Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review
Abstract
1. Introduction
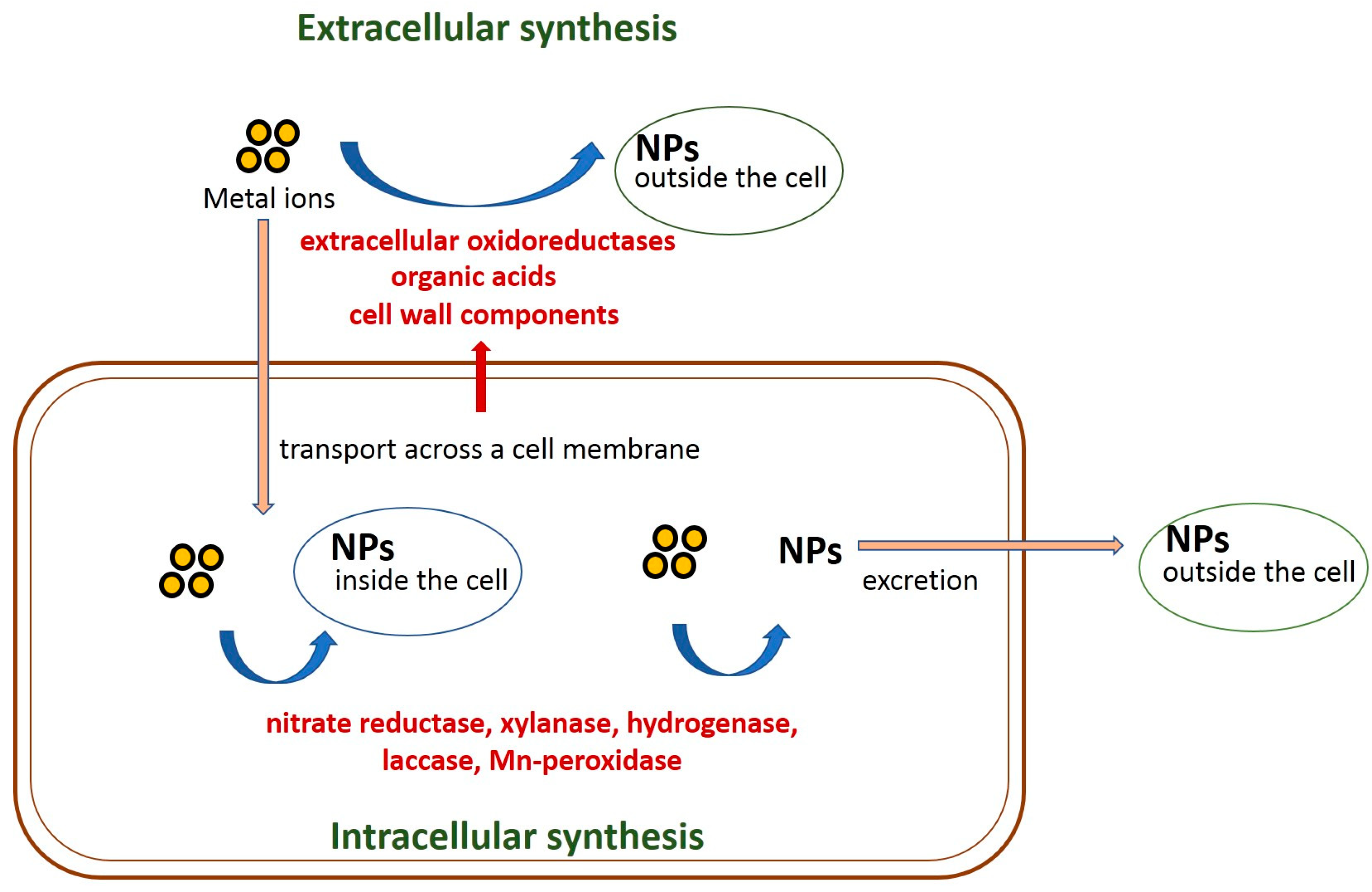
2. Fungi-Mediated Synthesis of Nanoparticles
2.1. Mycosynthesis of Silver Nanoparticles
2.2. Mycosynthesis of Gold Nanoparticles
2.3. Mycosynthesis of Platinum Nanoparticles
2.4. Mycosynthesis of Palladium Nanoparticles
2.5. Mycosynthesis of Copper Nanoparticles
2.6. Mycosynthesis of Iron Nanoparticles
2.7. Mycosynthesis of Selenium Nanoparticles
2.8. Mycosynthesis of Tellurium Nanoparticles
3. Mechanisms of Fungi-Mediated Nanoparticle Biosynthesis
4. Advantages of Fungi-Mediated Nanoparticle Synthesis and Prospects for Application of Mycogenic Nanoparticles
- Active production of reducing and capping compounds;
- High activity of enzymes involved in the bioreduction of various compounds resulting in nanoparticle formation;
- Resistance to high concentrations of metals and metalloids;
- Ability to biofabricate large quantities of nanoparticles mostly extracellularly;
- High speed of nanoparticle formation;
- Simplicity of cultivation, nanoparticle downstream processing and scaling up;
- Safety for human health (when using edible and medicinal mushrooms);
- Ability to produce nanoparticles with complex medical properties (when using medicinal mushrooms).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Jie Ying, A.N.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Krichevsky, G.E. Ecological «Green» biosynthesis of metal nanoparticles, reality and potential of their use in various fields of medicine. NBICS-Sci. Technol. 2018, 2, 85–106. [Google Scholar]
- Joseph, S.; Mathew, B. Microwave-assisted green synthesis of silver nanoparticles and the study on catalytic activity in the degradation of dyes. J. Mol. Liq. 2015, 204, 184–191. [Google Scholar] [CrossRef]
- Shakibaie, M.; Torabi-Shamsabad, R.; Forootanfar, H.; Amiri-Moghadam, P.; Amirheidari, B.; Adeli-Sardou, M.; Ameri, A. Rapid microwave-assisted biosynthesis of platinum nanoparticles and evaluation of their antioxidant properties and cytotoxic effects against MCF-7 and A549 cell lines. 3 Biotech 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.; Ayaz, M.; Ahmad, I.; Nethi, S.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Castro-Longoria, E. Fungal Biosynthesis of Nanoparticles, a Cleaner Alternative. In Fungal Applications in Sustainable Environmental Biotechnology; Purchase, D., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 323–351. [Google Scholar] [CrossRef]
- Majeed, A.; Ullah, W.; Anwar, A.W.; Shuaib, A.; Ilyas, U.; Khalid, P.; Mustafa, G.; Junaid, M.; Faheem, B.; Ali, S. Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: A review. Mater. Technol. 2018, 33, 313–320. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Bao, Z.; Lan, C.Q. Advances in biosynthesis of noble metal nanoparticles mediated by photosynthetic organisms—A review. Colloids Surf. B Biointerfaces 2019, 184, 110519. [Google Scholar] [CrossRef]
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Mashhadi Malekzadeh, A. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochemistry Res. 2018, 3, 1–16. [Google Scholar] [CrossRef]
- Singh, C.R.; Kathiresan, K.; Anandhan, S. A review on marine based nanoparticles and their potential applications. Afr. J. Biotechnol. 2015, 14, 1525–1532. [Google Scholar] [CrossRef]
- Jaganathan, A.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Dinesh, D.; Vadivalagan, C.; Aziz, A.T.; Chandramohan, B.; Suresh, U.; Rajaganesh, R.; et al. Earthworm-mediated synthesis of silver nanoparticles: A potent tool against hepatocellular carcinoma, Plasmodium falciparum parasites and malaria mosquitoes. Parasitol. Int. 2016, 65, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.-O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Azeez, M.A.; Alao, M.B.; Oke, A.M.; Aina, D.A. Fungi as veritable tool in current advances in nanobiotechnology. Heliyon 2021, 7, e08480. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Li, M.; Chen, C.; Gadd, G.M. Nanoparticle and nanomineral production by fungi. Fungal Biol. Rev. 2022, 41, 31–44. [Google Scholar] [CrossRef]
- Manimaran, M.; Kannabiran, K. Actinomycetes-mediated biogenic synthesis of metal and metal oxide nanoparticles: Progress and challenges. Lett. Appl. Microbiol. 2017, 64, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Tehri, N.; Gahlaut, A.; Hooda, V. Actinomycetes mediated synthesis, characterization, and applications of metallic nanoparticles. Inorg. Nano-Met. Chem. 2020, 51, 1386–1395. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-N.; Wang, R.; Ho, S.-H. Algae-mediated biosystems for metallic nanoparticle production: From synthetic mechanisms to aquatic environmental applications. J. Hazard. Mater. 2021, 420, 126625. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, G. Wang Biosynthesis of gold nanoparticles using chloroplasts. Int. J. Nanomed. 2011, 6, 2899. [Google Scholar] [CrossRef]
- Durán, M.; Silveira, C.P.; Durán, N. Catalytic role of traditional enzymes for biosynthesis of biogenic metallic nanoparticles: A mini-review. IET Nanobiotechnol. 2015, 9, 314–323. [Google Scholar] [CrossRef]
- Khanna, P.K.; Nair, C.K.K. Synthesis of Silver Nanoparticles Using Cod Liver Oil (Fish Oil): Green Approach to Nanotechnology. Int. J. Green Nanotechnol. Phys. Chem. 2009, 1, P3–P9. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, S.-H.; Govarthanan, M.; Hwang, P.-H.; Seo, Y.-S.; Cho, M.; Lee, W.-H.; Lee, J.-Y.; Kamala-Kannan, S.; Oh, B.-T. Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater. Lett. 2013, 105, 128–131. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef]
- González Fá, A.J.; Juan, A.; Di Nezio, M.S. Synthesis and Characterization of Silver Nanoparticles Prepared with Honey: The Role of Carbohydrates. Anal. Lett. 2017, 50, 877–888. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Valib, H.; Orsata, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Samanta, S.K.; Mondal, B.; Jana, S.; Ray, J.; Pandey, A.; Tripathy, T. Green synthesis of Ag@Au bimetallic composite nanoparticles using a polysaccharide extracted from Ramaria botrytis mushroom and performance in catalytic reduction of 4-nitrophenol and antioxidant, antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100341. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mejía-Rosales, S.; Deepak, F.L. Nanomaterial Properties: Size and Shape Dependencies. J. Nanomater. 2012, 2012, 180976. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Nikitina, V. Shape and Size Diversity of Gold, Silver, Selenium, and Silica Nanoparticles Prepared by Green Synthesis Using Fungi and Bacteria. Ind. Eng. Chem. Res. 2019, 58, 17207–17218. [Google Scholar] [CrossRef]
- Qu, M.; Yao, W.; Cui, X.; Xia, R.; Qin, L.; Liu, X. Biosynthesis of silver nanoparticles (AgNPs) employing Trichoderma strains to control empty-gut disease of oak silkworm (Antheraea pernyi). Mater. Today Commun. 2021, 28, 102619. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.H.A.; Zawawi, N.A.; Chundawat, T.S. Brief Review on Bioresources Green Synthesis of Silver Nanoparticles. J. Adv. Res. Mater. Sci. 2021, 76, 1–10. [Google Scholar]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Khan, S.; Fu, P. Fungus-(Alternaria sp.) Mediated Silver Nanoparticles Synthesis, Characterization, and Screening of Antifungal Activity against Some Phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Oladipo, I.C.; Adebayo, E.A.; Beukes, L.S.; Gueguim-Kana, E.B. Fungal xylanases-mediated synthesis of silver nanoparticles for catalytic and biomedical applications. IET Nanobiotechnol. 2018, 12, 857–863. [Google Scholar] [CrossRef]
- Nanda, A.; Nayak, B.K.; Krishnamoorthy, M. Antimicrobial properties of biogenic silver nanoparticles synthesized from phylloplane fungus, Aspergillus tamarii. Biocatal. Agric. Biotechnol. 2018, 16, 225–228. [Google Scholar] [CrossRef]
- Tyagi, S.; Tyagi, P.K.; Gola, D.; Chauhan, N.; Bharti, R.K. Extracellular synthesis of silver nanoparticles using entomopathogenic fungus: Characterization and antibacterial potential. SN Appl. Sci. 2019, 1, 1545. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Ping, L.Y.; Marcato, P.D.; Alves, O.L.; Silva, M.C.P.; Ruiz, R.C.; Melo, I.S.; Tasic, L.; De Souza, A.O. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl. Microbiol. Biotechnol. 2013, 97, 775–782. [Google Scholar] [CrossRef]
- Janakiraman, V.; Govindarajan, K.; CR, M. Biosynthesis of Silver Nanoparticles from Endophytic Fungi, and its Cytotoxic Activity. BioNanoScience 2019, 9, 573–579. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Possible Mosquito Control by Silver Nanoparticles Synthesized by Soil Fungus (Aspergillus niger 2587). Adv. Nanoparticles 2013, 02, 125–132. [Google Scholar] [CrossRef]
