Abstract
Medical-device-related infections are considered a worldwide public health problem. In particular, urinary catheters are responsible for 75% of cases of hospital urinary infections (a mortality rate of 2.3%) and present a high cost for public and private health systems. Some actions have been performed and described aiming to avoid it, including clinical guidelines for catheterization procedure, antibiotic prophylaxis, and use of antimicrobial coated-urinary catheters. In this review paper, we present and discuss the functionalization of urinary catheters surfaces with antimicrobial entities (e.g., photosensitizers, antibiotics, polymers, silver salts, oxides, bacteriophage, and enzymes) highlighting the immobilization of photosensitizing molecules for antimicrobial photodynamic applications. Moreover, the characterization techniques and (photo)antimicrobial effects of the coated-urinary catheters are described and discussed. We highlight the most significant examples in the last decade (2011–2021) concerning the antimicrobial coated-urinary catheter and their potential use, limitations, and future perspectives.
1. Introduction
Health-care associated infections (HAIs) lead to an increase in morbidity and financial burden, and in some cases, resulting in death [1,2]. Among them, infections associated with medical devices (e.g., intravenous lines, endotracheal tubes, central venous catheters, urinary catheter and others) are responsible for approximately 70% of cases of nosocomial infections resulting in complications that patients need even more medical protocols including the use of antibiotic therapy (contributing to microbial resistance to clinical antibiotics) [3,4,5].
The medical devices are widely used in routine medical procedures, increasing life expectancy, providing a better stay for patients in hospitals and facilitating their recovery [4,6]. Herein, we highlight the urinary catheters (temporary or long-term use) which are extremely applied (20% and 61% in non-intensive and intensive care units, respectively) [1] for the treatment and relief of hospitalized patients or those with diseases that need constant use [7,8]. The urinary catheter are made by different polymers (such as silicone, polyvinyl chloride (PVC), polyurethane (PU), latex rubber) and present the function of draining urine from the bladder [9], being used in cases of debilitated, paralyzed or comatose patients, presenting incontinence and urinary retention [2] or in anesthetized or sedated patients in surgical procedures [1].
Besides the urinary catheters benefits, its use is precursor to more than 75% of cases of hospital urinary infections [10,11] and responsible for up to 40% of nosocomial infections showing a mortality rate of 2.3% [12]. Catheter-associated urinary tract infection (CAUTIs) occur during insertion of the probe into the urinary canal, external bacteria can pass through the lumen of the catheter or through the outside of the catheter from the urethra to the bladder [13]. Moreover, on the outside surface of the catheter, the biofilm formation begins via adhesion of pathogenic bacteria (i.e., Escherichia coli, Staphylococcus aureu, Pseudomonas aeruginosa, Klebsiella spp., and others) and/or fungi (e.g., Candida spp.) to catheter surface but it dependent on the hydrophobicity of the bacteria and the catheter [4]. It is important to mention that biofilm is a complex polymeric matrix formed by exopolysaccharides, proteins, and microbes that attach themselves to a surface, making it more difficult to eradicate [14,15] (Figure 1a).
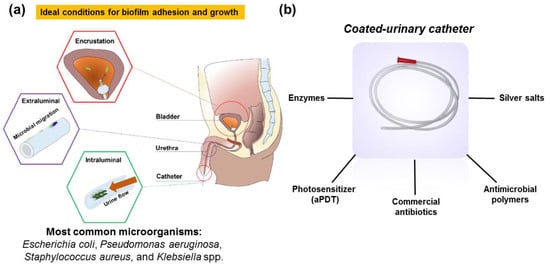
Figure 1.
(a) Pathogenesis of CAUTIs; (b) examples of antimicrobial coated-urinary catheter reported so far. Adapted from [23] (open access). Copyright 2021 MDPI.
Nowadays, in order to avoid a greater number of CAUTIs, there are clinical guidelines for the cases in which the probe should be used in hospital routine. It is recommended to apply only for specific cases, using for the time strictly necessary and also following aseptic catheter insertion technique (e.g., training, hand hygiene, adequate lubricant, and with smallest caliber catheter) [1,16,17]. Additionally, antibiotic prophylaxis has been largely performed, however, these strategies have not demonstrated sufficient efficiency due to the presence of multiresistant bacteria.
In this regard, several efforts have been made to propose a viable and efficient alternative to fight this nosocomial infection namely functionalization of urinary catheter surface with antimicrobial entities e.g., photosensitizers (for antimicrobial photodynamic therapy-aPDT) [18], antibiotics [19,20], polymers [21], silver salts [22], and others have been reported (Figure 1b). These antimicrobial catheters aim to avoid biofilm formation (including adhesion) and also biofilm eradication during the medical procedures.
Beside the complex composition of biofilm (proteins, extracellular DNA, enzymes, lipid, epithelial cells, and others) [17], the attachment of bacteria to a surface rapidly alters the expression of several genes responsible for the fabrication and maturation of extracellular exopolysaccharides, which results in a protective barrier against external chemical agents or the host’s defense system. In addition, the biofilm protects adherent bacteria from mechanical/physical stress, antimicrobial action, and the host’s immune defenses [24]. Herein, we analyze the past decade (2011–2021) concerning the functionalization and characterization of urinary catheter surfaces with antimicrobial entities namely photosensitizers (for aPDT), antibiotics, polymers, silver salts, oxides, bacteriophage, and enzymes. We present the most significant and illustrative examples for this period. For each example, we report in detail the functionalization processes, antimicrobial entities, characterization techniques, microorganisms used as well as their antimicrobial efficiency.
2. Functionalization of Urinary Catheter Surfaces with Antimicrobial Entities
2.1. Photosensitizing Molecules
Antimicrobial photodynamic therapy is an alternative and efficient tool able to photoinactivate pathogenic microorganisms such as bacteria, protozoa, fungi, and virus as demonstrated in several in vitro [25], in vivo [25] and clinical trials studies [26]. Due to its multi-target mode of action (unlike antimicrobial drugs), aPDT is described as a powerful tool showing a low probability to develop photoresistent strains and also able to kill antibiotic resistant bacteria [27].
