Establishment of an Enteric Inflammation Model in Broiler Chickens by Oral Administration with Dextran Sulfate Sodium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management, Diets, and Experimental Design
2.2. Modeling Broiler Enteric Inflamamtion by DSS
2.3. Sample Collection
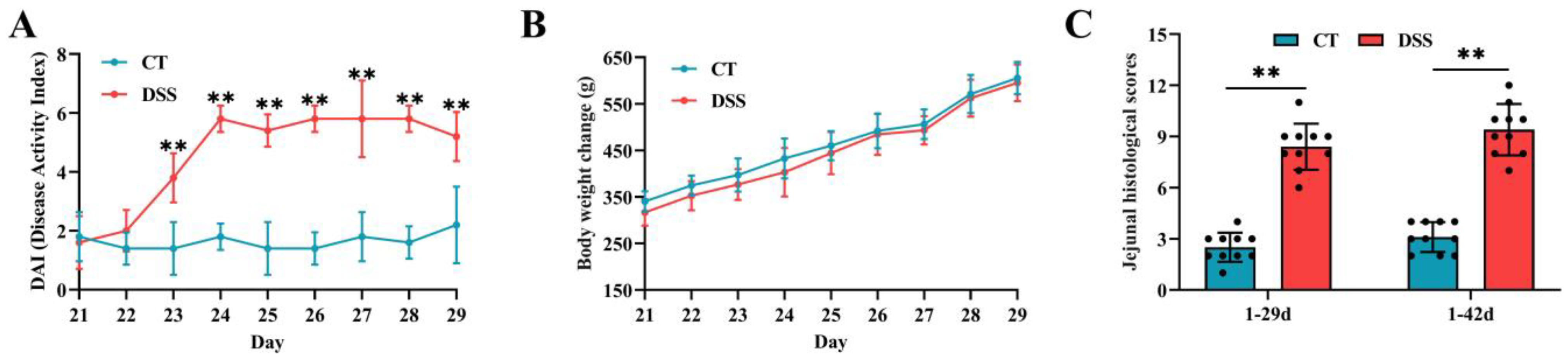
2.4. Criteria for DAI and Jejunal Histological Scores
2.5. Jejunal Gross Lesions
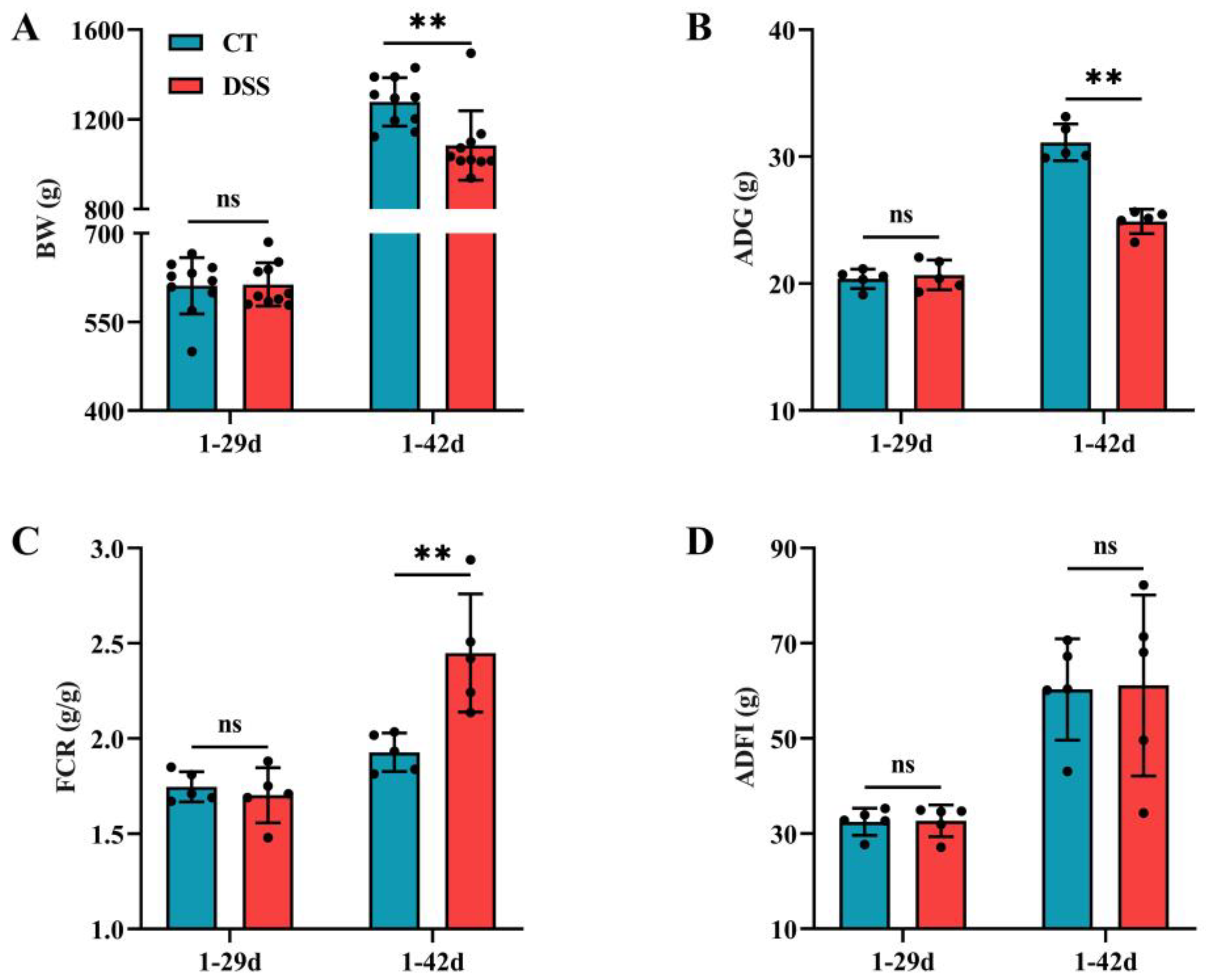
2.6. Broilers Growth Performance Parameters
2.7. Hematoxylin-Eosin (HE) Staining
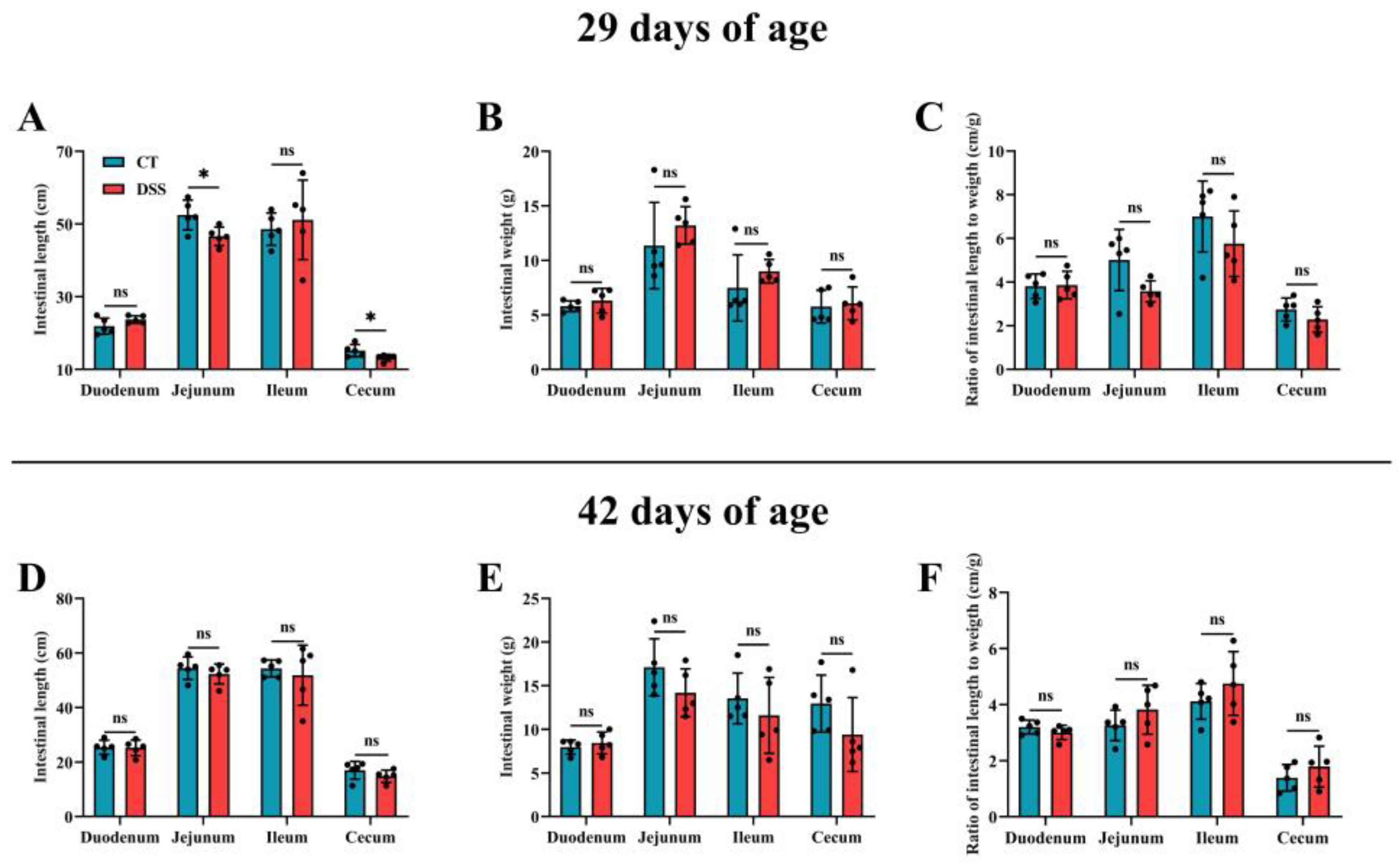
2.8. Intestinal Development
2.9. Statistical Analysis
3. Results
3.1. Effect of Oral Gavage of DSS on Signs of Enteric Inflammation
3.2. Effect of Oral Gavage of DSS on the Growth Performance of Broilers
3.3. Effect of Oral Gavage of DSS on Jejunal Lesion and Morphology
3.4. Effect of Oral Gavage of DSS on Intestinal Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, W.; Goes, E.C.; Wakaruk, J.; Barreda, D.R.; Korver, D.R. A poultry subclinical necrotic enteritis disease model based on natural Clostridium perfringens uptake. Front. Physiol. 2022, 13, 788592. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Pérez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Duran, D.; D’Angelo, M.; Solà-Oriol, D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Guo, L.; Xu, G.; Li, Z.; Appiah, M.O.; Yang, L.; Lu, W. Quercetin reduces inflammation and protects gut microbiota in broilers. Molecules 2022, 27, 3269. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Wang, Y.; Guo, Y.; Zhu, P.; Li, G.; Zhang, J.; Ma, Q.; Zhao, L. Dietary ellagic acid ameliorated Clostridium perfringens-induced subclinical necrotic enteritis in broilers via regulating inflammation and cecal microbiota. J. Anim. Sci. Biotechnol. 2022, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Vicuña, E.A.; Latorre, J.D.; Wolfenden, A.D.; Téllez, G.I.; Hargis, B.M.; Bielke, L.R. Evaluation of gastrointestinal leakage in multiple enteric inflammation models in chickens. Front. Vet. Sci. 2015, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Dal Pont, G.C.; Belote, B.L.; Lee, A.; Bortoluzzi, C.; Eyng, C.; Sevastiyanova, M.; Khadem, A.; Santin, E.; Farnell, Y.Z.; Gougoulias, C.; et al. Novel models for chronic intestinal inflammation in chickens: Intestinal inflammation pattern and biomarkers. Front. Immunol. 