- Manjunath Hulikere, M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus- Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Azmath, P.; Baker, S.; Rakshith, D.; Satish, S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 2016, 24, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yu, H.; He, D.; Yang, H.; Wang, W.; Wan, X.; Wang, L. Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess Biosyst. Eng. 2013, 36, 1613–1619. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Ishida, K.; Cipriano, T.F.; Rocha, G.M.; Weissmüller, G.; Gomes, F.; Miranda, K.; Rozental, S. Silver nanoparticle production by the fungus Fusarium oxysporum: Nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem. Inst. Oswaldo Cruz 2013, 109, 220–228. [Google Scholar] [CrossRef]
- Jebali, A.; Ramezani, F.; Kazemi, B. Biosynthesis of Silver Nanoparticles by Geotricum sp. J. Clust. Sci. 2011, 22, 225–232. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Kalaichelvan, P.T. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef]
- Talie, M.D.; Hamid Wani, A.; Ahmad, N.; Yaqub Bhat, M.; Mohd War, J. Green synthesis of silver nanoparticles (AgNPs) using Helvella leucopus Pers. and their antimycotic activity against fungi causing fungal rot of apple. Asian J. Pharm. Clin. Res. 2020, 13, 161–165. [Google Scholar] [CrossRef]
- Varshney, R.; Mishra, A.N.; Bhadauria, S.; Gaur, M.S. A novel microbial route to synthesize silver nanoparticles using fungus Hormoconis resinae. J. Nanomater. Biostruct. 2009, 4, 349–355. [Google Scholar]
- Syed, A.; Saraswati, S.; Kundu, G.C.; Ahmad, A. Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 144–147. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 2014, 9, 365. [Google Scholar] [CrossRef]
- Quester, K.; Avalos-Borja, M.; Castro-Longoria, E. Controllable Biosynthesis of Small Silver Nanoparticles Using Fungal Extract. J. Biomater. Nanobiotechnology 2016, 07, 118–125. [Google Scholar] [CrossRef]
- Devi, L.; Joshi, S. Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. J. Microsc. Ultrastruct. 2015, 3, 29. [Google Scholar] [CrossRef]
- Danagoudar, A.; Pratap, G.K.; Shantaram, M.; Ghosh, K.; Kanade, S.R.; Joshi, C.G. Characterization, cytotoxic and antioxidant potential of silver nanoparticles biosynthesised using endophytic fungus (Penicillium citrinum CGJ-C1). Mater. Today Commun. 2020, 25, 101385. [Google Scholar] [CrossRef]
- Wanarska, E.; Maliszewska, I. The possible mechanism of the formation of silver nanoparticles by Penicillium cyclopium. Bioorganic Chem. 2019, 93, 102803. [Google Scholar] [CrossRef]
- Pareek, V.; Bhargava, A.; Panwar, J. Biomimetic approach for multifarious synthesis of nanoparticles using metal tolerant fungi: A mechanistic perspective. Mater. Sci. Eng. B 2020, 262, 114771. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Seetharaman, P.K.; Chandrasekaran, R.; Periakaruppan, R.; Gnanasekar, S.; Sivaperumal, S.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Functional Attributes of Myco-Synthesized Silver Nanoparticles from Endophytic Fungi: A New Implication in Biomedical Applications. Biology 2021, 10, 473. [Google Scholar] [CrossRef]
- Neethu, S.; Midhun, S.J.; Sunil, M.A.; Soumya, S.; Radhakrishnan, E.K.; Jyothis, M. Efficient visible light induced synthesis of silver nanoparticles by Penicillium polonicum ARA 10 isolated from Chetomorpha antennina and its antibacterial efficacy against Salmonella enterica serovar Typhimurium. J. Photochem. Photobiol. B Biol. 2018, 180, 175–185. [Google Scholar] [CrossRef]
- Raheman, F.; Deshmukh, S.; Ingle, A.; Gade, A.; Rai, M. Silver Nanoparticles: Novel Antimicrobial Agent Synthesized from an Endophytic Fungus Pestalotia sp. Isolated from Leaves of Syzygium cumini (L). Nano Biomed. Eng. 2011, 3, 174–178. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gade, A.; Duran, N. Synthesis of silver nanoparticles by Phoma gardeniae and in vitro evaluation of their efficacy against human disease-causing bacteria and fungi. IET Nanobiotechnol. 2015, 9, 71–75. [Google Scholar] [CrossRef]
- Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S.; Dheyab, M.A. Mycogenic fabrication of silver nanoparticles using Picoa, Pezizales, characterization and their antifungal activity. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100612. [Google Scholar] [CrossRef]
- Korbekandi, H.; Mohseni, S.; Mardani Jouneghani, R.; Pourhossein, M.; Iravani, S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif. Cells Nanomed. Biotechnol. 2016, 44, 235–239. [Google Scholar] [CrossRef]
- Saxena, J.; Sharma, P.K.; Sharma, M.M.; Singh, A. Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. SpringerPlus 2016, 5, 861. [Google Scholar] [CrossRef]
- Moustafa, M.T. Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci. 2017, 31, 164–176. [Google Scholar] [CrossRef]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.-H. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int. J. Nanomed. 2019, 14, 3427–3438. [Google Scholar] [CrossRef]
- Owaid, M. Biosynthesis of Silver Nanoparticles from Truffles and Mushrooms and Their Applications as Nanodrugs. Curr. Appl. Sci. Technol. 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized Synthesis of Small and Stable Silver Nanoparticles Using Intracellular and Extracellular Components of Fungi: An Alternative for Bacterial Inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef]
- Raja, M.; Sharma, R.K.; Jambhulkar, P.; Sharma, K.R.; Sharma, P. Biosynthesis of silver nanoparticles from Trichoderma harzianum Th3 and its efficacy against root rot complex pathogen in groundnut. Mater. Today Proc. 2021, 43, 3140–3143. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Wang, M.-H. Trichoderma based synthesis of anti-pathogenic silver nanoparticles and their characterization, antioxidant and cytotoxicity properties. Microb. Pathog. 2018, 114, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Apte, M.; Sambre, D.; Gaikawad, S.; Joshi, S.; Bankar, A.; Kumar, A.R.; Zinjarde, S. Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express 2013, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, C.A.; Simões, M.F.; Fernandes, S.; dos Santos, J.G.; da Silva, E.S.; de Souza, R.F.B.; Maiorano, A.E. Screening of filamentous fungi for antimicrobial silver nanoparticles synthesis. AMB Express 2017, 7, 31. [Google Scholar] [CrossRef]
- Klaus, A.; Petrovic, P.; Vunduk, J.; Pavlovic, V.; Van Griensven, L.J.L.D. The Antimicrobial Activities of Silver Nanoparticles Synthesized from Medicinal Mushrooms. Int. J. Med. Mushrooms 2020, 22, 869–883. [Google Scholar] [CrossRef]
- Owaid, M.N.; Naeem, G.A.; Muslim, R.F.; Oleiwi, R.S. Synthesis, characterization and antitumor efficacy of silver nanoparticle from Agaricus bisporus pileus, Basidiomycota. Walailak J. Sci. Technol. 2020, 17, 75–87. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Khaniabadi, P.M.; Kareem, A.A.; Alrosan, M.; Ali, A.T.; Rabeea, M.A.; Mehrdel, B. Mycosynthesis of ultrasonically-assisted uniform cubic silver nanoparticles by isolated phenols from Agaricus bisporus and its antibacterial activity. Surf. Interfaces 2022, 29, 101774. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B. Edible mushroom extract engineered Ag NPs as safe antimicrobial and antioxidant agents with no significant cytotoxicity on human dermal fibroblast cells. Inorg. Chem. Commun. 2022, 139, 109362. [Google Scholar] [CrossRef]
- Abikoye, E.T.; Oloke, J.K.; Elemo, G.; Okorie, P.C.; Aier, S.; Oluwawole, O.F.; Barooah, M. Biosynthesis of silver nanoparticles in improved strain of Auricularia polytricha—An edible mushroom from Nigeria and its antimicrobial activities. Covenant J. Phys. Life Sci. (Spec. Ed.) 2019, 7, 47–55. [Google Scholar]
- Osorio-Echavarría, J.; Osorio-Echavarría, J.; Ossa-Orozco, C.P.; Gómez-Vanegas, N.A. Synthesis of silver nanoparticles using white-rot fungus Anamorphous Bjerkandera sp. R1: Influence of silver nitrate concentration and fungus growth time. Sci. Rep. 2021, 11, 3842. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Ö.; Gökşen Tosun, N.; Özgür, A.; Erden Tayhan, S.; Bilgin, S.; Türkekul, İ.; Gökce, İ. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: Characterization, anticancer, antimicrobial and wound healing activities. J. Drug Deliv. Sci. Technol. 2021, 64, 102641. [Google Scholar] [CrossRef]