Concerning its mechanism, aPDT acts based on a combination of a photosensitizing molecule (PS), molecular oxygen (O2), and a light source with appropriate wavelength with the purpose of produce reactive oxygen species (ROS) followed by oxidation of biomolecules present on pathogenic microorganisms. This mechanism of action can undergo two pathways and generate two groups of ROS: (i) H2O2 (hydrogen peroxide); O2− (superperoxide anion); OH (hydroxyl radical) (Type I mechanism); (ii) 1O2 (singlet oxygen) (type II mechanism), as described in Figure 2 [28].
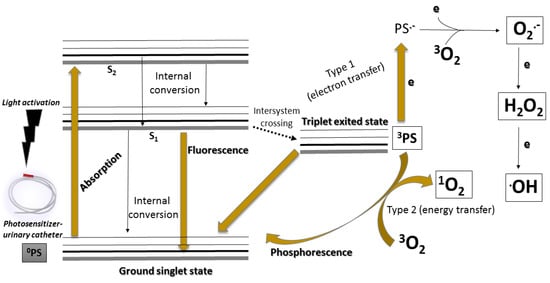
Figure 2.
Simplified Jablunski diagram that present the general mechanism of action of the aPDT.
In this regard, as a result of the high efficiency and low toxicity of aPDT, there are some studies which reported the functionalization of the urinary catheter surface with photosensitizers (organic and inorganics) [29]. This functionalization aims to produce an antimicrobial photodynamic action on functionalized-urinary catheter surface when illuminated avoiding the presence of pathogenic microorganisms and growth of biofilm [30]. Moreover, beyond the antimicrobial photodynamic action, the urinary catheter should maintain its ideal physicochemical (wettability and roughness) and mechanical (stiffness and flexibility) properties after the functionalization process in order to produce the exact clinical function. Figure 3 shows the 2D molecular structures of photosensitizers immobilized onto urinary catheters that are described in this work.
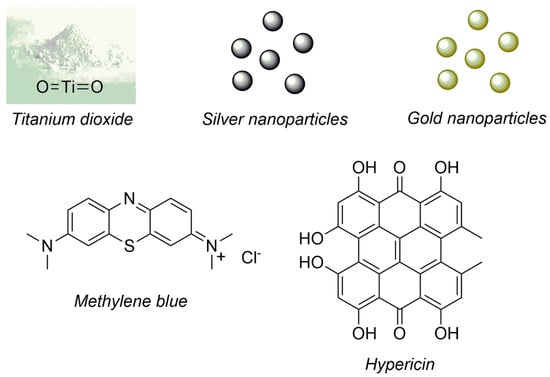
Figure 3.
Photosensitizers immobilized onto urinary catheters and described in this work.
In 2015, Bovis and collaborators described the incorporation of methylene blue and gold nanoparticles (AuNPs) on silicone urinary catheter surface by a simple methodology (swell–encapsulation–shrink’ technique) [31]. For that, the authors added AuNP and methylene blue (at 700 mg L−1) in acetone during 24 h. This functionalized urinary catheter was characterized by transmission electron microscopy (TEM), fluorescence microscopy, fiber optic confocal laser endomicroscopy, time-resolved EPR spectroscopy, continuous-wave electron paramagnetic resonance (EPR) spectroscopy, time-resolved 1O2 phosphorescence detection, 1O2 measurements with sensor green probe, and O2 consumption during laser irradiation. Furthermore, the functionalized silicone urinary catheter (segments) presented 3 log10 of photoreduction using Staphylococcus epidermidis bacteria as a bacteria model under illumination (at 660 nm, 45 J/cm2). The authors also reported that the prior sterilization of functionalized urinary catheter with ethylene oxide did not modify the photoantibacterial effect.
In 2019, Amaro and co-authors presented at the 17th International Photodynamic Association World Congress (MA, United States) a covalent functionalization process of silicone urinary catheter with a derivative metalloporphyrin (the exact molecular structure of the derivative metalloporphyrin was not described by the authors) [18]. Initially, the authors functionalized the urinary catheter with amine groups using 3-aminopropyltriethoxysilane (APTES) or 3-aminopropyltrimethoxysilane (APTMS) followed by reaction with the derivative metalloporphyrin. The metalloporphyrin-functionalized urinary catheter showed a considerable photoantibacterial effect against P. aeruginosa and S. epidermidis (inactivation up to 99.9%) under illumination (at 532 nm, 100 mW, during 30 and 60 min).
In 2019, Vögeling and co-authors evaluated the photoantibacterial action of a modified-polyurethane catheter [32]. The catheter surface was functionalized by inclusion of a complex (hypericin and 2-hydroxypropyl)-β-cyclodextrin) or a liposomal particle containing hypericin (a natural anthraquinone) as photosensitizer using layer by layer approach. This photoactivated-catheter was characterized by scanning electron microscope (SEM), atomic force microscopy (AFM) and the concentration of hypericin on its surface was also determined. Its photoantibacterial effect was evaluated against Staphylococcus saprophyticus subsp. bovis (DSM 18669) biofilm presenting a reduction of 4.3 log10 and improved by addition of ultrasound (bacterial reduction of 6.8 log10). In this regard, ultrasound has been described as an antimicrobial tool through activation of a sonosensitizer. Its mechanism is based on inertial cavitation produced by collapsing microbubbles and formation of free radicals [33].
The functionalization of photosensitizers onto urinary catheters shows as a promising tool to photoinactivate pathogenic microorganisms onto catheter surfaces. At the last years, few photosensitizers (such as methylene blue, gold nanoparticles, and silver nanoparticles) were evaluated without any systematic evaluation. Other classes of photosensitizers (e.g., porphyrin, chlorin, bacteriochlorin, and phthalocyanines) should be tested as well as their in vitro/in vivo safety studies. Moreover, the light source device should be designed aiming at the extracorporeal illumination of the urinary tract.
2.2. Antibiotics and Antimicrobial Agent
Antibiotics are natural, semi-synthetic or synthetic compounds that interfere with the development of bacteria, inhibiting their growth or even causing their death aiming to fight bacterial infections [34]. These are divided into classes, such as phosphomycin, nitrofurantoins, sulfonamides and beta-lactams, examples of antibiotics most used in cases of urinary infections [35]. In order to develop antibacterial urinary catheters, some authors described the immobilization of commercial antibiotics onto urinary catheters. This functionalization shows the same goal of the aPDT, in other words, to promote the inhibition of biofilm and pathogenic bacteria on the catheter surface. Figure 4 describes the 2D molecular structures of antibiotics (sparfloxacin and rifampicin) and an antimicrobial agent (triclosan) immobilized onto urinary catheters described and discussed in this work.