2021, 12, 676628. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Shen, Q.; Lyu, W.; Lv, L.; Wang, W.; Yu, M.; Yang, H.; Tao, S.; Xiao, Y. Clostridium butyricum and its derived extracellular vesicles modulate gut homeostasis and ameliorate acute experimental colitis. Microbiol. Spectr. 2022, 10, e0136822. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, J.; Sun, G.; Yang, H.; Sun, X.; Yao, X.; Lin, A.; Liu, H. 5-Aminosalicylic acid ameliorates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota and bile acid metabolism. Cell. Mol. Life Sci. CMLS 2022, 79, 460. [Google Scholar] [CrossRef]
- Dong, L.; Xie, J.; Wang, Y.; Jiang, H.; Chen, K.; Li, D.; Wang, J.; Liu, Y.; He, J.; Zhou, J.; et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat. Commun. 2022, 13, 4804. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, X.; Yin, M.; Li, C.; Han, L. Preventive effect of lycopene in dextran sulfate sodium-induced ulcerative colitis mice through the regulation of TLR4/TRIF/NF-κB signaling pathway and tight junctions. J. Agric. Food Chem. 2021, 69, 13500–13509. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, L.; Han, T.; Huang, H.; Chen, J. Dynamic changes in gut microbiome of ulcerative colitis: Initial study from animal model. J. Inflamm. Res. 2022, 15, 2631–2647. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef] [Green Version]
- Simon, K.; Arts, J.A.J.; de Vries Reilingh, G.; Kemp, B.; Lammers, A. Effects of early life dextran sulfate sodium administration on pathology and immune response in broilers and layers. Poult. Sci. 2016, 95, 1529–1542. [Google Scholar] [CrossRef]
- Nii, T.; Bungo, T.; Isobe, N.; Yoshimura, Y. Slight disruption in intestinal environment by dextran sodium sulfate reduces egg yolk size through disfunction of ovarian follicle growth. Front. Physiol. 2020, 11, 607369. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Berghman, L.R.; Vicuña, E.A.; Latorre, J.D.; Menconi, A.; Wolchok, J.D.; Wolfenden, A.D.; Faulkner, O.B.; Tellez, G.I.; Hargis, B.M.; et al. