Porous Materials from Thermally Activated Kaolinite: Preparation, Characterization and Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Porous Materials
2.2.2. Adsorption Tests
2.2.3. Instrumental Techniques
3. Results and Discussion
3.1. Preparation of Porous Materials
3.2. Characterization of Porous Materials
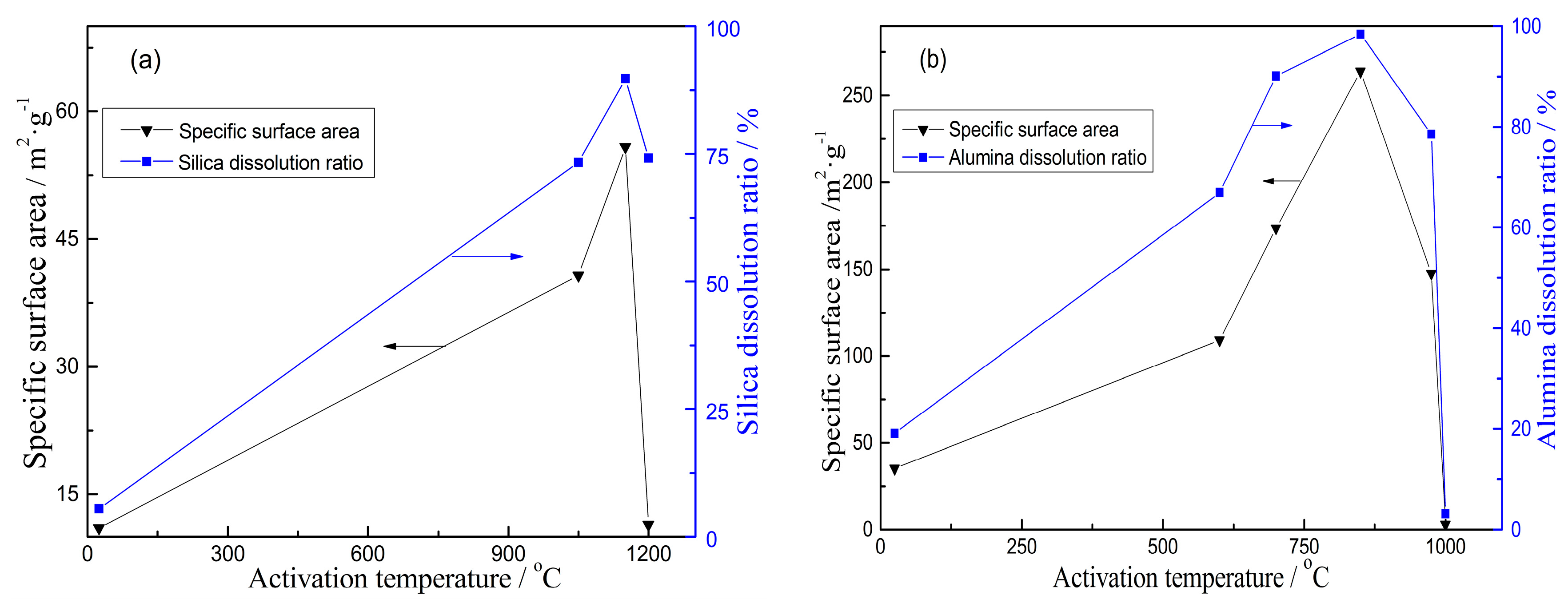
3.2.1. Specific Surface Area
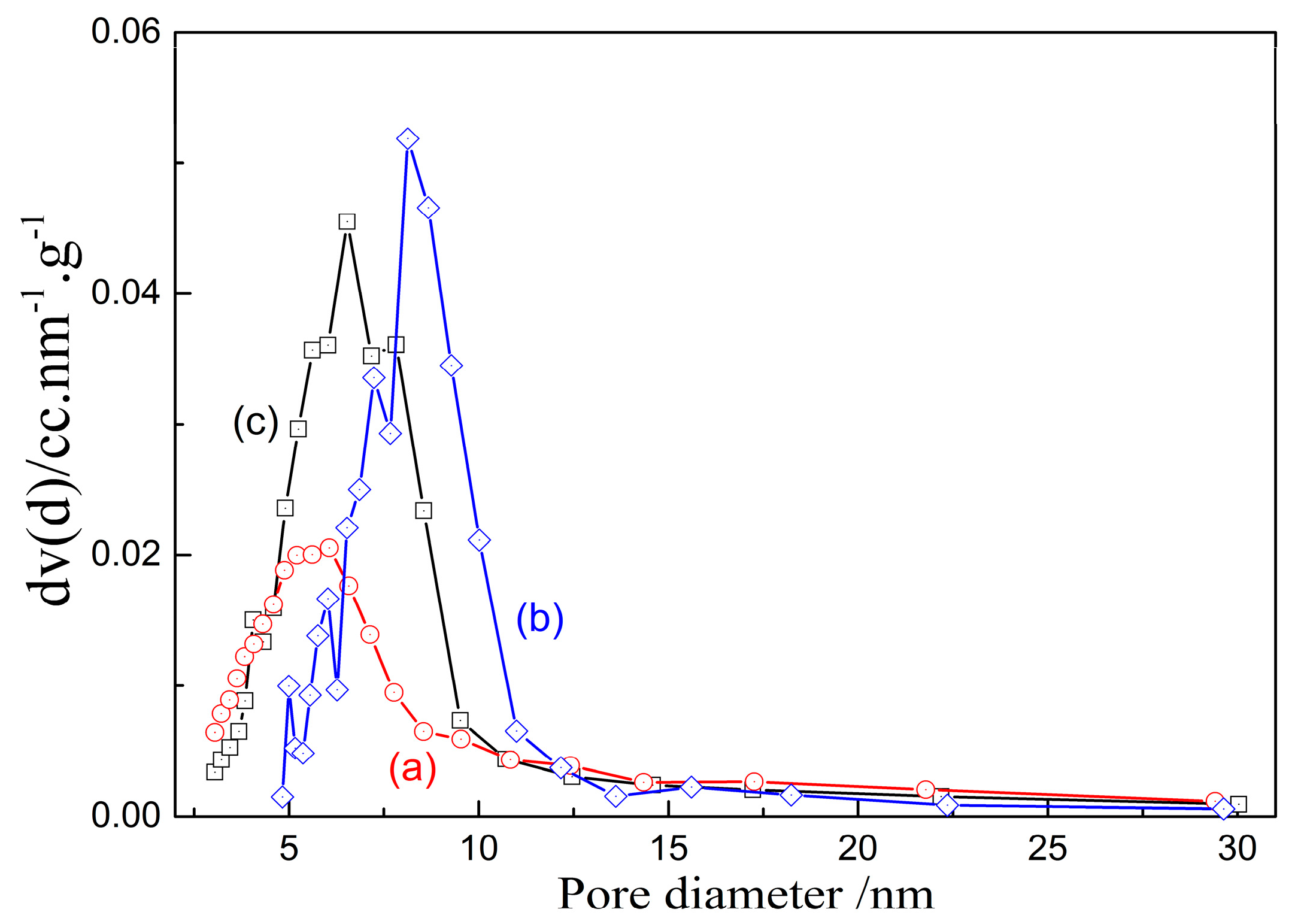
