteria. Res. Microbiol. 2002, 153, 205–212. [Google Scholar] [CrossRef]
- Benziman, M.; Rivetz, B. Factors affecting hexose phosphorylation in Acetobacter xylinum. J. Bacteriol. 1972, 111, 325–333. [Google Scholar] [PubMed]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [PubMed]
- Seesuriyachan, P.; Kuntiya, A.; Hanmoungjai, P.; Techapun, C.; Chaiyaso, T.; Leksawasdi, N. Optimization of exopolysaccharide overproduction by Lactobacillus confusus in solid state fermentation under high salinity stress. Biosci. Biotechnol. Biochem. 2012, 76, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Azuma, Y.; Hosoyama, A.; Nakazawa, H.; Matsutani, M.; Hasegawa, A.; Otsuyama, K.; Matsushita, K.; Fujita, N.; Shirai, M. Complete genome sequence of NBRC 3288, a unique cellulose-nonproducing strain of Gluconacetobacter xylinus isolated from vinegar. J. Bacteriol. 2011, 193, 6997–6998. [Google Scholar] [CrossRef] [PubMed]
- Komagataeibacter Medellinensis. Kyoto Encyclopedia of Genomes and Genes KEGG GENOME. Available online: http://www.genome.jp/kegg-bin/show_organism?menu_type=pathway_maps&org=gxy (accessed on 20 April 2017).
- Gama, F.; Gatenholm, P.; Klemm, D. (Eds.) Bacterial Nanocellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Tahara, N.; Tabuchi, M.; Watanabe, K.; Yano, H.; Morinaga, Y.; Yoshinaga, F. Degree of Polymerization of Cellulose from Acetobacter xylinum BPR2001 Decreased by Cellulase Produced by the Strain. Biosci. Biotechnol. Biochem. 1997, 61, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Sugiyama, J. Nanodomains of Iα and Iβ Cellulose in Algal Microfibrils. Macromolecules 1998, 31, 6275–6279. [Google Scholar] [CrossRef]

| Carbon Source | Product-Substrate Yield (g/g) |
|---|---|
| Fructose | 0.20 |
| Glucose | 0.19 |
| Sucrose | 0.59 |
| Carbon Source | Porosity (%) |
|---|---|
| Fructose | 45.23 (±1.92) |
| Glucose | 60.07 (±0.55) |
| Sucrose | 54.70 (±1.47) |
| Substrate | Allomorph Iα Fraction Estimation (A750/A710) |
|---|---|
| Fructose | 0.70 (±0.02) |
| Glucose | 0.74 (±0.05) |
| Sucrose | 0.71 (±0.00) |
| Substrate | 100 | 010 | 110 | CI | |||
|---|---|---|---|---|---|---|---|
| D-Spacing (nm) | ACS (nm) | D-Spacing (nm) | ACS (nm) | D-Spacing (nm) | ACS (nm) | ||
| Fructose | 0.60 | 8.83 | 0.51 | 8.07 | 0.39 | 10.66 | 0.90 (±0.02) |
| Glucose | 0.60 | 8.66 | 0.52 | 8.33 | 0.39 | 10.52 | 0.83 (±0.03) |
| Sucrose | 0.60 | 8.43 | 0.52 | 8.05 | 0.38 | 10.65 | 0.85 (±0.06) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Ramírez, C.; Castro, M.; Osorio, M.; Torres-Taborda, M.; Gómez, B.; Zuluaga, R.; Gómez, C.; Gañán, P.; Rojas, O.J.; Castro, C. Effect of Different Carbon Sources on Bacterial Nanocellulose Production and Structure Using the Low pH Resistant Strain Komagataeibacter Medellinensis. Materials 2017, 10, 639. https://doi.org/10.3390/ma10060639
Molina-Ramírez C, Castro M, Osorio M, Torres-Taborda M, Gómez B, Zuluaga R, Gómez C, Gañán P, Rojas OJ, Castro C. Effect of Different Carbon Sources on Bacterial Nanocellulose Production and Structure Using the Low pH Resistant Strain Komagataeibacter Medellinensis. Materials. 2017; 10(6):639. https://doi.org/10.3390/ma10060639
Chicago/Turabian StyleMolina-Ramírez, Carlos, Margarita Castro, Marlon Osorio, Mabel Torres-Taborda, Beatriz Gómez, Robin Zuluaga, Catalina Gómez, Piedad Gañán, Orlando J. Rojas, and Cristina Castro. 2017. "Effect of Different Carbon Sources on Bacterial Nanocellulose Production and Structure Using the Low pH Resistant Strain Komagataeibacter Medellinensis" Materials 10, no. 6: 639. https://doi.org/10.3390/ma10060639