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015, 94, 1220–1226. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium butyrate alleviates mouse colitis by regulating gut microbiota dysbiosis. Animals 2020, 10, 1154. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Tong, Y.; Li, Q.; Yang, W.; Wang, T.; Yang, Z. Trans-anethole alleviates subclinical necro-haemorrhagic enteritis-induced intestinal barrier dysfunction and intestinal inflammation in broilers. Front. Microbiol. 2022, 13, 831882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhong, Q.; Liu, N.; Song, P.; Zhu, P.; Zhang, C.; Sun, Z. Dietary glutamine supplementation alleviated inflammation responses and improved intestinal mucosa barrier of LPS-challenged broilers. Animals 2022, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Yilmaz, B. Microbial drivers of DSS variability. Nat. Microbiol. 2022, 7, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Zhang, T.; Li, B.; He, Q.; Kwok, L.; Zhang, H. Oral administration of pasteurized probiotic fermented milk alleviates dextran sulfate sodium-induced inflammatory bowel disease in rats. J. Funct. Foods 2022, 94, 105140. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Huang, S.; Li, T.; Zhang, X.; Pang, J.; Zhao, J.; Chen, L.; Zhang, B.; Wang, J.; et al. Milk fat globule membrane attenuates acute colitis and secondary liver injury by improving the mucus barrier and regulating the gut microbiota. Front. Immunol. 2022, 13, 865273. [Google Scholar] [CrossRef]
- Belote, B.L.; Tujimoto-Silva, A.; Hümmelgen, P.H.; Sanches, A.W.D.; Wammes, J.C.S.; Hayashi, R.M.; Santin, E. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poult. Sci. 2018, 97, 2287–2294. [Google Scholar] [CrossRef]
- Menconi, A.; Hernandez-Velasco, X.; Vicuña, E.A.; Kuttappan, V.A.; Faulkner, O.B.; Tellez, G.; Hargis, B.M.; Bielke, L.R. Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poult. Sci. 2015, 94, 906–911. [Google Scholar] [CrossRef]
- Zou, X.; Ji, J.; Wang, J.; Qu, H.; Shu, D.M.; Guo, F.Y.; Luo, C.L. Dextran sulphate sodium (DSS) causes intestinal histopathology and inflammatory changes consistent with increased gut leakiness in chickens. Br. Poult. Sci. 2018, 59, 166–172. [Google Scholar] [CrossRef]