3.2.2. Pore Size
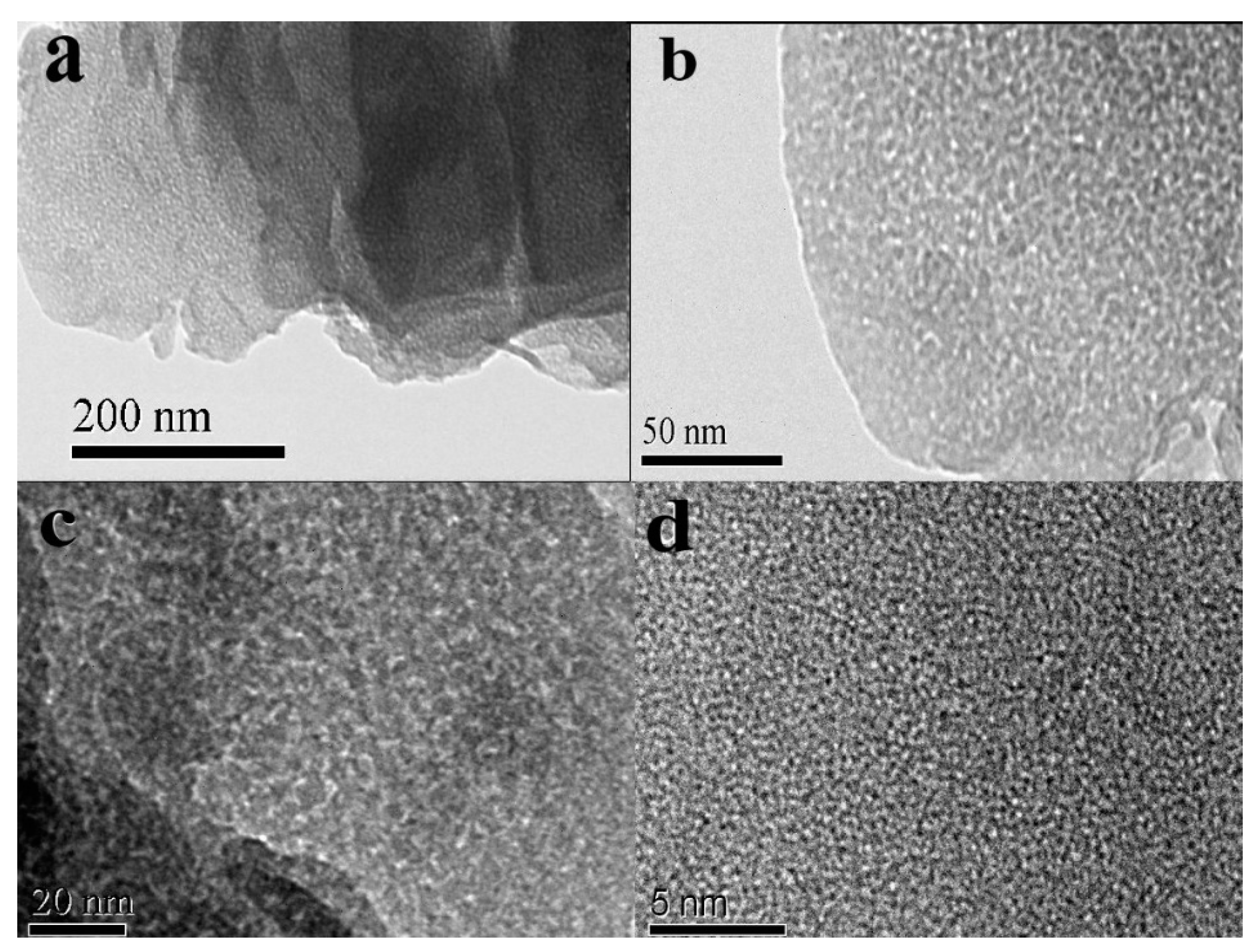
3.2.3. Microstructure
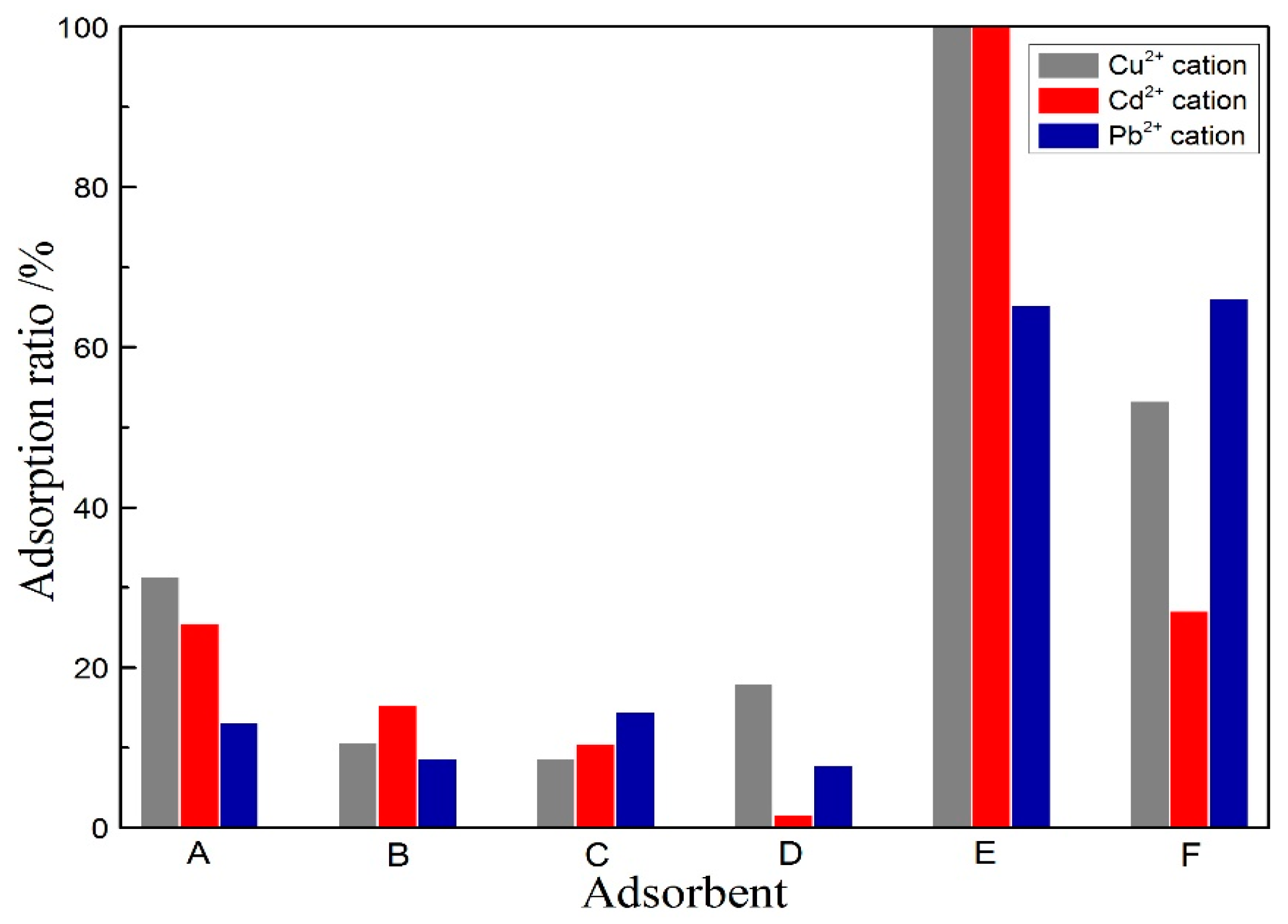
3.3. Adsorption of Cu2+, Cd2+ and Pb2+ in Aqueous Solution Using Porous Materials
4. Conclusions
- (1)
- The characterization of prepared porous materials indicates that their specific surface area (SSA) increases with the increasing dissolution of alumina/silica in aqueous leaching solution from activated kaolinite. The pore volume of porous alumina decreases with the increasing dissolution of silica, while there is no correlation between pore volume of porous silica and alumina dissolution of activated kaolinite. The prepared porous alumina/silica materials belong to the mesoporous materials, despite the slight change of their pore size with the dissolution of activated kaolinite.