- Mirunalini, S.; Arulmozhi, V.; Deepalakshmi, K.; Krishnaveni, M. Intracellular Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Edible Mushrooms. Not. Sci. Biol. 2012, 4, 55–61. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Faisal, S.; Khan, M.A.; Jan, H.; Shah, S.A.; Abdullah; Shah, S.; Rizwan, M.; Wajidullah; Akbar, M.T. Redaina Edible mushroom (Flammulina velutipes) as biosource for silver nanoparticles: From synthesis to diverse biomedical and environmental applications. Nanotechnology 2021, 32, 065101. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Y.; Wang, H.; Wu, F.; Zhao, Y.; Liu, X.; Wu, H.; Wang, L.; Su, H. Green synthesis of silver nanoparticles using mushroom Flammulina velutipes extract and their antibacterial activity against aquatic pathogens. Food Bioprocess Technol. 2020, 13, 1908–1917. [Google Scholar] [CrossRef]
- Rehman, S.; Farooq, R.; Jermy, R.; Mousa Asiri, S.; Ravinayagam, V.; Al Jindan, R.; Alsalem, Z.; Shah, M.A.; Reshi, Z.; Sabit, H.; et al. A Wild Fomes fomentarius for Biomediation of One Pot Synthesis of Titanium Oxide and Silver Nanoparticles for Antibacterial and Anticancer Application. Biomolecules 2020, 10, 622. [Google Scholar] [CrossRef]
- Rehman, S.; Jermy, R.; Mousa Asiri, S.; Shah, M.A.; Farooq, R.; Ravinayagam, V.; Azam Ansari, M.; Alsalem, Z.; Al Jindan, R.; Reshi, Z.; et al. Using Fomitopsis pinicola for bioinspired synthesis of titanium dioxide and silver nanoparticles, targeting biomedical applications. RSC Adv. 2020, 10, 32137–32147. [Google Scholar] [CrossRef]
- Mohanta, Y.; Nayak, D.; Biswas, K.; Singdevsachan, S.; Abd_Allah, E.; Hashem, A.; Alqarawi, A.; Yadav, D.; Mohanta, T. Silver Nanoparticles Synthesized Using Wild Mushroom Show Potential Antimicrobial Activities against Food Borne Pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef]
- Dandapat, S.; Kumar, M.; Ranjan, R.; Sinha, M.P. Ganoderma applanatum extract mediated synthesis of silver nanoparticles. Braz. J. Pharm. Sci. 2022, 58, e19173. [Google Scholar] [CrossRef]
- Shivashankar, M.; Premkumari, B.; Chandan, N. Biosynthesis, partial characterization and antimicrobial activities of silver nanoparticles from Pleurotus species. Int. J. Integr. Sci. Innov. Technol. 2013, 2, 13–23. [Google Scholar]
- Jaloot, A.S.; Owaid, M.N.; Naeem, G.A.; Muslim, R.F. Mycosynthesizing and characterizing silver nanoparticles from the mushroom Inonotus hispidus (Hymenochaetaceae), and their antibacterial and antifungal activities. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100313. [Google Scholar] [CrossRef]
- Vamanu, E.; Ene, M.; Biță, B.; Ionescu, C.; Crăciun, L.; Sârbu, I. In Vitro Human Microbiota Response to Exposure to Silver Nanoparticles Biosynthesized with Mushroom Extract. Nutrients 2018, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Javier, K.R.A.; Camacho, D.H. Dataset on the optimization by response surface methodology for the synthesis of silver nanoparticles using Laxitextum bicolor mushroom. Data Brief 2022, 45, 108631. [Google Scholar] [CrossRef] [PubMed]
- Debnath, G.; Das, P.; Saha, A.K. Characterization, Antimicrobial and α-Amylase Inhibitory Activity of Silver Nanoparticles Synthesized by using Mushroom Extract of Lentinus tuber-regium. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 37–45. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Santhosh, S.; Giyaullah, H.; Balakumaran, M.D. Mycosynthesis, characterization and antibacterial activity of silver nanoparticles from Microporus xanthopus: A macro mushroom. Int. J. Innov. Res. Sci. Eng. Technol. 2007, 2, 6262–6270. [Google Scholar]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Faraz, M.; Sherwani, M.A.; Fatma, T.; Prasad, R. Illuminating the Anticancerous Efficacy of a New Fungal Chassis for Silver Nanoparticle Synthesis. Front. Chem. 2019, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kalia, A.; Sodhi, H.S. Size controlled, time-efficient biosynthesis of silver nanoparticles from Pleurotus florida using ultra-violet, visible range, and microwave radiations. Inorg. Nano-Met. Chem. 2020, 50, 35–41. [Google Scholar] [CrossRef]
- Acay, H.; Baran, M. Determination of Antioxidant and cytotoxic activities of king oyster mushroom mediated AgNPs synthesized with environmentally friendly methods. Med. Sci. 2020, 9, 760–765. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Ellah, N.H.A.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.-S.; Hetta, H.F.; Singh, M.P. Pleurotus sajor-caju-Mediated Synthesis of Silver and Gold Nanoparticles Active against Colon Cancer Cell Lines: A New Era of Herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1406–1418. [Google Scholar] [CrossRef]
- Chan, Y.S.; Don, M.M. Biosynthesis and structural characterization of Ag nanoparticles from white rot fungi. Mater. Sci. Eng. C 2013, 33, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.A.; Cunha, M.D.C.; da Frota, S.M.; Mallmann, E.J.J.; Freire, T.M.; Costa, L.S.; Paula, A.J.; Menezes, E.A.; Fechine, P.B.A. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: Antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 2018, 34, 127. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Eyini, M.; Gunasekaran, P. Green synthesis of silver nanoparticles using the mushroom fungus Schizophyllum commune and its biomedical applications. Biotechnol. Bioprocess Eng. 2014, 19, 1083–1090. [Google Scholar] [CrossRef]
- Kobashigawa, J.M.; Robles, C.A.; Martínez Ricci, M.L.; Carmarán, C.C. Influence of strong bases on the synthesis of silver nanoparticles (AgNPs) using the ligninolytic fungi Trametes trogii. Saudi J. Biol. Sci. 2019, 26, 1331–1337. [Google Scholar] [CrossRef]
- Anthony, K.J.P.; Murugan, M.; Jeyaraj, M.; Rathinam, N.K.; Sangiliyandi, G. Synthesis of silver nanoparticles using pine mushroom extract: A potential antimicrobial agent against E. coli and B. subtilis. J. Ind. Eng. Chem. 2014, 20, 2325–2331. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Riaz Rajoka, M.S.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.M.; Dhasarathan, P.; Ranjitsingh, A.J.A.; Al-Humaid, L.A. Ganoderma lucidum inspired silver nanoparticles and its biomedical applications with special reference to drug resistant Escherichia coli isolates from CAUTI. Saudi J. Biol. Sci. 2020, 27, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef] [PubMed]
- Dat, T.D.; Viet, N.D.; Dat, N.M.; My, P.L.T.; Thinh, D.B.; Thy, L.T.M.; Huong, L.M.; Khang, P.T.; Hai, N.D.; Nam, H.M.; et al. Characterization and bioactivities of silver nanoparticles green synthesized from Vietnamese Ganoderma lucidum. Surf. Interfaces 2021, 27, 101453. [Google Scholar] [CrossRef]
- Iruoma, C.A.; Reginald, E.E.; Motunrayo, O.; Ezinwa, I.U. Green Synthesis of Silver Nanoparticles Using Pleurotus ostreatus. J. Appl. Life Sci. Int. 2021, 1–10. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Naraian, R. Green synthesis and characterization of silver NPs using oyster mushroom extract for antibacterial efficacy. J. Chem. Environ. Sci. Its Appl. 2020, 7, 13–18. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S.S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Eskandari-Nojedehi, M.; Jafarizadeh-Malmiri, H.; Rahbar-Shahrouzi, J. Hydrothermal green synthesis of gold nanoparticles using mushroom (Agaricus bisporus) extract: Physico-chemical characteristics and antifungal activity studies. Green Process. Synth. 2018, 7, 38–47. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Owaid, M.N.; Rabeea, M.A.; Aziz, A.A.; Jameel, M.S. Mycosynthesis of gold nanoparticles by the Portabello mushroom extract, Agaricaceae, and their efficacy for decolorization of Azo dye. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100312. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Bharathakumar, S.; Malaikozhundan, B.; Mahalingam, P.U. Mycofabrication of gold nanoparticles: Optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal. Agric. Biotechnol. 2021, 35, 102107. [Google Scholar] [CrossRef]
- Hemashekhar, B.; Chandrappa, C.P.; Govindappa, M.; Chandrashekar, N. Endophytic fungus Alternaria spp isolated from Rauvolfia tetraphylla root arbitrate synthesis of gold nanoparticles and evaluation of their antibacterial, antioxidant and antimitotic activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 035010. [Google Scholar] [CrossRef]
- Jha, P.; Saraf, A.; Sohal, J.K. Antimicrobial Activity of Biologically Synthesized Gold Nanoparticles from Wild Mushroom Cantharellus Species. J. Sci. Res. 2021, 65, 78–83. [Google Scholar] [CrossRef]
- Naeem, G.A.; Jaloot, A.S.; Owaid, M.N.; Muslim, R.F. Green Synthesis of Gold Nanoparticles from Coprinus comatus, Agaricaceae, and the Effect of Ultraviolet Irradiation on Their Characteristics. Walailak J. Sci. Technol. 2021, 18, 9396. [Google Scholar] [CrossRef]
- Rabeea, M.A.; Owaid, M.N.; Aziz, A.A.; Jameel, M.S.; Dheyab, M.A. Mycosynthesis of gold nanoparticles using the extract of Flammulina velutipes, Physalacriaceae, and their efficacy for decolorization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 103841. [Google Scholar] [CrossRef]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J. Mycol. Médicale 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef]
