1. Introduction
The existence of teeth in the oral cavity presents itself to be one of the most distinctive features of body anatomy. Saliva, mucosal, soft and hard tissue linings of teeth, and the diverse microbial communities are just some of the ecological niches that pertain to the oral cavity [
1]. The protrusion of teeth from the mucosal tissue in the oral cavity provides a solid surface for the formation of bacterial biofilms [
2]. Oral biofilms are formed by the complex network of interspecific competition between these microbial communities [
3] embedded in extracellular polymeric substances (EPS) matrices comprised of biological molecules, such as carbohydrates, proteins, and nucleic acids, which act as scaffolds to the structure [
4]. The microflora of the human body usually comprises saprophytic microorganisms, which start to colonize the human body even almost immediately after the birth of an individual, where bacteria are the dominant residents. They have an astonishing capability to attach and inhabit epithelial cells and to reproduce, and they are found readily in the human body. The oral microflora is home to more than 700 species of bacteria, as well as fungi, viruses, protozoa, and archaea [
5]. While bacterial taxa like Bacteroidetes and Firmicutes usually comprise the salivary microbiota, many other bacterial species are home to the oral cavity, which usually synergistically contribute with fungi to the formation of dental plaque and biofilms [
6,
7].
Dental caries is one of the most prevalent type of noncommunicable diseases, with more than 3.5 billion people affected worldwide [
8,
9]. Though significant improvements have been observed in regard to oral health and awareness, a high burden of this disease still subsists [
10]. It is a chronic disease marked by demineralization of dental surfaces, which are the result of acidic niches produced by metabolic products of biofilms [
11]. It can affect any gender and age and is notorious for causing pain, discomfort, and impacting routine life like other oral cavity-related diseases [
12]. Caries is a multi-stage disease that manifests over time with various agents of causation, such as dietary intake, lifestyle, smoking, socioeconomic status, and poor oral hygiene, all contributing significantly to its widespread distribution [
13]. Apart from these factors, the presence of bacterial species, carbohydrates, and vulnerable tooth surfaces are actively involved in the progression of caries [
14,
15]. Established treatments for dental caries include the use of fluorides and control of plaque through professional dental treatments and mouthwashes. However, the majority of affected people, especially from developing or third-world countries, lack sufficient awareness and dexterity to follow on these practices. Furthermore, agents like chlorhexidine gluconate in mouthwashes exhibit several undesirable side effects, such as elevated mineral uptake, irritation of mucosal surfaces, staining of teeth, and alteration of taste [
16].
Alternatively, using medicinal plants and their bioactive compounds for the effective management and prevention of oral diseases like dental caries has been investigated over the years, which implies that they have therapeutic applications and minimal side effects. While the earliest known use of herbal therapy to treat oral diseases is reported to date back to traditional Indian and Chinese medicine [
17], modern drugs comprising of single compound or a combination with others have proven plants and herbs to be effective against dental caries [
18].
Piper betle L. is a perennial native plant to Asian and Southeast Asian countries [
19]. It has characteristic heart-shaped leaves and is a member of the Piperaceae family, which houses more than a thousand plant species routinely found and grown in countries like India, Sri Lanka, and Bangladesh [
20]. Moreover, it has been used as a medicinal plant as its therapeutic potential has been reported in many studies conducted over the years. Betel leaves, due to their aroma, are routinely used in the treatment of bad breath and toothache. They are also used for the treatment of various medical conditions like conjunctivitis, itches, boils, abrasion, cuts, and wounds, as well as being used as a homeopathic medicine for treating female infertility [
21]. Along with its established antimicrobial activity, it also exhibits gastro- and hepato-protective activities, respectively [
22]. Betel leaves have significant value in the pharmaceutical industry as they are reported to possess aromatic, digestive, expectorant, euphoria-inducing, stimulative, antibacterial, antiprotozoal, carminative, aphrodisiac, and antifungal properties [
23].
This study aimed to evaluate the antibacterial, anti-biofilm, and antioxidative effect of
P. betle extract against bacteria isolated from dental caries. Furthermore, phytochemical characterization and in silico studies were performed to elucidate the types of phytochemical compounds present in
P. betle extract and to decipher the mechanism of action and binding affinity of these compounds with selected target proteins. The abbreviations used in this manuscript are given in
Table 1.