- (2)
- When the porous alumina is obtained via alkali leaching of kaolinite activated at 1150 °C for 15 min, the SSA, BJH pore size and pore volume are 55.8 m2/g, 6.06 nm and 0.1455 mL/g, respectively. For the porous silica prepared via acid leaching of kaolinite activated at 850 °C for 15 min, these values are 280.3 m2/g, 3.06 nm and 0.1945 mL/g, respectively. The adsorption tests confirm that the prepared porous alumina has a superior adsorption of Cu2+, Pb2+ and Cd2+, with maximums of 134 mg/g, 183 mg/g and 195 mg/g, respectively. However, the porous silica has difficulty adsorbing the above-mentioned heavy metal ions.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar] [CrossRef]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Keng, P.S.; Lee, S.L.; Ha, S.T.; Hung, Y.T.; Ong, S.T. Removal of hazardous heavy metals from aqueous environment by low-cost adsorption materials. Environ. Chem. Lett. 2014, 12, 15–25. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef]
- dos Anjos, V.E.; Rohwedder, J.R.; Cadore, S.; Abate, G.; Grassi, M.T. Montmorillonite and vermiculite as solid phases for the preconcentration of trace elements in natural waters: Adsorption and desorption studies of As, Ba, Cu, Cd, Co, Cr, Mn, Ni, Pb, Sr, V, and Zn. Appl. Clay Sci. 2014, 99, 289–296. [Google Scholar] [CrossRef]
- Filatova, E.G.; Pozhidaev, Yu.N.; Pomazkina, O.I. Investigation of adsorption of heavy metal ions by natural aluminosilicate. Protect. Met. Phys. Chem. Surf. 2016, 52, 438–442. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; Ferreira da Silva, E.; Velosa, A.; Rocha, F. The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Burch, R. Pillared interlayer clays. Appl. Catal. 1984, 12, 284–285. [Google Scholar] [CrossRef]