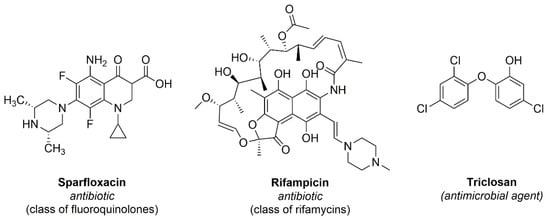
Figure 4.
Antibiotics and an antimicrobial agent immobilized onto urinary catheters and described in this work.
Table 1 shows a description of the functionalized urinary catheters with commercial antibiotics (2011–2021) and their evaluation parameters used for characterization, bacteria applied, and their efficiency.

Table 1.
Antibiotics- and antimicrobial agent-functionalized urinary catheters applied against bacteria.
Fischer and co-authors (in 2015) (Table 1, entry 1) [36] reported the impregnation of of antibiotics (e.g., rifampicin and sparfloxacin) and an antimicrobial agent (triclosan) on silicone-based urinary catheters and their evaluation against E. coli, P. mirabilis, K. pneumoniae, E. faecalis, and S. saprophyticus from clinical isolates from patients with CAUTIs (Figure 5). To analyze the functionalization processes applied, these antibiotic impregnated-urinary catheters were analyzed by XPS, AFM, and HPLC. Additionally, the release of the antibiotics from the functionalized urinary catheters were analyzed by HPLC and the following results were obtained: rifampicin was not detected until 28 days; 5.9–33.4% for triclosan and 11.1–44.3% for sparfloxacin. Furthermore, the authors evaluated the antimicrobial activity of these commercial antibiotics (e.g., rifampicin, triclosan, and sparfloxacin) on the urinary catheter surfaces and observed that no viable S. aureus, S. saprophyticus or K. pneumoniae were present after 24 h. Moreover, E. coli was completely killed after 48 h and E. faecalis in 72 h. P. mirabilis suffered a reduction of 99.9% after 72 h.
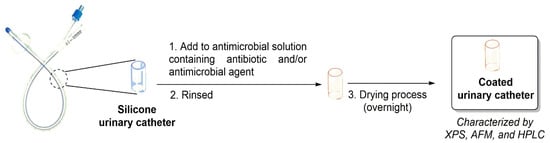
Figure 5.
General impregnation process of urinary catheter with antibiotics and antimicrobial agent.
In 2019, Belfield and co-authors [19] (Table 1, entry 2) evaluated an antimicrobial urinary catheter impregnated with a group of antibiotics (rifampicin (0.080% w/w), triclosan (1.084% w/w), and sparfloxacin (0.704% w/w)) against beta-lactamase producing E. coli, and carbapenemase-producing E. coli, P. mirabilis, S. saprophyticus, and E. coli collected from ureteral stents and indwelling urinary catheters. The functionalized urinary catheters were characterized by AFM, XPS, TEM, static model of encrustation, in vitro flow model of encrustation, SEM, and in vitro flow challenge model. The results demonstrated that the roughness of the catheter surface after the functionalization methodology was not altered. The authors observed that the antimicrobial urinary catheter inhibited for 12 weeks the establishment by methicillin-resistant S. epidermidis, MRSA, carbapenemase-producing E. coli, and extended-spectrum beta-lactamase producing E. coli.
In 2020, Burroughs and co-authors [20] (Table 1, entry 3) reported two different strategies for preventing bacterial biofilm attachment and formation onto urinary catheter surfaces. The authors performed the coating of silicone catheter with polyacrylate (polymerization reaction) impregnated with rifampicin, sparfloxacin and triclosan antibiotics using solutions with concentrations of 0.2%, 1.0%, and 1.0%, respectively. Besides, these coated-catheters were evaluated against E. coli and S. aureus bacteria (obtained from patients with CAUTI) showing a bacteria reduction and biofilm formation prevention. In order to characterize the functionalized urinary catheters, ToF-SIMS and SEM tests were carried out. The authors observed that the polyacrylate coatings did not kill planktonic bacteria, which is consistent with the mechanism of action of the polyacrylate acting specifically on biofilm formation prevention by an electrostatic interaction between the polymer and the biofilm matrix.
Overall, the use of antibiotics onto catheters is useful antimicrobial strategy, however the development of bacteria resistance should be considered and analyzed before their use on a large scale. Moreover, the leaching of the antibiotics and antimicrobial agents from the catheters should be analyzed and described.
2.3. Antimicrobial Polymers
Antimicrobial polymers (natural, semi-synthetic or synthetic) are agents whose surface hinders bacterial adhesion and, consequently, the biofilm formation [37]. These polymers also act by damaging the cell membrane of bacteria and also can release (delivery systems) antibiotics, nanoparticles, and other chemical or biological entities. Table 2 shows a description of antimicrobial coatings for urinary catheters (2011–2021) presenting their parameters applied for characterization, microorganisms, and efficiency.

Table 2.
Antimicrobial polymers applied against microorganisms and the parameters used.
In 2019, Matej and co-authors [38] (Table 2, Entry 1) immobilized colloidal polysaccharides (chitosan derivatives and hyaluronic acid combined with a lysine-based surfactant) onto silicone to create an antimicrobial coating. These polymers were tested against E. coli, Methicillin resistant S. aureus (MRSA), S. aureus, F+ conjugated E. coli, and Candida albicans. The materials surface were characterized by FTIR, XPS, CLSM and SEM. The most effective process to build the coating of polydimethylsiloxane with colloidal polysaccharide complex was the discontinuous 3-step dip-coating process. The developed coating demonstrated an inhibition of bacterial growth up to 86% and the hyaluronic acid surfactant-based coating also displayed a significant biofilm prevention.