- Abdul-Hadi, S.Y.; Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S. Rapid mycosynthesis and characterization of phenols-capped crystal gold nanoparticles from Ganoderma applanatum, Ganodermataceae. Biocatal. Agric. Biotechnol. 2020, 27, 101683. [Google Scholar] [CrossRef]
- Elumalai, D.; Suman, T.Y.; Hemavathi, M.; Swetha, C.; Kavitha, R.; Arulvasu, C.; Kaleena, P.K. Biofabrication of gold nanoparticles using Ganoderma lucidum and their cytotoxicity against human colon cancer cell line (HT-29). Bull. Mater. Sci. 2021, 44, 132. [Google Scholar] [CrossRef]
- Shukurov, I.; Mohamed, M.S.; Mizuki, T.; Palaninathan, V.; Ukai, T.; Hanajiri, T.; Maekawa, T. Biological Synthesis of Bioactive Gold Nanoparticles from Inonotus obliquus for Dual Chemo-Photothermal Effects against Human Brain Cancer Cells. Int. J. Mol. Sci. 2022, 23, 2292. [Google Scholar] [CrossRef] [PubMed]
- Farzana Fathima, M.R.; Usha Raja Nanthini, A.; Al-Khattaf, F.S.; Hatamleh, A.A.; Kabir, S.B. Mycosynthesis of Noble Metal Nanoparticle Using Laetiporus versisporus Mushroom and Analysis of Antioxidant Activity. J. Nanomater. 2022, 2022, 8086803. [Google Scholar] [CrossRef]
- Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S.; Dheyab, M.A. Mushroom-assisted synthesis of triangle gold nanoparticles using the aqueous extract of fresh Lentinula edodes (shiitake), Omphalotaceae. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100270. [Google Scholar] [CrossRef]
- Vetchinkina, E.P.; Loshchinina, E.A.; Vodolazov, I.R.; Kursky, V.F.; Dykman, L.A.; Nikitina, V.E. Biosynthesis of nanoparticles of metals and metalloids by basidiomycetes. Preparation of gold nanoparticles by using purified fungal phenol oxidases. Appl. Microbiol. Biotechnol. 2017, 101, 1047–1062. [Google Scholar] [CrossRef]
- Acay, H. Utilization of Morchella esculenta -mediated green synthesis golden nanoparticles in biomedicine applications. Prep. Biochem. Biotechnol. 2021, 51, 127–136. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Pakina, E.; Zargar, M. Evaluation of Phoma sp. Biomass as an Endophytic Fungus for Synthesis of Extracellular Gold Nanoparticles with Antibacterial and Antifungal Properties. Molecules 2022, 27, 1181. [Google Scholar] [CrossRef]
- Abdel-Kareem, M.M.; Zohri, A.A. Extracellular mycosynthesis of gold nanoparticles using Trichoderma hamatum: Optimization, characterization and antimicrobial activity. Lett. Appl. Microbiol. 2018, 67, 465–475. [Google Scholar] [CrossRef]
- do Nascimento, J.M.; Cruz, N.D.; de Oliveira, G.R.; Sá, W.S.; de Oliveira, J.D.; Ribeiro, P.R.S.; Leite, S.G.F. Evaluation of the kinetics of gold biosorption processes and consequent biogenic synthesis of AuNPs mediated by the fungus Trichoderma harzianum. Environ. Technol. Innov. 2021, 21, 101238. [Google Scholar] [CrossRef]
- Basu, A.; Ray, S.; Chowdhury, S.; Sarkar, A.; Mandal, D.P.; Bhattacharjee, S.; Kundu, S. Evaluating the antimicrobial, apoptotic, and cancer cell gene delivery properties of protein-capped gold nanoparticles synthesized from the edible mycorrhizal fungus Tricholoma crassum. Nanoscale Res. Lett. 2018, 13, 154. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef] [PubMed]
- Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A.; Kursky, V.F.; Nikitina, V.E. Nanoparticles synthesis by Agaricus soil basidiomycetes. J. Biosci. Bioeng. 2018, 126, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Preis, E.; Bakowsky, U.; Azzazy, H.M.E.-S. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules 2020, 25, 4981. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Diaz, R.; Gutiérrez de la Rosa, S.Y.; Gutiérrez Coronado, Ó.; Patakfalvi, R. Biogenic synthesis of platinum nanoparticles. Chem. Pap. 2022, 76, 2573–2594. [Google Scholar] [CrossRef]
- Sarkar, J.; Acharya, K. Alternaria alternata culture filtrate mediated bioreduction of chloroplatinate to platinum nanoparticles. Inorg. Nano-Met. Chem. 2017, 47, 365–369. [Google Scholar] [CrossRef]
- Riddin, T.L.; Gericke, M.; Whiteley, C.G. Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 2006, 17, 3482–3489. [Google Scholar] [CrossRef]
- Govender, Y.; Riddin, T.; Gericke, M.; Whiteley, C.G. Bioreduction of platinum salts into nanoparticles: A mechanistic perspective. Biotechnol. Lett. 2009, 31, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Govender, Y.; Riddin, T.L.; Gericke, M.; Whiteley, C.G. On the enzymatic formation of platinum nanoparticles. J. Nanoparticle Res. 2010, 12, 261–271. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2012, 97, 27–31. [Google Scholar] [CrossRef]
- Gupta, K.; Chundawat, T.S. Bio-inspired synthesis of platinum nanoparticles from fungus Fusarium oxysporum: Its characteristics, potential antimicrobial, antioxidant and photocatalytic activities. Mater. Res. Express 2019, 6, 1050d6. [Google Scholar] [CrossRef]
- Castro-Longoria, E. Production of Platinum Nanoparticles and Nanoaggregates Using Neurospora crassa. J. Microbiol. Biotechnol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.A.; Sheet, S.; Vinothkannan, M.; Yoo, D.J.; Lee, Y.S.; Belal, S.A.; Shim, K.S. One-Pot Facile Synthesis of Pt Nanoparticles Using Cultural Filtrate of Microgravity Simulated Grown P. chrysogenum and Their Activity on Bacteria and Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 3110–3125. [Google Scholar] [CrossRef] [PubMed]
- Borse, V.; Kaler, A.; Banerjee, U.C. Microbial Synthesis of Platinum Nanoparticles and Evaluation of Their Anticancer Activity. Int. J. Emerg. Trends Electr. Electron. 2015, 11, 26–31. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.-C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2019, 10, 66. [Google Scholar] [CrossRef]
- Fahmy, S.; Preis, E.; Bakowsky, U.; Azzazy, H.M. Palladium Nanoparticles Fabricated by Green Chemistry: Promising Chemotherapeutic, Antioxidant and Antimicrobial Agents. Materials 2020, 13, 3661. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Multi-Functional Biological Effects of Palladium Nanoparticles Synthesized Using Agaricus bisporus. J. Clust. Sci. 2020, 31, 391–400. [Google Scholar] [CrossRef]
- Gil, Y.-G.; Kang, S.; Chae, A.; Kim, Y.-K.; Min, D.-H.; Jang, H. Synthesis of porous Pd nanoparticles by therapeutic chaga extract for highly efficient tri-modal cancer treatment. Nanoscale 2018, 10, 19810–19817. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Biosynthesis of palladium nanoparticles using Saccharomyces cerevisiae extract and its photocatalytic degradation behaviour. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025018. [Google Scholar] [CrossRef]
- Saitoh, N.; Fujimori, R.; Yoshimura, T.; Tanaka, H.; Kondoh, A.; Nomura, T.; Konishi, Y. Microbial recovery of palladium by baker’s yeast through bioreductive deposition and biosorption. Hydrometallurgy 2020, 196, 105413. [Google Scholar] [CrossRef]
- Rafique, M.; Shaikh, A.J.; Rasheed, R.; Tahir, M.B.; Bakhat, H.F.; Rafique, M.S.; Rabbani, F. A Review on Synthesis, Characterization and Applications of Copper Nanoparticles Using Green Method. NANO: Brief Rep. Rev. 2017, 12, 1750043. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F. Biogenic copper nanoparticles and their applications: A review. SN Appl. Sci. 2020, 2, 505. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Prabowo, B.A.; Winarko, R. Bionanotechnology: The formation of copper nanoparticles assisted by biological agents and their applications as antimicrobial and antiviral agents. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100703. [Google Scholar] [CrossRef]
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: An in-vitro study. Colloid Interface Sci. Commun. 2020, 35, 100254. [Google Scholar] [CrossRef]
- Saitawadekar, A.; Kakde, U.B. Green synthesis of copper nanoparticles using Aspergillus flavus. J. Crit. Rev. 2020, 7, 9. [Google Scholar]
- Noor, S.; Shah, Z.; Javed, A.; Ali, A.; Hussain, S.B.; Zafar, S.; Ali, H.; Muhammad, S.A. A fungal based synthesis method for copper nanoparticles with the determination of anticancer, antidiabetic and antibacterial activities. J. Microbiol. Methods 2020, 174, 105966. [Google Scholar] [CrossRef]
- Ammar, H.A.; Rabie, G.H.; Mohamed, E. Novel fabrication of gelatin-encapsulated copper nanoparticles using Aspergillus versicolor and their application in controlling of rotting plant pathogens. Bioprocess Biosyst. Eng. 2019, 42, 1947–1961. [Google Scholar] [CrossRef]
- Majumder, D.R. Bioremediation: Copper Nanoparticles from Electronic-waste. Int. J. Eng. Sci. Technol. 2012, 4, 10. [Google Scholar]
- Salvadori, M.R.; Lepre, L.F.; Ando, R.A.; Oller do Nascimento, C.A.; Corrêa, B. Biosynthesis and Uptake of Copper Nanoparticles by Dead Biomass of Hypocrea lixii Isolated from the Metal Mine in the Brazilian Amazon Region. PLoS ONE 2013, 8, e80519. [Google Scholar] [CrossRef]