- Yang, S.; Liang, G.; Gu, A.; Mao, H. Facile synthesis and catalytic performance of Fe-containing silica-pillared clay derivatives with ordered interlayer mesoporous structure. Ind. Eng. Chem. Res. 2012, 51, 15593–15600. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, S.; Zuo, P. Horseradish peroxidase immobilized on aluminum-pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res. 2006, 40, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Perathoner, S. Catalysis by layered materials: A review. Microporous Mesoporous Mater. 2008, 107, 3–15. [Google Scholar] [CrossRef]
- Stefanova, R.Y. Metal removal by thermally activated clay marl. J. Environ. Sci. Health A 2001, 36, 293–306. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface 2008, 140, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, C.R.; Jacob, K.J.; Gray, A.C. Enhancement of Lewis acidity in layer aluminosilicates. Treatment with acetic acid. J. Chem. Soc. Faraday Trans. 1988, 84, 1509–1515. [Google Scholar] [CrossRef]
- Ravichandran, J. Properties and catalytic activity of acid-modified montmorillonite and vermiculite. Clays Clay Miner. 1997, 45, 854–858. [Google Scholar] [CrossRef]
- Breen, C.; Madejová, J.; Komadel, P. Correlation of catalytic activity with infra-red, 29Si MAS NMR and acidity data for HCl-treated fine fractions of montmorillonites. Appl. Clay Sci. 1995, 10, 219–230. [Google Scholar] [CrossRef]
- Suraj, G.; Iyer, C.S.P.; Lalithambika, M. Adsorption of cadmium and copper by modified kaolinites. Appl. Clay Sci. 1998, 13, 293–306. [Google Scholar] [CrossRef]