In 2020, Alves and co-authors [39] (Table 2, Entry 2) evaluated a polymer brush (a type of polymer characterized by the presence of long polymer chains covalently attached to a surface and chains mobile) made of poly [N-(2-hydroxypropyl) methacrylamide] (poly(HPMA)) aiming to prevent mineral encrustation and the formation of E. coli biofilm on catheter’s surface. The surface characterization was done by Contact angle measurement, AFM, SEM and measurement of the average roughness (Ra). The efficacy of the poly(HPMA) brush as an antimicrobial coating was analyzed using synthetic urine as a growth medium in a parallel plate flow chamber (PPFC). Moreover, the authors demonstrated a reduction up to 87% and that this process makes biofilms already formed more susceptible to the action of antibiotics.
Kisuk and co-authors [40] (Table 2, Entry 3) reported the functionalization of a set of polymers such as polyvinyl chloride (PVC, polyurethane (PU), and polydimethylsiloxane (PDMS) (used to preparation of urinary catheter) with chitosan, hyaluronic acid, and human serum albumin (in some cases impregnated with silver nanoparticles) and tested against E. coli and S. aureus. According to the authors, these functionalized polymers were characterized by SEM, contact angle measuring, scanning probe microscope (SPM), XPS and UV-Vis. The authors also observed a reduction in bacterial adhesion/biofilm formation (up to ~100%) against S. aureus and E. coli and using chitosan embedded with AgNPs on the PU polymer. Moreover, the authors showed that the coating’s antimicrobial properties can be modulated by adding antibacterial metallic nanoparticles (e.g., Ag+).
Costa and co-authors (Table 2, Entry 4) developed a marine cyanobacterial polymer-based coating (CyanoCoating) for urinary catheter and evaluated against a set of microorganisms (P. mirabilis, E. coli, MRSA, K. pneumoniae, and Candida albicans) [41]. The CyanoCoating was characterized by AFM, water contact angle (captive bubble method), SEM and EDAX. According to the authors, this coating showed a decrease on microbial adhesion up to 68 ± 28% for P. mirabilis; 95 ± 48% for K. pneumoniae; 80 ± 27% for S. aureus (MRSA); 69 ± 19% for C. albicans. A reduction on biofilm formation also was observed (up to 60% for E. coli, P. mirabilis, and C. albicans). Moreover, tests were performed using artificial urine, demonstrating a reduction of 65 ± 28% (E. coli), 98 ± 54% (K. pneumoniae), 95 ± 34% (S. aureus-MRSA) and 100% (C. albicans).
In 2020, Alves and co-authors [42] (Table 2, Entry 5) developed a poly [oligo(ethylene glycol) methyl ether methacrylate] (poly(MeOEGMA)) as an antimicrobial coating against E. coli. The authors performed the characterization of coated-catheter by OCT, and SEM. After 24 h, the poly(MeOEGMA) brush reduced by 57% the adhesion of E. coli and, adding ampicillin obtained a reduction up to 88% for E. coli.
Brill and collaborators [43] (Table 2, Entry 6) applied a solution of 0.02% polyhexanide onto urethral catheter and evaluated its antibacterial efficiency against E. coli, P. mirabilis, and methicillin-resistant S. aureus (MRSA). A reduction of 1.64 log10 (swab extraction) and 2.56 log10 for membrane filtration compared to the control catheter were observed. The swab extraction and membrane filtration methods are based on use of a sterile cotton swab to collect the bacteria from the inner lumen of the catheters and the application of a solution to extract bacteria of the catheter surfaces followed by microbial count, respectively. Moreover, the authors did not show any characterization data regarding the presence of polyhexanide on the catheter surface.
Khandwekar and co-authors (Table 2, Entry 7) reported a study of the anti-fouling capacity and effectiveness of polyurethane polymer modified with a polyvinylpyrrolidone complex (PVP-I) (Tecoflex®) against S. aureus and P. aeruginosa [44]. The functionalized-catheter was characterized by FTIR, AFM and SEM-EDAX and results showed an adhesion reduction of 86% for S. aureus and 80% for P. aeruginosa. Furthermore, the lubricity of the polymer modified with (Tecoflex®) was analyzed, demonstrating a greater lubricity in comparison with the non-adapted polyurethane.
A group of researchers [45] (Table 2, Entry 8) reported the functionalization of urinary catheter with methoxylated polyethylene glycol 3,4-dihydroxyphenylalanine (DOPA) plus AgNO3 or NaIO4 and its characterization by contact angle analysis, and testing against E. coli. The authors described that the best results were obtained using DOPA polymer with 0.25 mg/mL AgNO3, showing a reduction of 99.9% for E. coli. Moreover, the authors reported that the concentration used for AgNO3 is safe for application in humans.
In 2018, Raut and co-authors [21] (Table 2, Entry 9) reported the use of polyvinylpyrrolidone–iodine complex (PVPI) as coating for polyurethane (PU). This coated-polymer was applied against S. aureus, S. epidermidis and P. aeruginosa and characterized by contact angle, FTIR, CSLM, SEM-EDAX, TGA, and DSC. Antimicrobial tests were done using different concentrations of PVPI from 0.5 to 1.5% p/p in the PU/PVPI blends and a progressive increase of antimicrobial activity to the increase of PVPI concentration was observed. The PVPI concentration of 1.5% w/w demonstrated the best results, reducing the bacterial adhesion up to 99.8% for S. aureus, 99.0% for S. epidermidis and 89.0% for P. aeruginosa.
Mahata and co-authors [46] (Table 2, Entry 10) complexed N-glycidyl histidine ether with tannic acid followed by its functionalization onto the urinary catheter surface. This new catheter was characterized by a set of techniques (described in Table 2, entry 10) and a bacterial adhesion reduction (up to 90%) was observed for clinically isolated uropathogen E. coli. Wang and co-authors [47] (Table 2, Entry 11) reported the functionalization of silicone urinary catheter surface pre-treated with polydopamine (PDA) with a carboxymethyl chitosan derivative (CMCS) and its application against E. coli and P. mirabilis. The authors observed 90% of reduction for P. mirabilis and E. coli biofilm adhesion.