- Fatima, F.; Wahid, I. Eco-friendly synthesis of silver and copper nanoparticles by Shizophyllum commune fungus and its biomedical applications. Int. J. Environ. Sci. Technol. 2022, 19, 7915–7926. [Google Scholar] [CrossRef]
- Cuevas, R.; Durán, N.; Diez, M.C.; Tortella, G.R.; Rubilar, O. Extracellular Biosynthesis of Copper and Copper Oxide Nanoparticles by Stereum hirsutum, a Native White-Rot Fungus from Chilean Forests. J. Nanomater. 2015, 2015, 57. [Google Scholar] [CrossRef]
- Natesan, K.; Ponmurugan, P.; Gnanamangai, B.M.; Manigandan, V.; Joy, S.P.J.; Jayakumar, C.; Amsaveni, G. Biosynthesis of silica and copper nanoparticles from Trichoderma, Streptomyces and Pseudomonas spp. evaluated against collar canker and red root-rot disease of tea plants. Arch. Phytopathol. Plant Prot. 2021, 54, 56–85. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Ando, R.A.; Oller Do Nascimento, C.A.; Corrêa, B. Bioremediation from wastewater and extracellular synthesis of copper nanoparticles by the fungus Trichoderma koningiopsis. J. Environ. Sci. Health Part A 2014, 49, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Y.M.; Azzam, A.M.; Amin, B.H.; Safwat, N.A. Mycosynthesis of iron nanoparticles by Alternaria alternata and its antibacterial activity. Afr. J. Biotechnol. 2015, 14, 1234–1241. [Google Scholar] [CrossRef]
- Alamilla-Martínez, D.G.; Rojas-Avelizapa, N.G.; Domínguez-López, I.; Pool, H.; Gómez-Ramírez, M.; de Querétaro, A. Biosynthesis of iron nanoparticles (FeNPs) by Alternaria alternata MVSS-AH-5. Mex. J. Biotechnol. 2019, 4, 1–14. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R. Rapid, Low-Cost, and Ecofriendly Approach for Iron Nanoparticle Synthesis Using Aspergillus oryzae TFR9. J. Nanoparticles 2013, 2013, 141274. [Google Scholar] [CrossRef]
- Abdeen, S.; Isaac, R.R.; Geo, S.; Sornalekshmi, S.; Rose, A.; Praseetha, P.K. Evaluation of Antimicrobial Activity of Biosynthesized Iron and Silver Nanoparticles Using the Fungi Fusarium oxysporum and Actinomycetes sp. on Human Pathogens. Nano Biomed. Eng. 2013, 5, 39–45. [Google Scholar] [CrossRef]
- Mathur, P.; Saini, S.; Paul, E.; Sharma, C.; Mehtani, P. Endophytic fungi mediated synthesis of iron nanoparticles: Characterization and application in methylene blue decolorization. Curr. Res. Green Sustain. Chem. 2021, 4, 100053. [Google Scholar] [CrossRef]
- Manikandan, G.; Ramasubbu, R. Biosynthesis of Iron Nanoparticles from Pleurotus florida and its Antimicrobial Activity against Selected Human Pathogens. Indian J. Pharm. Sci. 2021, 83, 45–51. [Google Scholar] [CrossRef]
- Mazumdar, H.; Haloi, N. A study on Biosynthesis of Iron Nanoparticles by Pleurotus sp. J. Microbiol. Biotechnol. Res. 2011, 12, 39–49. [Google Scholar]
- Adeleye, T.; Kareem, S.; Kekere-Ekun, A. Optimization studies on biosynthesis of iron nanoparticles using Rhizopus stolonifer. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 012037. [Google Scholar] [CrossRef]
- Kareem, S.; Adeleye, T.; Ojo, R. Effects of pH, temperature and agitation on the biosynthesis of iron nanoparticles produced by Trichoderma species. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 012036. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Shoeibi, S.; Mozdziak, P.; Golkar-Narenji, A. Biogenesis of Selenium Nanoparticles Using Green Chemistry. Top. Curr. Chem. (Z) 2017, 375, 88. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Wilkinson, S.C.; Gadd, G.M. Reduction of selenium oxyanions by unicellular, polymorphic and filamentous fungi: Cellular location of reduced selenium and implications for tolerance. J. Ind. Microbiol. 1995, 14, 300–311. [Google Scholar] [CrossRef]
- Sarkar, J.; Dey, P.; Saha, S.; Acharya, K. Mycosynthesis of selenium nanoparticles. Micro Nano Lett. 2011, 6, 599. [Google Scholar] [CrossRef]
- Sarkar, J.; Saha, S.; Dey, P.; Acharya, K. Production of Selenium Nanorods by Phytopathogen, Alternaria alternata. Adv. Sci. Lett. 2012, 10, 111–114. [Google Scholar] [CrossRef]
- Bafghi, M.H.; Darroudi, M.; Zargar, M.; Zarrinfar, H.; Nazari, R. Biosynthesis of selenium nanoparticles by Aspergillus flavus and Candida albicans for antifungal applications. Micro Nano Lett. 2021, 16, 656–669. [Google Scholar] [CrossRef]
- Hussein, H.G.; El-Sayed, E.-S.R.; Younis, N.A.; Hamdy, A.E.H.A.; Easa, S.M. Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Express 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Zare, B.; Babaie, S.; Setayesh, N.; Shahverdi, A.R. Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomed. J. 2013, 1, 13–19. [Google Scholar]
- Liang, X.; Perez, M.A.M.-J.; Nwoko, K.C.; Egbers, P.; Feldmann, J.; Csetenyi, L.; Gadd, G.M. Fungal formation of selenium and tellurium nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 7241–7259. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, S.; Gadd, G.M.; McGrath, J.; Rooney, D.W.; Zhao, Q. Fungal-derived selenium nanoparticles and their potential applications in electroless silver coatings for preventing pin-tract infections. Regen. Biomater. 2022, 9, rbac013. [Google Scholar] [CrossRef] [PubMed]
- Asghari-Paskiabi, F.; Imani, M.; Razzaghi-Abyaneh, M.; Rafii-Tabar, H. Fusarium oxysporum, a bio-Factory for Nano Selenium Compounds: Synthesis and Characterization. Sci. Iran. 2018, 25, 1857–1863. [Google Scholar] [CrossRef]
- Vetchinkina, E.P.; Loshchinina, E.A.; Kurskyi, V.F.; Nikitina, V.E. Biological synthesis of selenium and germanium nanoparticles by xylotrophic basidiomycetes. Appl. Biochem. Microbiol. 2016, 52, 87–97. [Google Scholar] [CrossRef]
- Lian, S.; Diko, C.S.; Yan, Y.; Li, Z.; Zhang, H.; Ma, Q.; Qu, Y. Characterization of biogenic selenium nanoparticles derived from cell-free extracts of a novel yeast Magnusiomyces ingens. 3 Biotech 2019, 9, 221. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Rasouli, M. Biosynthesis of Selenium Nanoparticles using yeast Nematospora coryli and examination of their anti-candida and anti-oxidant activities. IET Nanobiotechnol. 2019, 13, 214–218. [Google Scholar] [CrossRef]
- Vahidi, H.; Kobarfard, F.; Kosar, Z.; Mahjoub, M.A.; Saravanan, M.; Barabadi, H. Mycosynthesis and characterization of selenium nanoparticles using standard Penicillium chrysogenum PTCC 5031 and their antibacterial activity: A novel approach in microbial nanotechnology. Nanomed. J. 2020, 7, 315–323. [Google Scholar] [CrossRef]
- Morad, M.Y.; El-Sayed, H.; Elhenawy, A.A.; Korany, S.M.; Aloufi, A.S.; Ibrahim, A.M. Myco-Synthesized Molluscicidal and Larvicidal Selenium Nanoparticles: A New Strategy to Control Biomphalaria alexandrina Snails and Larvae of Schistosoma mansoni with an In Silico Study on Induced Oxidative Stress. J. Fungi 2022, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, M.; El-Batal, A.I. Gentamicin-Assisted Mycogenic Selenium Nanoparticles Synthesized Under Gamma Irradiation for Robust Reluctance of Resistant Urinary Tract Infection-Causing Pathogens. Biol. Trace Elem. Res. 2020, 195, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.H.; Ahmed, H.Y.; El Gazzar, E.M.; Badawy, M.M.M. Enhancement the Mycosynthesis of Selenium Nanoparticles by Using Gamma Radiation. Dose-Response 2021, 19, 155932582110593. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, M.M.G.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, Cytotoxicity and Larvicidal Activity of Green Synthesized Selenium Nanoparticles Using Penicillium corylophilum. J. Clust. Sci. 2021, 32, 351–361. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef]
- Liang, X.; Perez, M.A.M.; Zhang, S.; Song, W.; Armstrong, J.G.; Bullock, L.A.; Feldmann, J.; Parnell, J.; Csetenyi, L.; Gadd, G.M. Fungal transformation of selenium and tellurium located in a volcanogenic sulfide deposit. Environ. Microbiol. 2020, 22, 2346–2364. [Google Scholar] [CrossRef]
- Ashengroph, M.; Tozandehjani, S. Optimized resting cell method for green synthesis of selenium nanoparticles from a new Rhodotorula mucilaginosa strain. Process Biochem. 2022, 116, 197–205. [Google Scholar] [CrossRef]
- Joshi, S.; De Britto, S.; Jogaiah, S.; Ito, S. Mycogenic Selenium Nanoparticles as Potential New Generation Broad Spectrum Antifungal Molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Diko, C.S.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Mater. Chem. Phys. 2020, 246, 122583. [Google Scholar] [CrossRef]