3. Discussion
Oral cavity is the largest organ after the gut that inhabits more than 700 microorganisms [
24] including bacteria, fungi, etc. [
5,
25]. Some of these organisms actively form biofilms on various oral surfaces such as dental prostheses and epithelial cells [
26]. They are also found to be the causative agents of oral diseases including tooth decay (caries), gum-related infections (gingivitis and periodontitis), and root canal infections (endodontitis). These diseases, which are most commonly occurring oral diseases in humans of all ages [
27], comprise a serious global health issue [
28]. Demineralization of tooth enamel is caused by the elevated acid production during glycolysis after intaking high carbohydrate food. The tooth enamel is restored by remineralization, which occurs after alkalinization and ultimately leads to the diffusion of acids from biofilms. These acids are buffered by salivary bicarbonate, salivary peptides, and the bacterial metabolism of urea and arginine. The stage comes where the acidification outweighs the alkalinization, which leads to the dental caries. Ultimately, the pH values lower and thus prolonged dental caries persists [
29]. The NIH recommends brushing, flossing, and mouthwash usage on the regular basis in order to avoid the oral cavity diseases [
30].
In this study, total 2500 cariogenic samples were collected, among which 1900 were positive cultures, while 600 were negative cultures (
Table 2). In the 165 positive culture samples, a different frequency of the various bacterial species was observed. Their molecular characterization and prevalence are given in the
Table 3. According to this study, the highest frequency of
S. mutans was observed, followed by
S. sobrinus,
S. aureus,
B. gaemokensis,
B. cereus,
B. subtilis,
S. haemolyticus, and
B. flexus, and the lowest frequency was
L. salivarius,
L. rhamnosus, and
P. stutzeri (
Table 3).
S. aureus, as a resident of the oral cavity of healthy adults, was already reported [
31]. The role of
Bacillus acidophilus as a cariogenic agent was reported earlier this century [
32,
33]. Early studies reported diverse microorganisms as cariogenic agents like
Streptococcus mutans [
34,
35,
36],
S. sanguinis,
Bacillus cereus [
37],
L. acidophilus [
38],
S. aureus [
39,
40,
41,
42],
S. sobrinus [
43,
44],
Pseudomonas stutzeri [
45],
S. haemolyticus [
46].
L. rhamnosus, and
L. salivarus found in the current study, which belongs to the casei group of
Lactobacillus [
47]. The biofilms formed by
B. cereus were reported by Majed et al. [
48].
B. subtilis from the oral cavity was reported previously [
38,
49,
50,
51]. Biofilm formation by oral cavity-inhabited
B. subtilis was reported by Jain et al. [
52]. According to Shaw [
53],
Bacillus fusiformis was one of the causative agents of acute necrotizing ulcerative gingivitis, leading to infection [
54].
B. licheniformis as a dental cariogenic agent was reported by Rostinawati et al. [
55].
B. acidophilus was reported as cariogenic agent by Tucker [
56]. Biofilm formation by
Bacillus species was reported previously [
57].
Bacillus subtilis from the oral cavity was reported by Yamane et al. [
58]. Biofilm of dental
B. subtilis [
52] and
B. licheniformis [
55] was reported. The role of
Bacillus in dental caries or
Bacillus as a cariogenic agent can be hypothesized from its adherence to the dental enamel, followed by its colonization, which ultimately results in the form of film or biofilm containing a large number of
Bacillus cells. Their source of energy is the remaining of the food particles that are left behind after taking meals. Out of these 15 bacterial species isolated in this study,
Bacillus gaemokensis was selected on the basis of its thick biofilm feature. In simple words, first the bacterial species were isolated and characterized at the molecular level; then, their biofilm forming ability was observed. Biofilm formation was the selective criteria for the selection of bacteria for further studies (
Figure 7). Here,
B. gaemokensis was selected on this basis.
B. gaemokensis is environmental microorganism. It was first reported by Jung et al. [
59] from tidal flat sediment of the Yellow Sea. Similarly,
Bacillus pumilis and
Bacillus flexus are the environmental microorganisms, among which the latter is known for its biofilm production [
57].