- Okada, K.; Kawashima, H.; Saito, Y.; Hayashi, S.; Yasumori, A. New preparation method for mesoporous γ-alumina by selective leaching of calcined kaolin minerals. J. Mater. Chem. 1995, 5, 1241–1244. [Google Scholar] [CrossRef]
- Okada, K.; Shimai, A.; Takei, T.; Hayashi, S.; Yasumori, A.; MacKenzie, K.J.D. Preparation of microporous silica from metakaolinite by selective leaching method. Microporous Mesoporous Mater. 1998, 21, 289–296. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K.J.D.; Jadambaa, T. Charaterization of porous silica prepared from mechanically amorphized kaolinite by selective leaching. Powder Technol. 2001, 121, 259–262. [Google Scholar] [CrossRef]
- Makó, É.; Senkár, Z.; Kristóf, J.; Vágvölgyi, V. Surface modification of mechanochemically activated kaolinites by selective leaching. J. Colloid Interface Sci. 2006, 294, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, A.G.S.; Castelló, R.; Luengo, M.A.M.; Vizcayno, C. Acid activation of mechanically and thermally modified kaolins. Mater. Res. Bull. 2009, 44, 2103–2111. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; Jadambaa, T.; Mackenzie, K.J.D.; Amarsanaa, J. Effect of grinding on the preparation of porous material from talc by selective leaching. J. Mater. Sci. Lett. 2002, 21, 1607–1609. [Google Scholar] [CrossRef]
- Yang, H.; Du, C.; Hu, Y.; Jin, S.; Yang, W.; Tang, A.; Avvakumov, E.G. Preparation of porous material from talc by mechanochemical treatment and subsequent leaching. Appl. Clay Sci. 2006, 31, 290–297. [Google Scholar] [CrossRef]
- Li, G.; Luo, J.; Jiang, T.; Li, Z.; Peng, Z.; Zhang, Y. Digestion of alumina from non-magnetic material obtained from magnetic separation of reduced iron-rich diasporic bauxite with sodium salts. Metals 2016, 6, 294. [Google Scholar] [CrossRef]