- Arunthirumeni, M.; Veerammal, V.; Shivakumar, M.S. Biocontrol Efficacy of Mycosynthesized Selenium Nanoparticle Using Trichoderma sp. on Insect Pest Spodoptera litura. J. Clust. Sci. 2022, 33, 1645–1653. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kursky, V.; Nikitina, V. Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J. Microbiol. 2013, 51, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; Al-Hagar, O.E.A.; Abdelkhalek, E.S.; Sidkey, N.M. Synthesis and investigations on tellurium myconanoparticles. Biotechnol. Rep. 2018, 18, e00247. [Google Scholar] [CrossRef]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. 2018, 17, 87–97. [Google Scholar]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Guyot, F.; van Hullebusch, E.D.; Lens, P.N.L. Biomineralization of tellurium and selenium-tellurium nanoparticles by the white-rot fungus Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2017, 124, 258–266. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Forootanfar, H. Biosynthesis and characterization of gold nanoparticles produced by laccase from Paraconiothyrium variabile. Colloids Surf. B Biointerfaces 2011, 87, 23–27. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P.; Puri, S. Enzymatic Formation of Gold Nanoparticles Using Phanerochaete Chrysosporium. Adv. Chem. Eng. Sci. 2011, 1, 154–162. [Google Scholar] [CrossRef]
- El-Batal, A.I.; ElKenawy, N.M.; Yassin, A.S.; Amin, M.A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol. Rep. 2015, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Shabani, M.; Akbarzadeh, A.; Norouzian, D.; Amini, A.; Gholami-Shabani, Z.; Imani, A.; Chiani, M.; Riazi, G.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Antimicrobial Activity and Physical Characterization of Silver Nanoparticles Green Synthesized Using Nitrate Reductase from Fusarium oxysporum. Appl. Biochem. Biotechnol. 2014, 172, 4084–4098. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Cuevas, R.; Cordi, L.; Rubilar, O.; Diez, M.C. Biogenic silver nanoparticles associated with silver chloride nanoparticles (Ag@AgCl) produced by laccase from Trametes versicolor. SpringerPlus 2014, 3, 645. [Google Scholar] [CrossRef]
- Alharbi, R.M.; Alshammari, S.O.; Abd El Aty, A.A. Statically improved fungal laccase-mediated biogenesis of silver nanoparticles with antimicrobial applications. J. Appl. Pharm. Sci. 2022, 001–014. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.-K.; Wu, J.-Y. Characterization and antibacterial activity of silver nanoparticles prepared with a fungal exopolysaccharide in water. Food Hydrocolloids 2016, 53, 69–74. [Google Scholar] [CrossRef]
- Nair, V.; Sambre, D.; Joshi, S.; Bankar, A.; Ravi Kumar, A.; Zinjarde, S. Yeast-Derived Melanin Mediated Synthesis of Gold Nanoparticles. J. Bionanosci. 2013, 7, 159–168. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of Metal Nanoparticles from Fungi and Metal Salts: Scope and Application. Nanoscale Res. Lett. 2016, 11, 98. [Google Scholar] [CrossRef]
- Fungal Nanobionics: Principles and Applications; Prasad, R., Kumar, V., Kumar, M., Wang, S., Eds.; Springer: Gateway East, Singapore, 2018; 316p, p. 316. [Google Scholar] [CrossRef]
- Nanobiotechnology in Neurodegenerative Diseases; Rai, M., Yadav, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; 398p, p. 398. [Google Scholar] [CrossRef]
- Simões, M.F.; Ottoni, C.A.; Antunes, A. Mycogenic metal nanoparticles for the treatment of mycobacterioses. Antibiotics 2020, 9, 569. [Google Scholar] [CrossRef]
- Yadav, R.N.; Chitara, M.K.; Zaidi, N.W.; Khan, A.I.; Singh, U.S.; Singh, H.B. Novel facets and challenges in the management of phytopathogens using myconanoparticles. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3296–3308. [Google Scholar] [CrossRef]
- Sundaravadivelan, C.; Padmanabhan, M.N. Effect of mycosynthesized silver nanoparticles from filtrate of Trichoderma harzianum against larvae and pupa of dengue vector Aedes aegypti L. Environ. Sci. Pollut. Res. 2014, 21, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Prakash, S. Microbial synthesis of spherical nanosilver and nanogold for mosquito control. Ann. Microbiol. 2014, 64, 1099–1111. [Google Scholar] [CrossRef]
- Salunkhe, R.B.; Patil, S.V.; Patil, C.D.; Salunke, B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol. Res. 2011, 109, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zayed, K.M.; Guo, Y.-H.; Lv, S.; Zhang, Y.; Zhou, X.-N. Molluscicidal and antioxidant activities of silver nanoparticles on the multi-species of snail intermediate hosts of schistosomiasis. PLoS Negl. Trop. Dis. 2022, 16, e0010667. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic metal nanoparticles: Progress and applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef]
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable production of nanoparticles via mycogenesis for biotechnological applications: A critical review. Environ. Res. 2022, 204, 111963. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; AboDalam, H.M.; Ahmed, F.K.; Ravichandran, M.; Kalia, A.; Rai, M. Trichoderma: An eco-friendly source of nanomaterials for sustainable agroecosystems. J. Fungi 2022, 8, 367. [Google Scholar] [CrossRef]
- Sonawane, H.; Shelke, D.; Chambhare, M.; Dixit, N.; Math, S.; Sen, S.; Borah, S.N.; Islam, N.F.; Joshi, S.J.; Yousaf, B.; et al. Fungi-derived agriculturally important nanoparticles and their application in crop stress management—Prospects and environmental risks. Environ. Res. 2022, 212, 113543. [Google Scholar] [CrossRef]
- Akther, T.; Hemalatha, S. Mycosilver Nanoparticles: Synthesis, Characterization and its efficacy against plant pathogenic fungi. BioNanoScience 2019, 9, 296–301. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Silva, L.P.; Ferraz, C.M.; Tobias, F.L.; de Araújo, J.V.; Loureiro, B.; Braga, G.M.; Veloso, F.B.; de Freitas Soares, F.E.; Fronza, M.; et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 2019, 14, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Gaurav, S.S.; Rani, V.; Singh, A.; Rani, P.; Verma, P.; Kumar, B. Evaluation of larvicidal effect of mycogenic silver nanoparticles against white grubs (Holotrichia sp). J. Adv. Sci. Res. 2020, 11, 296–304. [Google Scholar]
- Joshi, S.M.; De Britto, S.; Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J. Biotechnol. 2021, 325, 196–206. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Chen, G.; Zeng, G.; Huang, Z.; Guo, Z.; Huang, T.; Peng, M.; Shi, J.; Hu, L. Applications of white rot fungi in bioremediation with nanoparticles and biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 4853–4862. [Google Scholar] [CrossRef]
- Shakya, M.; Rene, E.R.; Nancharaiah, Y.V.; Lens, P.N.L. Fungal-Based Nanotechnology for Heavy Metal Removal. In Nanotechnology, Food Security and Water Treatment; Gothandam, K.M., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2018; pp. 229–253. [Google Scholar] [CrossRef]
- Sabuda, M.C.; Rosenfeld, C.E.; DeJournett, T.D.; Schroeder, K.; Wuolo-Journey, K.; Santelli, C.M. Fungal bioremediation of selenium-contaminated industrial and municipal wastewaters. Front. Microbiol. 2020, 11, 2105. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Shakya, M.; Jain, R.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Sorption of zinc onto elemental selenium nanoparticles immobilized in Phanerochaete chrysosporium pellets. Environ. Sci. Pollut. Res. 2016, 23, 21619–21630. [Google Scholar] [CrossRef]
- Kalia, A.; Singh, S. Myco-decontamination of azo dyes: Nano-augmentation technologies. 3 Biotech 2020, 10, 384. [Google Scholar] [CrossRef]
- Deka, A.C.; Sinha, S.K. Mycogenic silver nanoparticle biosynthesis and its pesticide degradation potentials. Int. J. Technol. Enhanc. Emerg. Eng. Res. 2015, 3, 108–113. [Google Scholar]
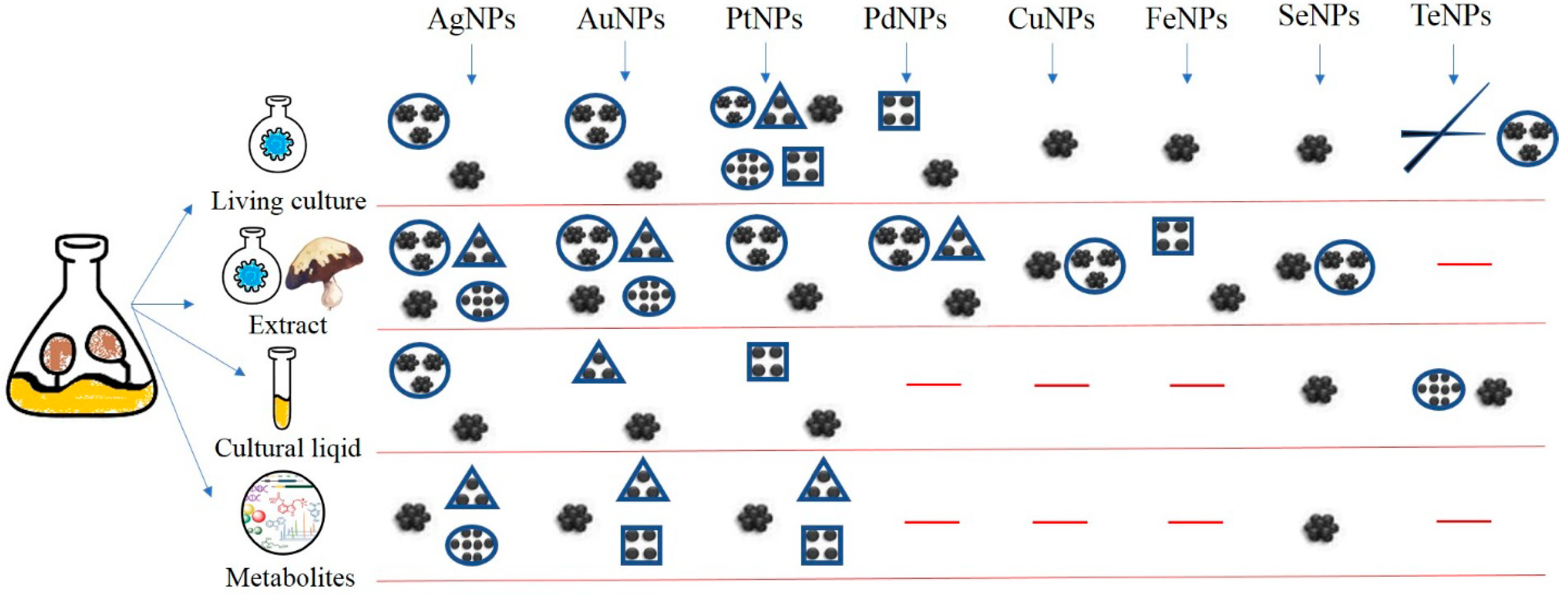
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Agaricus arvensis | Living culture | AgNO3 | Spherical (10–20 nm) | [41] |
| Cultural liquid | AgNO3 | Irregular spherical (10–100 nm) | ||
| Mycelial extract | AgNO3 | Spherical (1–10 nm) | ||
| Agaricus bisporus | Living culture | AgNO3 | Spherical (10–20 nm) | [41] |
| Cultural liquid | AgNO3 | Irregular spherical (10–100 nm) | ||
| Mycelial extract | AgNO3 | Spherical (1–10 nm) | ||
| Agaricus bisporus | Crude polysaccharide extract | AgNO3 | Irregularly quasi-spherical (20–40 nm) | [88] |
| Agaricus bisporus | Fruit body extract | AgNO3 | Face-centered cubic (average size of 43.9 nm) | [89] |
| Agaricus bisporus | Fruit body extract | AgNO3 | Cubic (average size of 50.44 nm) | [90] |
| Agaricus bisporus | Fruit body extract | AgNO3 | Spherical (average size of 16 nm) | [91] |
| Agaricus brasiliensis | Crude polysaccharide extract | AgNO3 | Irregularly quasi-spherical (20–40 nm) | [88] |
| Alternaria sp. | Mycelial extract | AgNO3 | Spherical (3–10 nm) | [47] |
| Aspergillus niger | Crude xylanase | AgNO3 | Spherical, cylindrical, oval (15.21–77.49 nm) | [48] |
| Auricularia polytricha | Mycelial extract | AgNO3 | Spherical (5–50 nm) | [92] |
| Beauveria bassiana | Mycelial extract | AgNO3 | Triangular, circular, hexagonal (10–50 nm) | [50] |