- Nunes, N.S.; Chandran, P.; Sundby, M.; Visioli, F.; da Costa Gonçalves, F.; Burks, S.R.; Paz, A.H.; Frank, J.A. Therapeutic ultrasound attenuates DSS-induced colitis through the cholinergic anti-inflammatory pathway. EBioMedicine 2019, 45, 495–510. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Chen, Z.; Xu, Z.; Xu, H. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett. 2011, 585, 3113–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, M.; Shojadoost, B.; Boodhoo, N.; Astill, J.; Taha-Abdelaziz, K.; Hodgins, D.C.; Kulkarni, R.R.; Sharif, S. Necrotic enteritis in chickens: A review of pathogenesis, immune responses and prevention, focusing on probiotics and vaccination. Anim. Health Res. Rev. 2021, 22, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Khalique, A.; Zeng, D.; Shoaib, M.; Wang, H.; Qing, X.; Rajput, D.S.; Pan, K.; Ni, X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. AMB Express 2020, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Akerele, G.; Al Hakeem, W.G.; Lourenco, J.; Selvaraj, R.K. The effect of necrotic enteritis challenge on production performance, cecal microbiome, and cecal tonsil transcriptome in broilers. Pathogens 2022, 11, 839. [Google Scholar] [CrossRef]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.M.; Wang, Y.; Liu, T.F.; Li, Y.; Luo, C.L. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef]
- Chen, J.Y.; Yu, Y.H. Bacillus subtilis-fermented products ameliorate the growth performance, alleviate intestinal inflammatory gene expression, and modulate cecal microbiota community in broilers during the starter phase under dextran sulfate sodium challenge. J. Poult. Sci. 2022, 59, 260–271. [Google Scholar] [CrossRef]
- Chen, Y.; Zha, P.; Xu, H.; Zhou, Y. An evaluation of the protective effects of chlorogenic acid on broiler chickens in a dextran sodium sulfate model: A preliminary investigation. Poult. Sci. 2022, 102, 102257. [Google Scholar] [CrossRef]
- Wassie, T.; Lu, Z.; Duan, X.; Xie, C.; Gebeyew, K.; Yumei, Z.; Yin, Y.; Wu, X. Dietary enteromorpha polysaccharide enhances intestinal immune response, integrity, and caecal microbial activity of broiler chickens. Front. Nutr. 2021, 8, 783819. [Google Scholar] [CrossRef]