2.4. Silver Salts
Silver (Ag+) presents a long history as an antimicrobial agent [48]. In this regard, silver (e.g., salt form, colloidal, nanoparticles, and other pharmaceutical formulations) has been used as an antiseptic for surgical procedures, wound treatment, dentistry, water purification, and in medical devices surfaces. Regarding its toxicity, there are some studies describing that Ag+ is toxic against mammalian cells (in vitro) but research is needed to evaluate its toxicity in a controlled and randomized clinical study [49,50]. Due to its efficiency against a vast group of pathogenic microorganisms, some authors promoted its functionalization onto medical devices such as urinary catheters. Table 3 shows a description of functionalized urinary catheters with silver (2011–2021) and their evaluation parameters used for characterization, microorganisms, and efficiency.

Table 3.
Silver-functionalized urinary catheters applied against microorganisms.
Kumar and co-authors [51] (Table 3, Entry 1) reported the use of Kocuran, an exopolysaccharide produced by Kocuria rosea strain BS-1 to synthesize silver glyconanoparticles by a green protocol. The functionalized medical device was characterized by SAED, colloidal stability at different pH, CSLM, and SPR. This functionalized catheter was evaluated against S. aureus and E. coli showing an inhibition of biofilm development up to 90%. Moreover, the authors evaluated the cytotoxicity of the functionalized catheter using human gingival fibroblasts presenting a low toxicity.
Evliyaoglu and co-authors [52] (Table 3, Entry 2) reported the synthesis of Ag-incorporated nano-HA coated urinary catheters and their evaluation against prophylaxis of biofilm formation and also bacteriuria using rabbit models (3, 5 and 7 days of the urethral catheterization time in rabbits). The authors described the evaluation of a control group (siliconized latex-based urethral catheters) for comparison with the functionalized urinary catheter. For bacterial analysis, urine and catheter surface were used for quantification of efficiency of the coated catheter. Concerning the results obtained, the authors showed that at the end of 7 days (catheterization protocol), the number of the rabbits with the functionalized catheter bacteriuria was significantly lower when compared to the control group.
Wang and co-authors [53] (Table 3, Entry 3) developed a nanocomposite of silver-polytetrafluoroethylene (Ag-PTFE) as coating and its deposition on a silicone urethral catheter surface. This coated-catheter was tested against E. coli and anti-encrustation performances against P. mirabilis and characterized by SEM, EDX and contact angle measurement. To evaluate this antimicrobial coating, two concentrations of bacterial suspension challenged each model and results were used and demonstrated an increase in the time to begin biofilm formation compared to the control group (6 to 41 days).
Wang and co-authors [22] (Table 3, Entry 4) developed a coating for urinary catheter using polydopamine (PDA) and silver nanoparticles (AgNPs) and promoted its characterization using FESEM, XPS analysis, CLSM, spread plate method and contact angle measurement. The coated-catheter was tested against E. coli, P. aeruginosa and P. mirabilis. Results demonstrated that the release of silver depends on the number of PDA-AgNPs bilayers on the modified catheter surface. One AgNP-PDA by layer is capable of resisting encrustation for 12 days, while two bilayers in combination with poly(SBMA-co-AAm) as a final graft layer could resist up to 45 days and reduced by 99% the adhesion of bacteria on catheter surface.
The catheters immobilized with silver salts should be functionalized with efficient protocols due to the leaching of silver from the catheters. This leaching can produce an accumulation and consequent in vivo toxicity. In vitro and in vivo experiments should be performed using analytical techniques to analyze it.
2.5. Antimicrobial Derivative Compounds, Bacteriophages, and Enzymes
Besides the investigation of aPDT, antibiotics, antibacterial polymers, and silver salts as antimicrobial coating for urinary catheter, there are other strategies and compounds being applied so far. Table 4 shows a description of functionalized urinary catheters with antimicrobial compounds immobilized on nanoparticles, molecules extracted from soil, use of bacteriophages, and antimicrobial enzymes (2011–2021) and their evaluation parameters used for characterization, microorganisms, and their efficiency.

Table 4.
Functionalization of urinary catheters applied against microorganisms.
Kanugala and co-authors [54] (Table 4, Entry 1) reported the synthesis of mesoporous silica nanoparticles via base-catalyzed sol-gel process followed by immobilization of phenazine-1-carboxamide onto their surface. The nanoparticles were characterized by FT-IR, UV-vis, XRD spectroscopic techniques, DLS, SEM, TGA, TEM, and BET analysis. Regarding the antimicrobial evaluation, the phenazine-1-carboxamide functionalized mesoporous silica nanoparticles as a coating layer for ureteral catheters was evaluated against C. albicans-S. aureus polymicrobial biofilm. The authors described that the PCN-MSNPs-immobilized urethral catheters at a concentration of 2.23 mM showed no formation of C. albicans-S. aureus biofilms. However, the author did not present any evaluation concerning its toxicity.
Janek and co-authors [55] (Table 4, Entry 2) used a biosurfactant produced by Rhodococcus fascians BD8, isolated from Arctic soil, characterized by HPLC, and surface tension reduction and reported its antiadhesive and antimicrobial properties against several pathogenic microorganisms. Results showed a reduction of 95% of C. albicans and 70% for E. coli biofilm adhesion to a silicone and polystyrene surface. This biosurfactant presents potential to be applied as an antimicrobial coating for urinary catheter.
Lehman and co-authors [56] (Table 4, Entry 3) reported a pre-treated silicone urethral catheter with a hydrogel containing a blend of P. aeruginosa and P. mirabilis bacteriophages and tested against multiresistant bacteria (clinical isolates) in a continuous-flow in vitro model using artificial urine. The authors evaluated biofilm growth at 72–96 h and observed a reduction up to 4 log10 CFU/cm2 and >2 log10 CFU/cm2 for P. aeruginosa and P. mirabilis, respectively.
Cadavid and co-authors [57] (Table 4, Entry 4) conducted a study to demonstrate the formation of K. pneumoniae biofilm under the effect of several natural substances. To define which compounds would follow in the study, different concentrations of the compounds were tested for the viability of the bacteria. The compounds that reached 85% reduction in viability went on to analyze the ability to inhibit biofilm formation. The best results were obtained of 20-hydroxycinnamic acid and 3-methyl-2(5H)-furanone, which inhibited the formation of biofilm by 65.06% and 67.38%, respectively. Moreover, the authors observed alterations in the adherence and biofilm formation on the PVC-based urethral catheter by SEM.