- Brown, I.W.M.; Mackenzie, K.J.D.; Bowden, M.E.; Meinhold, R.H. Outstanding problems in the kaolinite-mullite reaction sequence investigated by 29Si and 27Al solid-state nuclear magnetic resonance: II, high-temperature transformations of metakaolinite. J. Am. Ceram. Soc. 1985, 68, 298–301. [Google Scholar] [CrossRef]
- Qiu, G.; Jiang, T.; Li, G.; Fan, X.; Huang, Z. Activation and removal of silicon in kaolinite by thermochemical process. Scand. J. Metall. 2004, 33, 121–128. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Havlica, J.; Brandštetr, J. The kinetic analysis of the thermal decomposition of kaolinite by DTG technique. Powder Technol. 2011, 208, 20–25. [Google Scholar] [CrossRef]
- Jiang, T.; Li, G.; Qiu, G.; Fan, X.; Huang, Z. Thermal activation and alkali dissolution of silicon from illite. Appl. Clay Sci. 2008, 40, 81–89. [Google Scholar] [CrossRef]
- Li, G.; Zeng, J.; Luo, J.; Liu, M.; Jiang, T.; Qiu, G. Thermal transformation of pyrophyllite and alkali dissolution behavior of silicon. Appl. Clay Sci. 2014, 99, 282–288. [Google Scholar] [CrossRef]
- Rahmani, A.; Mousavi, H.Z.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination 2010, 253, 94–100. [Google Scholar] [CrossRef]
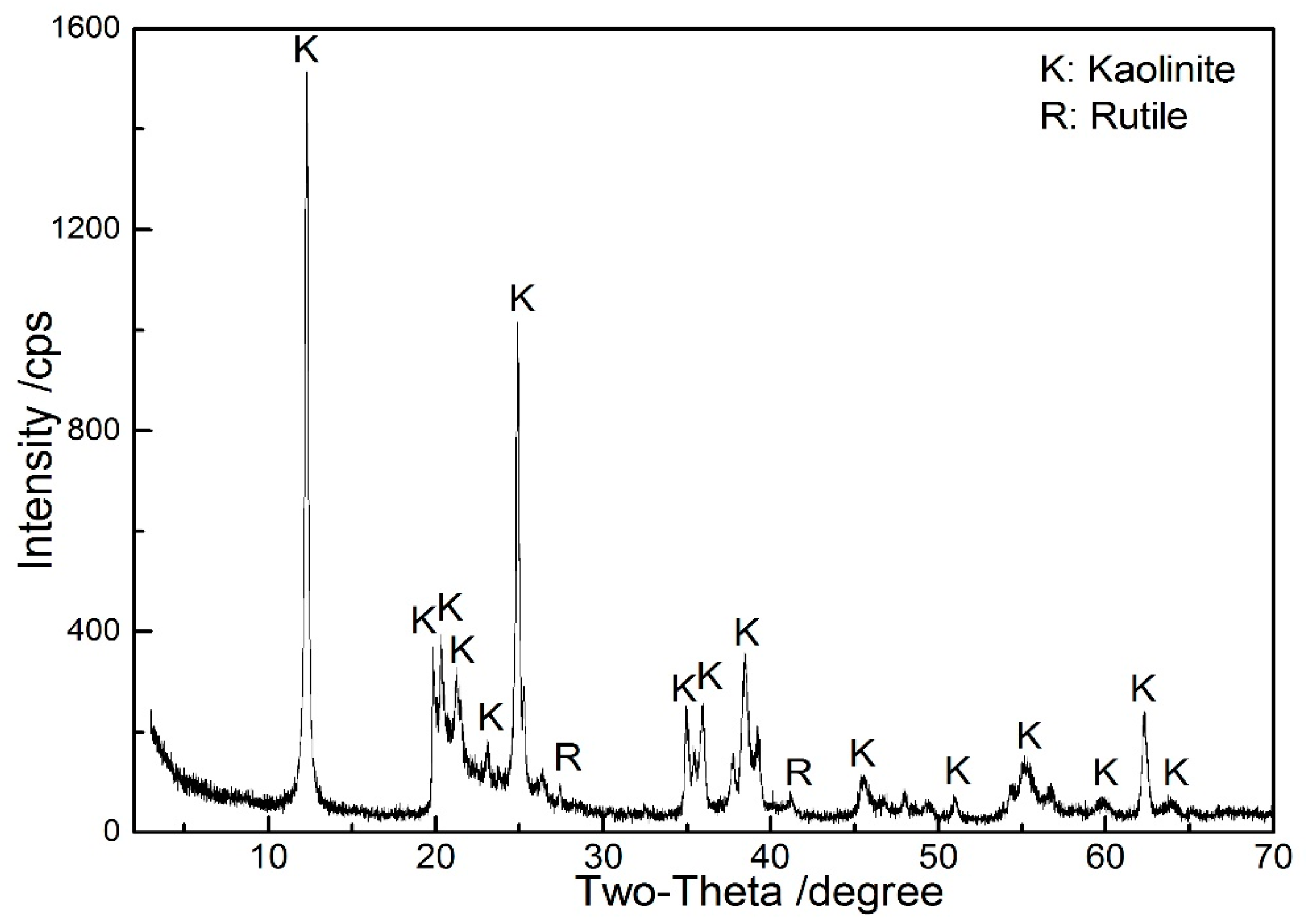
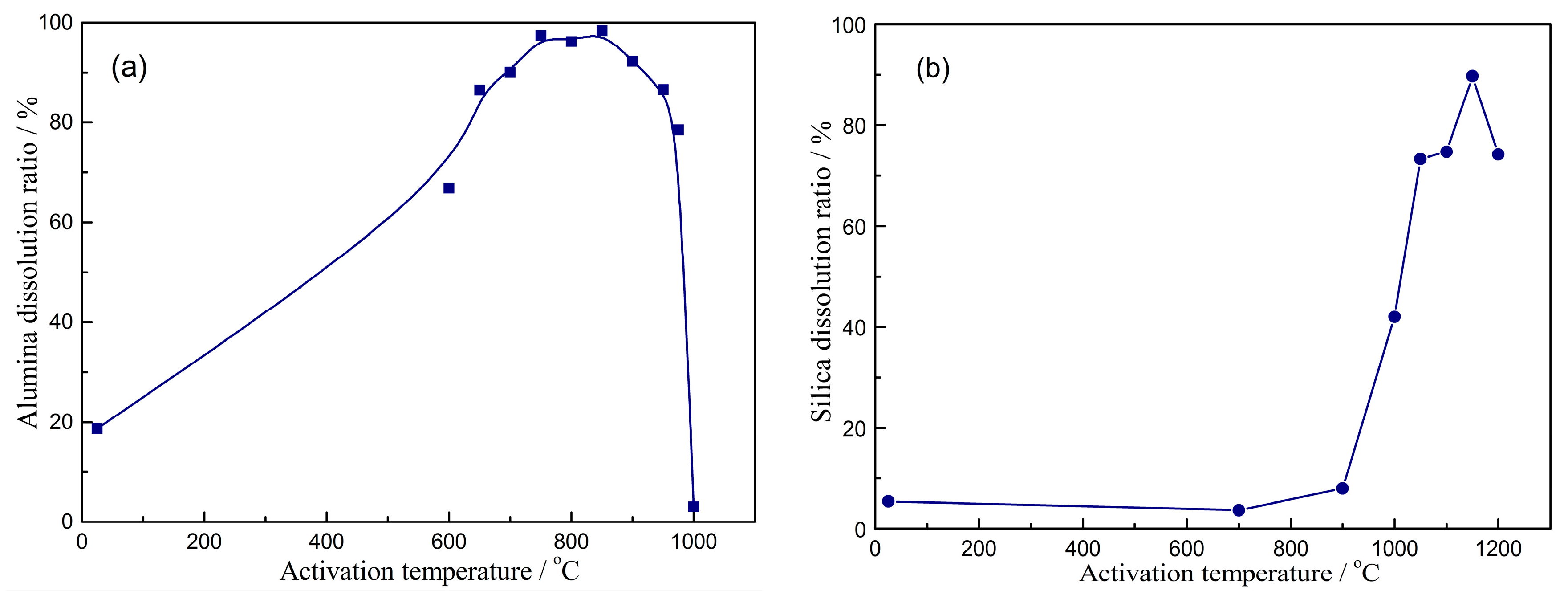
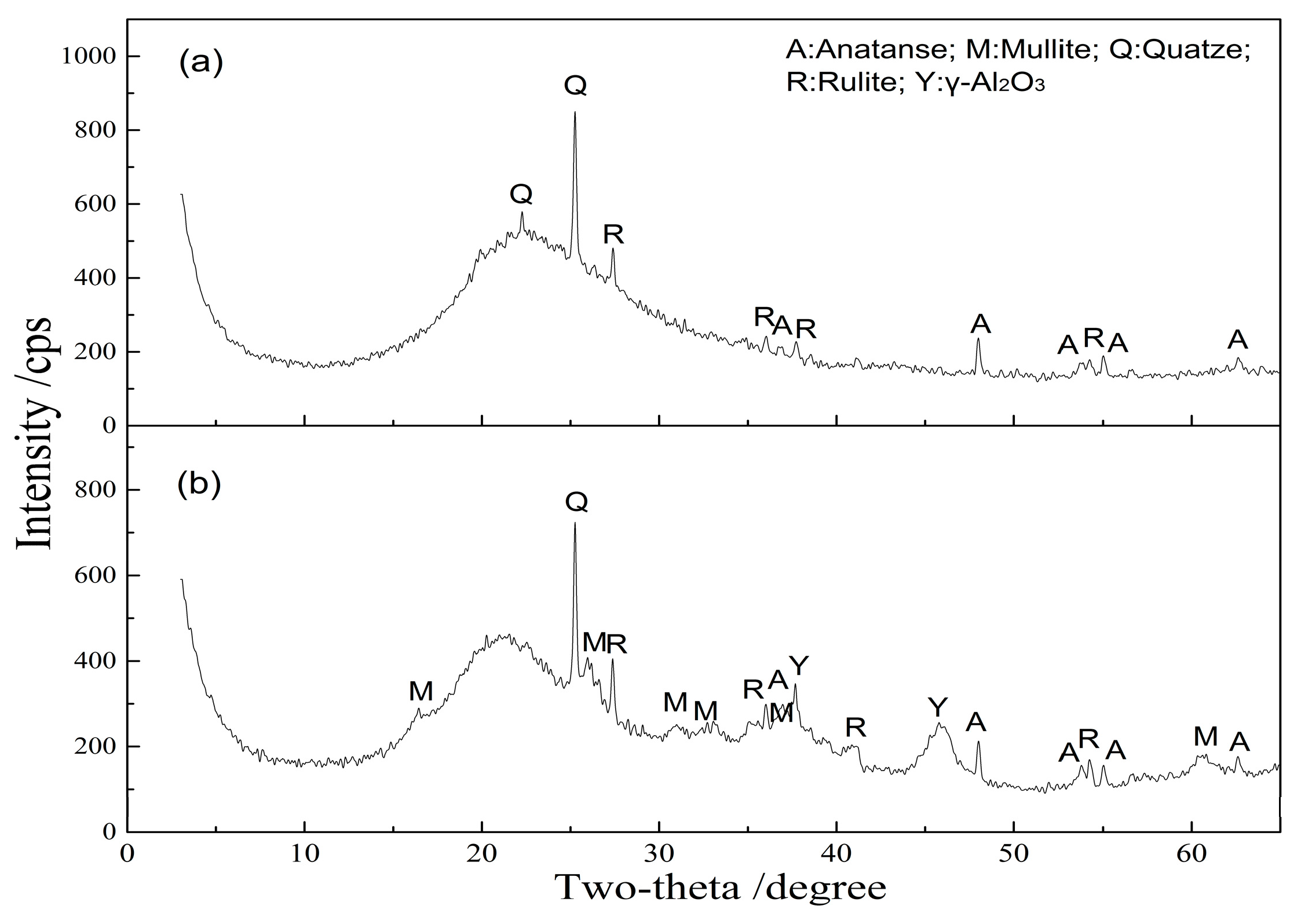
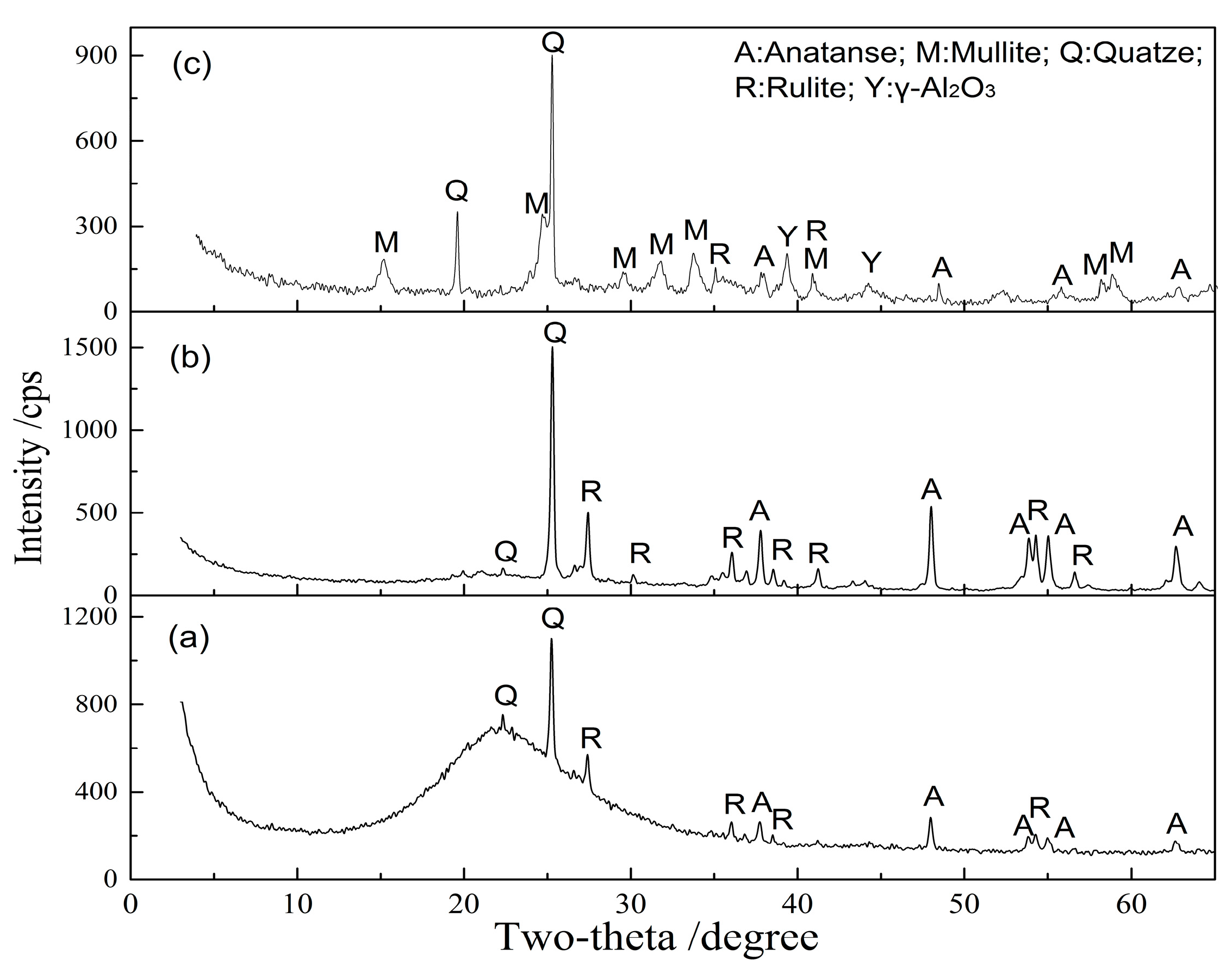



| Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | LOI 1 |
|---|---|---|---|---|---|---|---|---|
| 38.8 | 42.2 | 0.5 | 4.0 | 0.2 | 0.1 | 0.1 | 0.3 | 13.7 |
| Activation Temperature/°C | Silica Dissolution/% | BJH Pore Size/nm | Pore Volume/mL·g−1 |