- Lackeyram, D.; Mine, Y.; Archbold, T.; Fan, M.Z. The small intestinal apical hydrolase activities are decreased in the piglet with bowel inflammation induced by dextran sodium sulfate. J. Anim. Sci. 2012, 90, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Bungo, T.; Isobe, N.; Yoshimura, Y. Intestinal inflammation induced by dextran sodium sulphate causes liver inflammation and lipid metabolism disfunction in laying hens. Poult. Sci. 2020, 99, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Dzierzewicz, Z.; Cwalina, B.; Weglarz, L.; Wiśniowska, B.; Szczerba, J. Susceptibility of Desulfovibrio desulfuricans intestinal strains to sulfasalazine and its biotransformation products. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004, 10, Br185–Br190. [Google Scholar]
- Sanches, A.W.D.; Belote, B.L.; Hümmelgen, P.; Heemann, A.C.W.; Soares, I.; Tujimoto-Silva, A.; Tirado, A.G.C.; Cunha, A.F.; Santin, E. Basal and infectious enteritis in broilers under the I See Inside methodology: A chronological evaluation. Front. Vet. Sci. 2019, 6, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, L.; Qi, J.; Shao, B.; Ruan, Z.; Ren, Y.; Sui, S.; Wu, X.; Sun, X.; Liu, S.; Li, S.; et al. Microbial hydrogen economy alleviates colitis by reprogramming colonocyte metabolism and reinforcing intestinal barrier. Gut Microbes 2022, 14, 2013764. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Salvi, P.S.; Cowles, R.A. Butyrate and the intestinal epithelium: Modulation of proliferation and inflammation in homeostasis and disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The immunomodulatory functions of butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene alleviates dss-induced colitis and behavioral disorders via mediating microbes-gut-brain axis balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Zhang, Z.; Liu, T.; Fan, Y.; Liu, T.; Peng, N. Bacillus coagulans in combination with chitooligosaccharides regulates gut microbiota and ameliorates the Dss-induced colitis in mice. Microbiol. Spectr. 2022, 10, e0064122. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Nutritional Levels 2 | ||
|---|---|---|---|
| Corn | 54.19 | Metabolism energy (MJ/kg) | 12.12 |