Ivanova and co-authors [58] (Table 4, Entry 5) promoted the functionalization of a silicone urinary catheter with acylase enzyme via layer-by-layer technique (negatively charged enzyme and positively charged polyethyleneimine) to inhibit the growth of P. aeruginosa biofilm. The surface characterization of the acylase coated catheter was carried out by FTIR, AFM, water contact angle measurements, and fluorescence microscopy. The acylase-immobilized urinary catheter was able to reduce the P. aeruginosa biofilm (up to ~50%) and did not affect the viability of human fibroblasts (over 7 days).
Colleta and co-authors [59] (Table 4, Entry 6) carried out a functionalization process of silicone Foley catheter with S-Nitroso-N-acetylpenicillamine (SNAP) (a release nitric oxide (NO) polymer) and its application on reduction of microbial biofilm formation (S. epidermidis and P. mirabilis). The NO is widely described as a potent antimicrobial agent and vasodilator. The authors characterized the coated-catheter with UV-Vis, Sievers chemiluminescence and fluorescence microscopy and a generation of NO surface-fluxes of 0.7 × 10−10 mol min−1 cm−2. Concerning the antimicrobial evaluation, the best result of S. epidermidis was after 14 days, with a biofilm formation reduction of 3.7 log. To P. mirabilis the best result was also after 14 days, with a reduction of 6 log.
3. Conclusions and Future Perspectives
The main challenges in treating infections caused by microorganisms associated with biofilms are their challenging diagnosis and in the clinic, biofilms can also be difficult to eradicate due to high antibiotic tolerance [24]. In this context, the control of microbial biofilm-associated infections in medical devices requires that new and effective approaches be developed [17]. Strategies for the control of biofilms can be divided between the prevention of biofilm formation by inhibiting or blocking the adherence of microorganisms and the eradication of pre-formed biofilms through the use of strategies such as aPDT, nanotechnology, direct combat, electric current, and the development of antimicrobial-coated medical devices [60].
In order to address infections in the urinary tract, over the last years (2010–2021) a set of antimicrobial coatings have been prepared and applied onto the urinary catheter surface. These coatings are based on use of covalent and electrostatic strategies using photosensitizing entities for application in aPDT protocol (production of ROS), commercial antibiotics, synthetic and natural polymers, the traditional silver salt, and other antimicrobial entities such as enzymes, and bacteriophages. Regarding the antimicrobial evaluation and efficiency, the authors have evaluated these coated-urinary catheters against planktonic and biofilm (different species and composition) aiming to demonstrate the inhibition of growth and elimination (% or log unit) and satisfactory results have been described in the literature. However, different protocols for biofilm formation have been applied and a standardization on experimental protocol should be done to facilitate the comparison of the results. Concerning the safety tests for the new coated-urinary catheter, some studies have presented in vitro cytotoxicity tests using fibroblast cells showing low toxicity but more tests (e.g., animal models) should be made to prove the real safety of the new coated-urinary catheters. According to the literature and the results presented in this review paper, we propose the following perspectives for the next studies for the urinary catheters: (i) to explore the use of photodynamic action as described for others medical devices (such as endotracheal tube) [61]; (ii) combination of commercial antibiotics and antimicrobial coatings; (iii) to present a systematic safety study of the coated-urinary catheter; (iv) to promote the standardization of the biofilm formation protocols; (v) to evaluate the mechanical properties (i.e., tensile property) of the functionalized-urinary catheters; (vi) to transpose the coated-urinary catheter described in literature for in vivo evaluation (e.g., animal models); (vii) to evaluate the cost and viability of the proposal antimicrobial coatings.
In sum, those antimicrobial coatings for urinary catheters can be useful for decrease of number and severity of UTIs but the health professionals should apply the clinical guidelines for catheter management in order to prevent the catheter-associated urinary tract infections [12,62].
Author Contributions
Conceptualization, L.D.D.; methodology, L.D.D., H.B.N. and V.S.B.; validation, L.D.D., L.S.D., P.L.F.N., H.B.N. and V.S.B.; formal analysis, L.D.D., L.S.D., P.L.F.N., H.B.N. and V.S.B.; data curation, L.D.D., L.S.D., P.L.F.N., H.B.N. and V.S.B.; writing—original draft preparation, L.D.D. and L.S.D.; writing—review and editing, L.D.D., L.S.D., P.L.F.N., H.B.N. and V.S.B. visualization, L.D.D., L.S.D., P.L.F.N., H.B.N. and V.S.B.; supervision, L.D.D.; project administration, L.D.D.; funding acquisition, L.D.D., L.S.D., P.L.F.N., H.B.N. and V.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (CEPOF 2013/07276-1) and INCT “Basic Optics and Applied to Life Sciences” (FAPESP 2014/50857-8, CNPq465360/2014-9). L. S. Duarte thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for Scientific Initiation grant 138853/2020-7. L. D. Dias thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for Post-doc grant 151188/2022-0.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, M.J.; Flores-Mireles, A.L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef]
- Anjum, S.; Singh, S.; Lepoittevin, B.; Roger, P.; Panigrahi, M.; Gupta, B. Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances. Glob. Chall. 2018, 2, 1700068. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.M.C.; Lemos, S.M.C.; Monge, N.; Ribeiro, I.A.C. A scope at antifouling strategies to prevent catheter-associated infections. Adv. Colloid Interface Sci. 2020, 284, 102230. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, H.C.; Yu, H.; Yan, S.J.; Luan, S.F. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020, 8, 4095–4108. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, D.M.; Darouiche, R.O. New strategies to prevent catheter-associated urinary tract infections. Nat. Rev. Urol. 2012, 9, 305–314. [Google Scholar] [CrossRef]