|---|---|---|---|
| 900 | 8.9 | 6.54 | 0.2221 |
| 1050 | 73.3 | 6.57 | 0.2215 |
| 1150 | 89.7 | 6.06 | 0.1455 |
| Activation Temperature/°C | Alumina Dissolution/% | BJH Pore Size/nm | Pore Volume/mL·g−1 |
|---|---|---|---|
| 600 | 67 | 1.53 | 0.1963 |
| 850 | 98 | 3.05 | 0.1945 |
| 975 | 79 | 3.06 | 0.1313 |
| Number | Adsorbent |
|---|---|
| A | Raw kaolinite ore |
| B | Activated kaolinite at 850 °C for 15 min |
| C | Porous silica prepared via acid leaching of activated kaolinite at 850 °C for 15 min |
| D | Activated kaolinite at 1150 °C for 15 min |
| E | Porous alumina prepared via alkali leaching of activated kaolinite at 1150 °C for 15 min |
| F | Activated carbon |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Jiang, T.; Li, G.; Peng, Z.; Rao, M.; Zhang, Y. Porous Materials from Thermally Activated Kaolinite: Preparation, Characterization and Application. Materials 2017, 10, 647. https://doi.org/10.3390/ma10060647
Luo J, Jiang T, Li G, Peng Z, Rao M, Zhang Y. Porous Materials from Thermally Activated Kaolinite: Preparation, Characterization and Application. Materials. 2017; 10(6):647. https://doi.org/10.3390/ma10060647
Chicago/Turabian StyleLuo, Jun, Tao Jiang, Guanghui Li, Zhiwei Peng, Mingjun Rao, and Yuanbo Zhang. 2017. "Porous Materials from Thermally Activated Kaolinite: Preparation, Characterization and Application" Materials 10, no. 6: 647. https://doi.org/10.3390/ma10060647
APA StyleLuo, J., Jiang, T., Li, G., Peng, Z., Rao, M., & Zhang, Y. (2017). Porous Materials from Thermally Activated Kaolinite: Preparation, Characterization and Application. Materials, 10(6), 647. https://doi.org/10.3390/ma10060647