In our study, the most potent biofilm producer among the bacterial isolates was identified to be
Bacillus gaemokensis, which was also found to be the fourth most frequent isolate among collected samples (
Table 3). The use of medicinal plants for treating dental caries and other diseases has been well reported over the years, mediated by the action of various bioactive compounds reported in the composition of these plants [
58]. The antibacterial activity of
Piper betle and its extracts has been reported against various pathogens [
60,
61,
62,
63]. In this context, we investigated the antibacterial and anti-biofilm potential of
Piper betle extract against
B. gaemokensis isolated from dental caries. Nature has gifted plants with the presence of different substances that aid in the biological activities of living beings. These substances, known as phytochemical compounds, are largely responsible for the close association between their bioactivity and their therapeutic potential. These substances hold no nutritional value but are equipped with potent antimicrobial and other properties, which enable them to ward off disease [
64]. In this study,
P. betle extract was demonstrated to contain various phytochemical compounds (
Table 4). TLC analysis elucidated the presence of three compounds with different Rf values (
Figure 2). Aara et al. [
65] reported the Rf value of eugenol to be 0.84, which is almost equivalent to the Rf value observed in our study, thereby confirming the presence of eugenol. FTIR analysis demonstrated the absorbance peak at 3428.25 cm
−1, which corresponds to the –OH group found in phenolic compounds (
Figure 3). Additional bands of 2986.03 and 2903.03 cm
−1 are due to the C-O-H bonds. The rest of the bands (up to 1212.17 cm
−1) mark the presence of aromatic compounds, respectively. The band at 1013.50 cm
−1 is the sharpest, which can be associated with C-O stretching. Singh et al. [
66] also confirmed the presence of compounds like alcohols, phenolic compounds, alkanes, and alkenes, which was similar to our study. GC-MS analysis elucidated the presence of 20 phytocompounds, the majority of which are widely reported bioactive compounds having known antibacterial potential (
Table 5). The isolated fractions were investigated for their antibacterial activities against
B. gaemokensis, of which Spirost-8-en-11-one,3-hydroxy(3β,5α,14β,20β,22β,25R) was observed to demonstrate the most effective antibacterial action against the bacterium (
Figure 4). It has been suggested that the formation of the distinct structure of
B. subtilis biofilms relies on the ability of the bacterial cells to heterogeneously differentiate into motile, ECM-producing, and spore-forming cells, all within the bacterial colonies. This ability and the eventual phenotype are fundamentally pre-determined by various factors, such as temperature, availability of nutrients, oxygen, growth media, and availability of sugars, along with other factors [
67,
68]. The attachment of bacterial cells to the tooth surface leads to the formation of film or biofilm, which is composed of the dietary particles as well. Among various dietary constituents, sucrose is considered to be the most cariogenic in nature owing to its fermentable nature, resulting in low pH of the dental premises [
69]. The shift in resident microflora to more cariogenic one is in accordance with ecological plaque hypothesis [
70]. It ultimately leads to dental demineralization. Dental biofilms are directly affected by the dietary fermentable sugars including glucose, sucrose, maltose, fructose, etc. However, the clear direct effect of sucrose on physiology and biochemistry of the biofilm formation leading to enhancing dental caries has already been reported [
69]. Sucrose is a cariogenic dietary carbohydrate [
71]. Its metabolism leads to the acid production, which results in an acidic environment, thus promoting cariogenic aciduric bacterial flora and not alkali-producing bacteria, which causes dental demineralization due to formation of biofilms by aciduric bacterial species as observed in this study (
Table 3,
Figure 7) when the growth media (LB and TSB) were supplemented with sucrose.