- Al-Qahtani, M.; Safan, A.; Jassim, G.; Abadla, S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: A review on recent updates. J. Infect. Public Health 2019, 12, 760–766. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 108, 110319. [Google Scholar] [CrossRef]
- Dellimore, K.H.; Helyer, A.R.; Franklin, S.E. A scoping review of important urinary catheter induced complications. J. Mater. Sci.-Mater. Med. 2013, 24, 1825–1835. [Google Scholar] [CrossRef]
- Werneburg, G.T.; Nguyen, A.; Henderson, N.S.; Rackley, R.R.; Shoskes, D.A.; Le Sueur, A.L.; Corcoran, A.T.; Katz, A.E.; Kim, J.; Rohan, A.J.; et al. The Natural History and Composition of Urinary Catheter Biofilms: Early Uropathogen Colonization with Intraluminal and Distal Predominance. J. Urol. 2020, 203, 357–363. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Wang, Z.P.; Li, S.H.; Yuan, X. Antimicrobial strategies for urinary catheters. J. Biomed. Mater. Res. Part A 2019, 107, 445–467. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Andrade, V.L.F.; Fernandes, F.A.V. Prevention of catheter-associated urinary tract infection: Implementation strategies of international guidelines. Rev. Lat.-Am. Enferm. 2016, 24, e2678. [Google Scholar] [CrossRef][Green Version]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef]
- Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; Alahmari, F.S.; Baykal, A.; et al. Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 572. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Palanivel, V.; Thangavelu, S.; Paramasivam, R.; Muthupandian, S. Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS ONE 2021, 16, e0256748. [Google Scholar] [CrossRef]
- Conway, L.J.; Larson, E.L. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung 2012, 41, 271–283. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.Y.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Amaro, F.; Gomez-Mendoza, M.; Descalzo, A.B.; Rivas, L.; Orellana, G. Self-sterilizing photoactivated catheters to prevent nosocomial infections. In Proceedings of the 17th World Congress of the International-Photodynamic-Association (IPA), Cambridge, MA, USA, 28 June–4 July 2019. [Google Scholar]
- Belfield, K.; Chen, X.Y.; Smith, E.F.; Ashraf, W.; Bayston, R. An antimicrobial impregnated urinary catheter that reduces mineral encrustation and prevents colonisation by multi-drug resistant organisms for up to 12 weeks. Acta Biomater. 2019, 90, 157–168. [Google Scholar] [CrossRef]
- Burroughs, L.; Ashraf, W.; Singh, S.; Martinez-Pomares, L.; Bayston, R.; Hook, A.L. Development of dual anti-biofilm and anti-bacterial medical devices. Biomater. Sci. 2020, 8, 3926–3934. [Google Scholar] [CrossRef]
- Raut, P.W.; Khandwekar, A.P.; Sharma, N. Polyurethane-polyvinylpyrrolidone iodine blends as potential urological biomaterials. J. Mater. Sci. 2018, 53, 11176–11193. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2015, 103, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.J.; Gadd, G.M.; Zhao, Q. Marine Microbial-Derived Antibiotics and Biosurfactants as Potential New Agents against Catheter-Associated Urinary Tract Infections. Mar. Drugs 2021, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Bacterial Infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Stringasci, M.D.; Requena, M.B.; Blanco, K.C.; Dias, L.D.; Correa, T.Q.; Bagnato, V.S. Randomized and Controlled Clinical Studies on Antibacterial Photodynamic Therapy: An Overview. Photonics 2022, 9, 340. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C-Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Lellouche, J.; Friedman, A.; Lahmi, R.; Gedanken, A.; Banin, E. Antibiofilm surface functionalization of catheters by magnesium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 1175–1188. [Google Scholar] [CrossRef]
- Al Rugaie, O.; Abdellatif, A.A.H.; El-Mokhtar, M.A.; Sabet, M.A.; Abdelfattah, A.; Alsharidah, M.; Aldubaib, M.; Barakat, H.; Abudoleh, S.M.; Al-Regaiey, K.A.; et al. Retardation of Bacterial Biofilm Formation by Coating Urinary Catheters with Metal Nanoparticle-Stabilized Polymers. Microorganisms 2022, 10, 1297. [Google Scholar] [CrossRef]
- Bovis, M.J.; Noimark, S.; Woodhams, J.H.; Kay, C.W.M.; Weiner, J.; Peveler, W.J.; Correia, A.; Wilson, M.; Allan, E.; Parkin, I.P.; et al. Photosensitisation studies of silicone polymer doped with methylene blue and nanogold for antimicrobial applications. Rsc Adv. 2015, 5, 54830–54842. [Google Scholar] [CrossRef]
- Vogeling, H.; Plenagl, N.; Seitz, B.S.; Duse, L.; Pinnapireddy, S.R.; Dayyoub, E.; Jedelska, J.; Brussler, J.; Bakowsky, U. Synergistic effects of ultrasound and photodynamic therapy leading to biofilm eradication on polyurethane catheter surfaces modified with hypericin nanoformulations. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 103, 109749. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhou, Q.F.; Deng, Z.T.; Pan, M.; Liu, X.; Wu, J.R.; Yan, F.; Zheng, H.R. IR-780 Dye as a Sonosensitizer for Sonodynamic Therapy of Breast Tumor. Sci. Rep. 2016, 6, 25968. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Lelie- van der Zande, R.; Bouvy, M.; Teichert, M. Adherence to guideline recommendations for urinary tract infections in adult women: A cross-sectional study. Prim. Health Care Res. Dev. 2021, 22, e11. [Google Scholar] [CrossRef]
- Fisher, L.E.; Hook, A.L.; Ashraf, W.; Yousef, A.; Barrett, D.A.; Scurr, D.J.; Chen, X.Y.; Smith, E.F.; Fay, M.; Parmenter, C.D.J.; et al. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J. Control. Release 2015, 202, 57–64. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef]
- Bracic, M.; Sauperl, O.; Strnad, S.; Kosalec, I.; Plohl, O.; Zemljic, L.F. Surface modification of silicone with colloidal polysaccharides formulations for the development of antimicrobial urethral catheters. Appl. Surf. Sci. 2019, 463, 889–899. [Google Scholar] [CrossRef]