| Botryodiplodia theobromae | Mycelial extract | AgNO3 | 66.75–111.23 nm | [52] |
| Mycelial biomass | AgNO3 | 62.77–103 nm | ||
| Flammulina velutipes | Fungal extract | AgNO3 | Spherical (average size of 21.4 nm) | [97] |
| Flammulina velutipes | Fruit body extract | AgNO3 | Spherical (average size of 22 nm) | [98] |
| Fomes fomentarius | Fruit body extract | AgNO3 | Spherical (10–20 nm) | [99] |
| Fomitopsis pinicola | Fruit body extract | AgNO3 | Spherical (10–30 nm) | [100] |
| Ganoderma applanatum | Fruit body extract | AgNO3 | Spherical (average size of 58.77 nm) | [102] |
| Ganoderma lucidum | Living culture | AgNO3 | Spherical (10–20 nm) | [41] |
| Cultural liquid | AgNO3 | Irregular spherical (10–100 nm) | ||
| Mycelial extract | AgNO3 | Spherical (1–10 nm) | ||
| Fruit body extract | AgNO3 | Near-cubic (20–200 nm) | ||
| Ganoderma lucidum | Fungal extract | AgNO3 | Spherical (23–58 nm) | [123] |
| Ganoderma lucidum | Fruit body extract | AgNO3 | Spherical (15–22 nm) | [124] |
| Ganoderma lucidum | Fruit body extract | AgNO3 | Spherical (average size of 11.38 nm) | [125] |
| Ganoderma sessile | Mycelial extract | AgNO3 | Quasi-spherical (average size of 5.4 or 8.9 nm depending on the extraction method) | [82] |
| Ganoderma sessiliforme | Fruit body extract | AgNO3 | Spherical (average size of 45 nm) | [101] |
| Grifola frondosa | Living culture | AgNO3 | Spherical (10–20 nm) | [41] |
| Cultural liquid | AgNO3 | Irregular spherical (10–100 nm) | ||
| Mycelial extract | AgNO3 | Spherical (1–10 nm) | ||
| Helvella leucopus | Fruit body extract | AgNO3 | Spherical (80–100 nm), aggregated | [61] |
| Lactarius piperatus | Fruit body extract | AgNO3 | Spherical (average size of 49 nm) | [105] |
| Lentinus edodes | Living culture | AgNO3 | Spherical (10–20 nm) | [41] |
| Cultural liquid | AgNO3 | Irregular spherical (10–100 nm), spherical conglomerates 50–250) | ||
| Mycelial extract | AgNO3 | Spherical (1–10 nm) | ||
| Lentinus tuber-regium | Fruit body extract | AgNO3 | Spherical (5–35 nm) | [107] |
| Penicillium citrinum | Mycelial extract | AgNO3 | Spherical (2–5 nm) | [67] |
| Penicillium cyclopium | Mycelial biomass | AgNO3 | Mostly irregular (12–25 nm) | [68] |
| Penicillium janthinellum | Mycelial extract | AgNO3 | Spherical (1–30 nm) | [69] |
| Penicillium oxalicum | Mycelial extract | AgNO3 | Spherical (60–80 nm) | [70] |
| Penicillium oxalicum | Mycelial extract | AgNO3 | Spherical (average size of 52.26 nm) | [71] |
| Penicillium polonicum | Mycelial extract | AgNO3 | Mostly spherical (10–15 nm), hexagonal, polyhedral (above 30 nm) | [72] |
| Phaenerochaete chrysosporium | Mycelial extract | AgNO3 | Spherical, oval (34–90 nm) | [109] |
| Phellinus linteus | Crude polysaccharide extract | AgNO3 | Irregularly quasi-spherical (20–40 nm) | [88] |
| Picoa sp. | Fruit body extract | AgNO3 | Irregular (average size of 19.5 nm) | [76] |
| Pleurotus djamor | Fruit body extract | AgNO3 | Spherical (average size of 55.76 nm) | [114] |
| Pleurotus eryngii | Fruit body extract | AgNO3 | Spherical (average size of 18.45 nm) | [112] |
| Pleurotus florida | Fruit body extract | AgNO3 | Spherical (average size of 10 nm) | [111] |
| Pleurotus ostreatus | Living culture | AgNO3 | Spherical (10–20 nm) | [41] |
| Cultural liquid | AgNO3 | Irregular spherical (10–100 nm) | ||
| Mycelial extract | AgNO3 | Spherical (1–10 nm) | ||
| Pleurotus ostreatus | Fruit body extract | AgNO3 | Spherical, hexagonal (18–82 nm) | [126] |
| Pleurotus ostreatus | Fruit body extract | AgNO3 | Spherical (average size of 28.44 nm) | [114] |
| Pleurotus sajor caju | Fruit body extract | AgNO3 | Spherical (11–44 nm) | [127] |
| Pleurotus sajor caju | Fruit body extract | AgNO3 | Spherical (average size of 15–20 nm) | [113] |
| Tirmania sp. | Fruit body extract | AgNO3 | Irregular, spherical (average size of 72 nm) | [81] |
| Trametes trogii | Mycelial extract | AgNO3 | Mostly spherical (5–65 nm) | [118] |
| Trichoderma atroviride | Mycelial extract | AgNO3 | 15–25 nm | [84] |
| Trichoderma atroviride | Cultural liquid | AgNO3 | Spherical (20–30 nm) | [42] |
| Mycelial extract | AgNO3 | Spherical (15–35 nm) | ||
| Trichoderma harzianum | Mycelial extract | AgNO3 | Spherical (10–25 nm) | [83] |
| Trichoderma harzianum | Mycelial extract | AgNO3 | Quasi-spherical (average size of 9.6 or 19.1 nm depending on the extraction method) | [82] |
| Trichoderma longibrachiatum | Crude xylanase | AgNO3 | Spherical, cylindrical, oval (15.21–77.49 nm) | [48] |
| Trichoderma longibrachiatum | Cultural liquid | AgNO3 | Spherical (5–15 nm) | [42] |
| Mycelial extract | AgNO3 | Spherical (10–25 nm) |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Agaricus arvensis | Living culture | HAuCl4 | Spherical (5–50 nm) | [41] |
| Cultural liquid | HAuCl4 | Spherical (2–10 nm) | ||
| Mycelial extract | HAuCl4 | Irregular spherical (25–20 nm) | ||
| Agaricus bisporus | Fruit body extract | HAuCl4 | Spherical (average size of 25 nm) | [132] |
| Agaricus bisporus | Living culture | HAuCl4 | Spherical (5–50 nm) | [41] |
| Cultural liquid | HAuCl4 | Spherical (2–10 nm) | ||
| Mycelial extract | HAuCl4 | Spherical (10–50 nm), hexagonal, tetragonal, triangular (30–100 nm) | ||
| Agaricus bisporus | Fruit body extract | HAuCl4 | Oval, spherical, drum-like, hexagonal, triangular (average size of 53 nm) | [133] |
| Agaricus bisporus | Fruit body extract | HAuCl4 | Spherical (10–50 nm) | [134] |
| Alternaria spp. | Fungal extract | HAuCl4 | Triangular, circular (average size of 28 nm) | [135] |
| Cantharellus sp. | Fungal extract | HAuCl4 | Spherical (average size of 60.6 nm) | [136] |
| Coprinus comatus | Fruit body extract | HAuCl4 | Face-centered cubic (average size of 17.39 nm) | [137] |
| Flammulina velutipes | Fruit body extract | HAuCl4 | Triangular, spherical, irregular (average size of 74.32 nm) | [138] |
| Fusarium oxysporum | Cultural liquid | HAuCl4 | Spherical, hexagonal (22–30 nm) | [139] |
| Fusarium solani | Biomass extract | HAuCl4 | Needle and flower-like structures with spindle shape (40–45 nm) | [140] |
| Ganoderma applanatum | Isolated phenolic compounds | HAuCl4 | Face-centered cubic (average size of 18.70 nm) | [141] |
| Ganoderma lucidum | Living culture | HAuCl4 | Spherical (5–50 nm) | [41] |
| Cultural liquid | HAuCl4 | Spherical (5–60 nm) | ||
| Mycelial extract | HAuCl4 | Spherical (10–50 nm), hexagonal, tetragonal, triangular (30–100 nm) | ||
| Ganoderma lucidum | Fruit body extract | HAuCl4 | Spherical, oval, irregular (1–100 nm) | [142] |
| Grifola frondosa | Living culture | HAuCl4 | Spherical (5–50 nm) | [41] |
| Cultural liquid | HAuCl4 | Spherical (2–10 nm) | ||
| Mycelial extract | HAuCl4 | Spherical (10–50 nm), hexagonal, tetragonal, triangular (30–100 nm) | ||
| Inonotus obliquus | Fruit body extract | HAuCl4 | Mostly spherical (below 20 nm) | [143] |
| Laetiporus versisporus | Fruit body extract | HAuCl4 | Spherical (average size of 10 nm) | [144] |
| Lentinus edodes | Fruit body extract | HAuCl4 | Triangular, hexagonal, spherical, irregular (average size of 72 nm) | [145] |
| Lentinus edodes | Living culture | HAuCl4 | Spherical (5–50 nm) | [41,146] |
| Cultural liquid | HAuCl4 | Spherical (2–20 nm) | ||
| Mycelial extract | HAuCl4 | Spherical (10–50 nm), hexagonal, tetragonal, triangular (30–200 nm) | ||
| Intracellular Mn-peroxidase | HAuCl4 | Spherical (2–20 nm) | ||
| Intracellular laccases and tyrosinases | HAuCl4 | Irregular spherical, triangular, tetrahedral (5–120 nm) | ||
| Morchella esculenta | Fruit body extract | HAuCl4 | Face-centered cubic (average size of 16.51 nm) | [147] |
| Penicillium janthinellum | Mycelial extract | HAuCl4 | Spherical (1–40 nm) | [69] |
| Phoma sp. | Mycelial biomass | HAuCl4 | Spherical (10–100 nm) | [148] |
| Pleurotus ostreatus | Living culture | HAuCl4 | Spherical (5–50 nm) | [41] |
| Cultural liquid | HAuCl4 | Spherical (2–20 nm) | ||
| Mycelial extract | HAuCl4 | Spherical (10–50 nm), hexagonal, tetragonal, triangular (30–200 nm) | ||
| Pleurotus sajor-caju | Fruit body extract | HAuCl4 | Spherical (average size of 16–18 nm) | [113] |
| Trichoderma hamatum | Mycelial extract | HAuCl4 | Spherical, pentagonal, hexagonal (5–30 nm) | [149] |
| Trichoderma harzianum | Mycelial biomass | HAuCl4 | Spherical (below 30 nm) | [150] |
| Tricholoma crassum | Mycelial extract | HAuCl4 | Circular, rhomboid (5 nm or less), hexagonal, cubic, triangular (4.36–22.94 nm) | [151] |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Alternaria alternata | Cultural liquid | H2PtCl6 | Irregular (50–315) | [157] |
| Fusarium oxysporum | Mycelial biomass | H2PtCl6 | Hexagonal, pentagonal, circular, square, rectangular (10–100 nm) | [158] |