| Soybean meal | 28.20 | Crude protein | 21.02 |
| Fish meal | 5.00 | Lysine | 1.30 |
| Corn gluten meal | 2.00 | Methionine | 0.56 |
| Bentonite | 3.00 | Calcium | 1.00 |
| Soybean oil | 3.00 | Available phosphorus | 0.48 |
| L-Lysine | 0.14 | ||
| DL-Methionine | 0.19 | ||
| Limestone | 1.50 | ||
| Dicalcium phosphate | 1.27 | ||
| Sodium chloride | 0.25 | ||
| Choline chloride | 0.26 | ||
| Vitamin and mineral premix 1 | 1.00 | ||
| Score | Severity | Body Weight Loss (%) | Fecal Quality | Fecal Blood |
|---|---|---|---|---|
| 0 | None | 0 | Normal | Normal |
| 1 | Mild | 1–5 | Soft feces | Blood on the fecal surface |
| 2 | Moderate | 5–10 | Irregular shaped feces | Blood on the fecal inner |
| 3 | Severe | >10 | Watery feces with gas | Bloody feces |
| Score | Inflammatory Cell Infiltration | Jejunal Structure Damage | ||
|---|---|---|---|---|
| Severity | Extent | Tissue Disruption | VH:CD Ratio | |
| 0 | None | None | None | >6 |
| 1 | Mild | Mucosa | Mild | 4–6 |
| 2 | Moderate | Submucosa | Moderate | 2–4 |
| 3 | Severe | Muscle layer | Severe | <2 |
| Criterion | Number of Hemorrhagic Spots |
|---|---|
| None | 0 |
| Mild | 1–2 |
| Moderate | 2–5 |
| Severe | 5–10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Sui, W.; Yang, Y.; Liu, L.; Li, Q.; Guo, A. Establishment of an Enteric Inflammation Model in Broiler Chickens by Oral Administration with Dextran Sulfate Sodium. Animals 2022, 12, 3552. https://doi.org/10.3390/ani12243552
Liu L, Sui W, Yang Y, Liu L, Li Q, Guo A. Establishment of an Enteric Inflammation Model in Broiler Chickens by Oral Administration with Dextran Sulfate Sodium. Animals. 2022; 12(24):3552. https://doi.org/10.3390/ani12243552
Chicago/Turabian StyleLiu, Lixuan, Wenjing Sui, Yajin Yang, Lily Liu, Qingqing Li, and Aiwei Guo. 2022. "Establishment of an Enteric Inflammation Model in Broiler Chickens by Oral Administration with Dextran Sulfate Sodium" Animals 12, no. 24: 3552. https://doi.org/10.3390/ani12243552
APA StyleLiu, L., Sui, W., Yang, Y., Liu, L., Li, Q., & Guo, A. (2022). Establishment of an Enteric Inflammation Model in Broiler Chickens by Oral Administration with Dextran Sulfate Sodium. Animals, 12(24), 3552. https://doi.org/10.3390/ani12243552