- Alves, P.; Gomes, L.C.; Vorobii, M.; Rodriguez-Emmenegger, C.; Mergulhao, F.J. The potential advantages of using a poly(HPMA) brush in urinary catheters: Effects on biofilm cells and architecture. Colloids Surf. B-Biointerfaces 2020, 191, 110976. [Google Scholar] [CrossRef]
- Yang, K.; Kim, K.; Lee, E.A.; Liu, S.S.; Kabli, S.; Alsudir, S.A.; Albrahim, S.; Zhou, A.; Park, T.G.; Lee, H.; et al. Robust Low Friction Antibiotic Coating of Urethral Catheters Using a Catechol-Functionalized Polymeric Hydrogel Film. Front. Mater. 2019, 6, 274. [Google Scholar] [CrossRef]
- Costa, B.; Mota, R.; Tamagnini, P.; Martins, M.C.L.; Costa, F. Natural Cyanobacterial Polymer-Based Coating as a Preventive Strategy to Avoid Catheter-Associated Urinary Tract Infections. Mar. Drugs 2020, 18, 279. [Google Scholar] [CrossRef]
- Alves, P.; Gomes, L.C.; Rodriguez-Emmenegger, C.; Mergulhao, F.J. Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia coli Biofilm Formation and Susceptibility. Antibiotics 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Brill, F.H.H.; Gabriel, H.; Brill, H.; Klock, J.H.; Steinmann, J.; Arndt, A. Decolonization potential of 0.02% polyhexanide irrigation solution in urethral catheters under practice-like in vitro conditions. BMC Urol. 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khandwekar, A.P.; Doble, M. Physicochemical characterisation and biological evaluation of polyvinylpyrrolidone-iodine engineered polyurethane (Tecoflex (R)). J. Mater. Sci. -Mater. Med. 2011, 22, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- MacPhee, R.A.; Koepsel, J.; Tailly, T.; Vangala, S.K.; Brennan, L.; Cadieux, P.A.; Burton, J.P.; Wattengel, C.; Razvi, H.; Dalsin, J. Application of Novel 3,4-Dihydroxyphenylalanine-Containing Antimicrobial Polymers for the Prevention of Uropathogen Attachment to Urinary Biomaterials. J. Endourol. 2019, 33, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Mahata, D.; Mandal, S.M.; Basak, A.; Nando, G.B. Self-assembled capsules of poly-N-glycidyl histidine ether-tannic acid for inhibition of biofilm formation in urinary catheters. RSC Adv. 2015, 5, 69215–69219. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Shi, Z.L.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Inhibition of escherichia coli and proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol. Bioeng. 2012, 109, 336–345. [Google Scholar] [CrossRef]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tabaran, F.A.; Mocan, L. Review on Silver Nanoparticles as a Novel Class of Antibacterial Solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Niskanen, J.; Shan, J.; Tenhu, H.; Jiang, H.; Kauppinen, E.; Barranco, V.; Pico, F.; Yliniemi, K.; Kontturi, K. Synthesis of copolymer-stabilized silver nanoparticles for coating materials. Colloid Polym. Sci. 2010, 288, 543–553. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Kumar, C.G.; Sujitha, P. Green synthesis of Kocuran-functionalized silver glyconanoparticles for use as antibiofilm coatings on silicone urethral catheters. Nanotechnology 2014, 25, 325101. [Google Scholar] [CrossRef]
- Evliyaoglu, Y.; Kobaner, M.; Celebi, H.; Yelsel, K.; Dogan, A. The efficacy of a novel antibacterial hydroxyapatite nanoparticle-coated indwelling urinary catheter in preventing biofilm formation and catheter-associated urinary tract infection in rabbits. Urol. Res. 2011, 39, 443–449. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Keatch, R.; Corner, G.; Nabi, G.; Murdoch, S.; Davidson, F.; Zhao, Q. In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J. Hosp. Infect. 2019, 103, 55–63. [Google Scholar] [CrossRef]
- Kanugala, S.; Jinka, S.; Puvvada, N.; Banerjee, R.; Kumar, C.G. Phenazine-1-carboxamide functionalized mesoporous silica nanoparticles as antimicrobial coatings on silicone urethral catheters. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Janek, T.; Krasowska, A.; Czyznikowska, Z.; Lukaszewicz, M. Trehalose Lipid Biosurfactant Reduces Adhesion of Microbial Pathogens to Polystyrene and Silicone Surfaces: An Experimental and Computational Approach. Front. Microbiol. 2018, 9, 2441. [Google Scholar] [CrossRef]
- Lehman, S.M.; Donlan, R.M. Bacteriophage-Mediated Control of a Two-Species Biofilm Formed by Microorganisms Causing Catheter-Associated Urinary Tract Infections in an In Vitro Urinary Catheter Model. Antimicrob. Agents Chemother. 2015, 59, 1127–1137. [Google Scholar] [CrossRef]
- Cadavid, E.; Echeverri, F. The Search for Natural Inhibitors of Biofilm Formation and the Activity of the Autoinductor C6-AHL in Klebsiella pneumoniae ATCC 13884. Biomolecules 2019, 9, 49. [Google Scholar] [CrossRef]
- Ivanova, K.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Enzyme multilayer coatings inhibit Pseudomonas aeruginosa biofilm formation on urinary catheters. Appl. Microbiol. Biotechnol. 2015, 99, 4373–4385. [Google Scholar] [CrossRef]
- Colletta, A.; Wu, J.F.; Wo, Y.Q.; Kappler, M.; Chen, H.; Xi, C.W.; Meyerhoff, M.E. S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device To Prevent Catheter-Associated Urinary Tract Infections. ACS Biomater. Sci. Eng. 2015, 1, 416–424. [Google Scholar] [CrossRef]
- Huang, D.N.; Wang, J.; Ren, K.F.; Ji, J. Functionalized biomaterials to combat biofilms. Biomater. Sci. 2020, 8, 4052–4066. [Google Scholar] [CrossRef]
- Zangirolami, A.C.; Dias, L.D.; Blanco, K.C.; Vinagreiro, C.S.; Inada, N.M.; Arnaut, L.G.; Pereira, M.M.; Bagnato, V.S. Avoiding ventilator-associated pneumonia: Curcumin-functionalized endotracheal tube and photodynamic action. Proc. Natl. Acad. Sci. USA 2020, 117, 22967–22973. [Google Scholar] [CrossRef]
- Musco, S.; Giammo, A.; Savoca, F.; Gemma, L.; Geretto, P.; Soligo, M.; Sacco, E.; Del Popolo, G.; Li Marzi, V. How to Prevent Catheter-Associated Urinary Tract Infections: A Reappraisal of Vico’s Theory-Is History Repeating Itself? J. Clin. Med. 2022, 11, 3415. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).