| Fusarium oxysporum | Purified mycelial enzyme | PtCl2 | Rectangular, triangular (100–180 nm) | [159] |
| Purified mycelial enzyme | H2PtCl6 | Spherical (100–140 nm) | ||
| Fusarium oxysporum | Mycelial extract | H2PtCl6 | Irregular (30–40 nm) | [160] |
| Purified mycelial enzyme | H2PtCl6 | Circular, triangular, pentagonal, hexagonal, often as nanoplates (40–60 nm) | ||
| Fusarium oxysporum | Mycelial biomass | H2PtCl6 | Spherical (15–30 nm) | [161] |
| Fusarium oxysporum | Cultural liquid | H2PtCl6 | Face-centered cubic (average size of 25 nm) | [162] |
| Neurospora crassa | Mycelial biomass | H2PtCl6 | Quazi-spherical single PtNPs (4–35 nm) and spherical nanoaggregates (20–110 nm) | [163] |
| Mycelial extract | H2PtCl6 | Spherical nanoaggregates (17–76 nm), containing individual single crystals 2–3 nm in diameter | ||
| Penicillium chrysogenum | Cultural liquid | H2PtCl6 | Spherical (5–40 nm) | [164] |
| Saccharomyces boulardii | Cell free extract | H2PtCl6 | Spherical (80–150 nm) | [165] |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Agaricus bisporus | Mushroom extract | [Pd(OAc)2]n | Triangular and spherical (13–18 nm) | [168] |
| Inonotus obliquus | Fruit body powder extract | PdCl42− | Porous spherical | [169] |
| Saccharomyces cerevisiae | Biomass extract | [Pd(OAc)2]n | Hexagonal (average size of 32 nm), agglomerated | [170] |
| Saccharomyces cerevisiae | Biomass | Na2PdCl4 | Spherical (10–20 nm) | [171] |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Agaricus bisporus | Fruit body extract | Cu(NO3)2 | Spherical (10–60 nm) | [175] |
| Aspergillus flavus | Mycelial biomass | CuSO4 | Spherical (2–60 nm) | [176] |
| Aspergillus niger | Mycelial extract | CuSO4 | Spherical (5–100 nm) | [177] |
| Aspergillus versicolor | Mycelial extract | CuSO4 | Spherical, polygonal (23–82 nm) | [178] |
| Fusarium oxysporum | Mycelial biomass | Copper-containing waste | Spherical (93–115 nm) | [179] |
| Hypocrea lixii | Mycelial biomass | CuCl2 | Spherical (average size of 24.5 nm) | [180] |
| Shizophyllum commune | Mycelial biomass | CuCl2 | Spherical (40–65 nm) | [181] |
| Stereum hirsutum | Mycelial extract | CuCl2 | Spherical (5–20 nm) | [182] |
| Trichoderma atroviride | Mycelial extract | CuSO4 | Irregular spherical (5–25 nm) | [183] |
| Trichoderma koningiopsis | Mycelial biomass | CuCl2 | Spherical (average size of 87.5 nm) | [184] |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Alternaria alternata | Mycelial extract | Fe(NO3)3 | Cubic (average size of 9 nm) | [187] |
| Alternaria alternata | Mycelial extract | FeSO4 | Semi-oval (20–40 nm)/spherical (10–80 nm) | [188] |
| Aspergillus oryzae | Mycelial extract | FeCl3 | Spherical (10–24.6 nm) | [189] |
| Fusarium oxysporum | Mycelial biomass | K3Fe(CN)6 | Spherical (20–40 nm) | [190] |
| K4Fe(CN)6 | ||||
| Penicillium oxalicum | Mycelial extract | FeSO4 | Spherical (average size of 140 nm) | [191] |
| Pleurotus florida | Fruit body extract | FeCl3 | Spherical (100 nm) | [192] |
| Pleurotus sp. | Mycelial biomass | FeSO4 | – | [193] |
| Rhizopus stolonifer | Mycelial extract | FeCl3 | – | [194] |
| Trichoderma sp. | Mycelial extract | FeCl3 | – | [195] |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Agaricus arvensis | Cultural liquid | Na2SeO3 | Spherical (150–550 nm) | [41,153] |
| Mycelial extract | Na2SeO3 | Spherical (100–250 nm) | ||
| Agaricus bisporus | Cultural liquid | Na2SeO3 | Spherical (100–250 nm) | [41,153] |
| Mycelial extract | Na2SeO3 | Spherical (40–140 nm) | ||
| Alternaria alternata | Cultural liquid | Na2SeO4 | Spherical (30–150 nm) | [200] |
| Alternaria alternata | Cultural liquid | Na2SeO4 | Nanorods (200–800 nm in length, 50–70 nm in width) | [201] |
| Aspergillus flavus | Cultural liquid | Na2SeO4 | Spherical (average size of 51.5 nm) | [202] |
| Aspergillus ochraceus | Living culture | Na2SeO3 | Spherical (average size of 45.22 nm) | [203] |
| Aspergillus quadrilineatus | Living culture | Na2SeO3 | Spherical (average size of 55.37 nm) | [203]] |
| Aspergillus terreus | Cultural liquid | Se4+ ions solution | Spherical (average size of 47 nm) | [204] |
| Aspergillus terreus | Living culture | Na2SeO3 | Spherical (average size of 30.98 nm) | [203] |
| Aureobasidium pullulans | Living culture | Na2SeO3 | Spherical (average size of 60 nm) | [205] |
| Aureobasidium pullulans | Cultural liquid | Na2SeO3 | Spherical (20–120 nm) | [206] |
| Candida albicans | Cultural liquid | Na2SeO4 | Spherical (average size of 64 nm) | [202] |
| Fusarium equiseti | Living culture | Na2SeO3 | Spherical and rod-shaped (average size of 30.11 nm) | [203] |
| Fusarium oxysporum | Biomass | H2SeO3 | Spherical (34.32–231.98 nm) | [207] |
| Ganoderma lucidum | Living culture | Na2SeO3 | Spherical (20–50 nm) | [208] |
| Ganoderma lucidum | Cultural liquid | Na2SeO3 | Spherical (20–50 nm) | [41] |
| Mycelial extract | Na2SeO3 | Spherical (100–300 nm) | ||
| Grifola frondosa | Living culture | Na2SeO3 | Spherical (50–320 nm) | [208] |
| Grifola frondosa | Cultural liquid | Na2SeO3 | Spherical (20–50 nm) | [41] |
| Mycelial extract | Na2SeO3 | Spherical (100–300 nm) | ||
| Lentinus edodes | Living culture | Na2SeO3 | Spherical (50–320 nm) | [208] |
| Lentinus edodes | Cultural liquid | Na2SeO3 | Spherical (50–150 nm) | [41] |
| Mycelial extract | Na2SeO3 | Irregular spherical (50–150 nm) | ||
| Magnusiomyces ingens | Biomass extract | SeO2 | Spherical, quasi-spherical (70–90 nm) | [209] |
| Mariannaea sp. | Living culture | SeO2 | Spherical (average size of 45.19/212.65 nm depending on the nanoparticle location) | [210] |
| Mortierella humilis | Living culture | Na2SeO3 | Spherical (average size of 48 nm) | [205] |
| Nematospora coryli | Biomass | Na2SeO3 | Spherical (50–250 nm) | [211] |
| Penicillium chrysogenum | Cultural liquid | Na2SeO3 | Spherical (average size of 24.65 nm) | [212] |
| Penicillium chrysogenum | Cultural liquid | Na2SeO3 | Spherical (44–78 nm) | [213] |
| Penicillium chrysogenum | Cultural liquid | Na2SeO4 | Spherical (average size of 33.84 nm) | [214] |
| Penicillium citrinum | Biomass | HNaO3Se | Spherical (various sizes depending on the conditions) | [215] |
| Penicillium corylophilum | Cultural liquid | Na2SeO3 | Spherical (29.1–48.9 nm) | [216] |
| Penicillium crustosum | Cultural liquid | Na2SeO3 | Spherical (3–22 nm) | [217] |
| Penicillium expansum | Cultural liquid | SeO2 | Spherical (4–12.7 nm) | [218] |
| Phoma glomerata | Living culture | Na2SeO3 | Spherical (100–200 nm) | [219] |
| Pleurotus ostreatus | Living culture | Na2SeO3 | Spherical (50–320 nm) | [208] |
| Pleurotus ostreatus | Cultural liquid | Na2SeO3 | Spherical (50–150 nm) | [41] |
| Mycelial extract | Na2SeO3 | Irregular spherical (50–150 nm) | ||
| Rhodotorula mucilaginosa | Biomass | Na2SeO3 | Spherical, rod-shaped (83–478 nm depending on the precursor concentration) | [220] |
| Trichoderma atroviride | Mycelial extract | Na2SeO3 | Spherical (60.48–123.16 nm) | [221] |
| Trichoderma harzianum | Mycelial extract | Na2SeO3 | Irregular (average size of 60 nm) | [222] |
| Trichoderma sp. | Living culture | SeO2 | Spherical, pseudo-spherical (20–220 nm) | [223] |
| Trichoderma sp. | Mycelial extract | – | Spherical (40–100 nm) | [224] |
| Species | Source | Precursor | Nanoparticles | References |
|---|---|---|---|---|
| Aspergillus welwitschiae | Cultural liquid | K2TeO3 | Oval to spherical (60.80 nm) | [227] |
| Aureobasidium pullulans | Living culture | Na2TeO3 | Granular | [205] |
| Mortierella humilis | Living culture | Na2TeO3 | Granular | [205] |
| Penicillium chrysogenum | Cultural liquid | K2TeO3 | Spherical (average size of 50.16 nm) | [228] |
| Phanerochaete chrysosporium | Living culture | K2TeO3 | Needles (20–465 nm) | [229] |
| Phoma glomerata | Living culture | Na2TeO3 | Pillars, needles | [205] |
| Phoma glomerata | Living culture | Na2TeO3 | Rods (10–80 nm) | [219] |
| Trichoderma harzianum | Living culture | Na2TeO3 | Pillars, needles, agglomerated rods | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A. Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review. Biomimetics 2023, 8, 1. https://doi.org/10.3390/biomimetics8010001
Loshchinina EA, Vetchinkina EP, Kupryashina MA. Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review. Biomimetics. 2023; 8(1):1. https://doi.org/10.3390/biomimetics8010001
Chicago/Turabian StyleLoshchinina, Ekaterina A., Elena P. Vetchinkina, and Maria A. Kupryashina. 2023. "Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review" Biomimetics 8, no. 1: 1. https://doi.org/10.3390/biomimetics8010001
APA StyleLoshchinina, E. A., Vetchinkina, E. P., & Kupryashina, M. A. (2023). Diversity of Biogenic Nanoparticles Obtained by the Fungi-Mediated Synthesis: A Review. Biomimetics, 8(1), 1. https://doi.org/10.3390/biomimetics8010001