The MIC of the extract was reported to be 100 mg mL
−1, which was then selected as the concentration to conduct further experiments (
Table 6). Antibacterial activity of
P. betle extracts (ethanol, methanol, and chloroform) demonstrated effective zones of inhibition, with the most effective activity being of the chloroform extract, which was then chosen for further analysis (
Table 7). In a previous research work,
P. betle extract was examined against dental plaque bacteria, which demonstrated its bacteriostatic effect against frequent oral pathogens [
72]. Rahman et al. [
73] also reported
P. betle leaf extract to be effective against
B. subtilis and
Staphylococcus aureus, respectively. The inhibitory action of
P. betle extract was observed on
B. gaemokensis and its established biofilm (
Figure 8a,b). In the biofilm experiments, the SEM images of 24 and 48 h old culture of
B. gaemokensis is shown in
Figure 9(a–c). The protective effects of
P. betle in the oral cavity have rendered the plant to be effective in the prevention of biofilm formation and reduction of gingival inflammation [
74,
75]. The time-kill assay also marked a trend of decrease in bacterial growth when
P. betle extract was added, which was also observed in other similar research findings [
76,
77] (
Figure 6). Various antioxidants like SOD, CAT, and GPx prevent, repair, and regulate the detrimental effects of oxidative stress by acting on their radical scavenging activity [
78]. Therefore, the role of antioxidants in the regulation of oxidative stress holds promise from a therapeutic standpoint [
79]. The expression of enzymes like APOX, POX, SOD, and GR in
B. gaemokensis were observed to be remarkably induced when bacterial cells were treated with
P. betle extract (
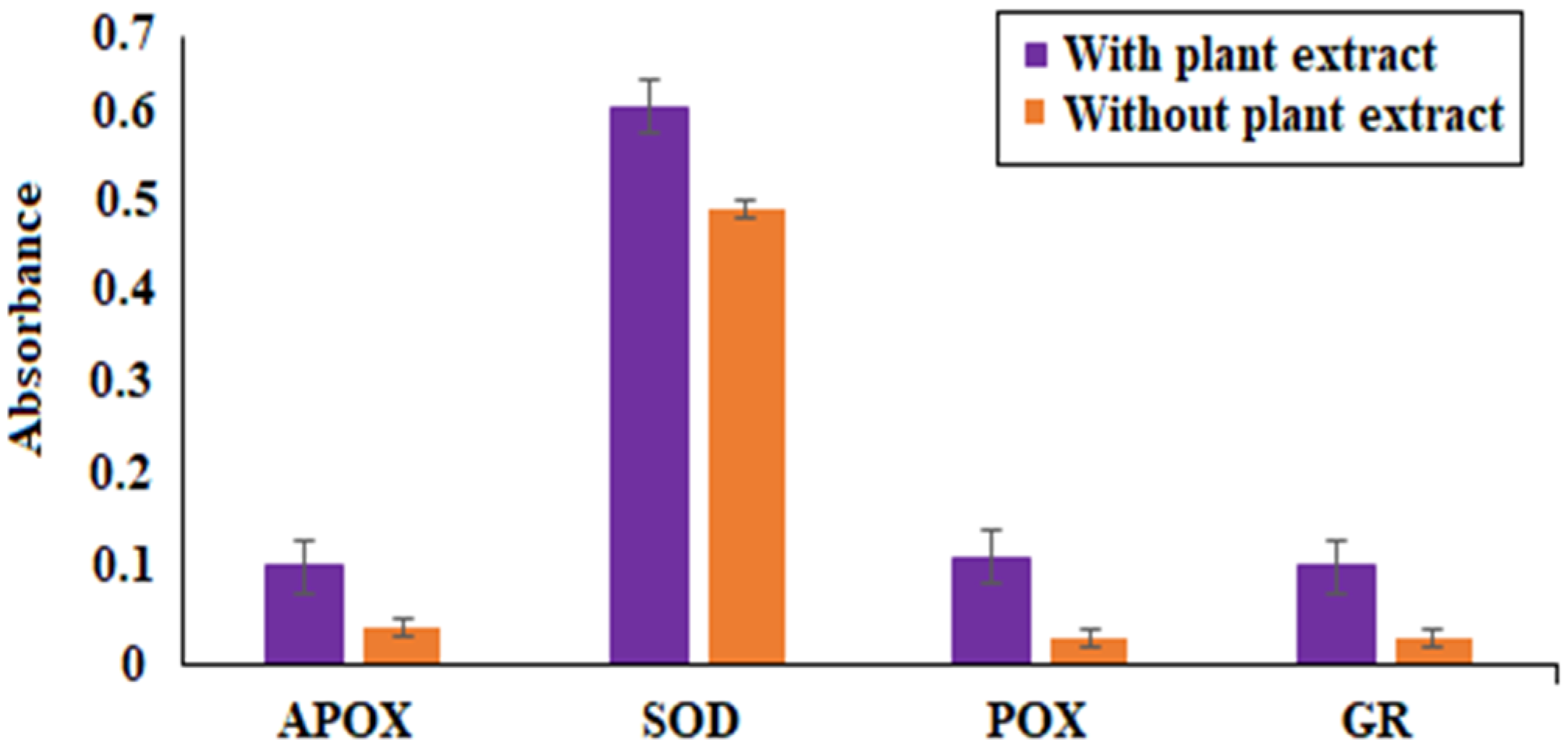
Figure 5). SOD activity was the most pronounced, while APOX, POX, and GR activity were roughly the same. The findings of our study agreed with Abrahim et al. [
80], where the increased expression of SOD was reported in the presence of
P. betle leaf extract. The elevated expression of the SOD enzyme demonstrates their ability to remove or scavenge superoxide anions, leading to the alleviation of reactive oxygen species (ROS). Profiling of bacterial proteins gives an insight into their complex genome, due to which the quantification and evaluating the expression of whole protein (treated and/or untreated) also serves a significant role when performing comparative analyses [
81]. In our study, the suppression of bacterial proteins in extract-treated cells suggests the proteolytic activity of the extract (
Figure 10). This activity can be attributed to the degradation of proteins by antibacterial (bioactive) compounds [
82,
83]. Moreover, it is important to note that the disappearance of whole cell proteins in treated bacterial cells indicates that their synthesis is not affected, but rather there is an inhibition of protective enzymes that sustain the cellular integrity of the bacterium [
84]. SEM analysis of treated and untreated
B. gaemokensis cells with
P. betle extract suggests noticeable morphological changes to the structure of bacterial cells (
Figure 11). Untreated cells were observed to be smooth and intact, while treatment with
P. betle extract rendered distortion in the cell structure, whereby cells became swollen and disintegrated. Formation of pores was apparent, as well as cell lysis. Cellular debris in the surroundings of the cells were also visible after treatment. Ramasamy et al. [
85] also reported the disintegration of cellular structure after treatment with plant extract.
The mechanism of action of
P. betle extract against
B. gaemokensis was ascertained by in silico studies, while docking was used to predict the binding of phytocompounds with potential target sites found in the bacterium. A systematic study comprising network construction and its visualization aided in understanding the signaling pathways involved in the action of
P. betle against dental caries. The PPIN revealed a diverse array of interacting moieties with
P. betle and its primary bioactive compounds like eugenol, caryophyllene, and phytol. Mediators like ESR1, SRC, and IL6 demonstrated the strongest association to bioactive compounds, stating their role in the mechanism of action of
P. betle (
Table 9). Dental fluorosis is linked to human ESR1 because estrogen or its receptors affect the activity of ameloblasts, which directly leads to the development of dental fluorosis. Similarly, GO terms and KEGG pathways are also crucial in understanding which pathways and genes are involved in the mechanism of action and up/downregulation of biological pathways involved with
P. betle (
Figure 12).
Molecular docking is a method that unravels the interactive abilities of a molecule with its target, which is customarily a protein. In this study, 20 compounds were selected for molecular docking, as revealed by the GC-MS analysis of
Piper betle extract (
Table 10). FabI is a well-reported protein that is involved in cell wall and cell membrane integration through the fatty acid biosynthesis pathway (FAS-II), which is attributable to the synthesis of lipids and fatty acids, the major primary constituents of the bacterial cell wall [
86]. FtsZ serves its key role in the cytokinetic machinery of the bacterial cytoskeleton, via the formation of a “Z” ring located at the center of the cell, which functions to constrict the cell division of the cell [
87]. In this study, Spirost-8-en-11-one,3-hydroxy(3β,5α,14β,20β,22β,25R) was observed to exhibit the highest binding energy against the three target sites. This in silico approach can be validated by previously mentioned results, whereby the compound was isolated and investigated for this antibacterial activity. Therefore, the docking analysis predicted that the compounds work with these proteins, which are involved in the essential regulation of the metabolism of bacteria, suggesting that targeting this mechanism may be one of the main routes that plant compounds use to exert their pharmacological and antimicrobial actions and pathological aspects on bacterial species and human beings. In vivo studies can help us to gain an insight into the practicability of Spirost-8-en-11-one,3-hydroxy(3β,5α,14β,20β,22β,25R) as an anticariogenic agent.