Abstract
Alcoholic liver disease (ALD), caused by excessive alcohol consumption, leads to high mortality. We investigated the hepatoprotective effect of Levilactobacillus brevis MG5311 in C57BL/6 mice with liver injuries induced by chronic ethanol plus binge feeding. L. brevis MG5311 was administered orally at a dose of 1 × 109 CFU/mouse once daily for 32 days. L. brevis MG5311 administration significantly reduced serum ALT, AST, and triglyceride (TG) levels in ethanol-fed mice. L. brevis MG5311 also decreased malondialdehyde levels and increased glutathione peroxidase (GPx) activity in liver tissues. In addition, hepatic TG content and histopathological scores were significantly reduced. L. brevis MG5311 increased the protein expression of SIRT1, PPARα, SOD1, CAT, and GPx 1/2 in liver tissue, while inhibiting CYP2E1 and SREBP-1c. These results indicated that L. brevis MG5311 alleviated ethanol-induced liver injury by inhibiting hepatic oxidative stress and promoting lipid metabolism. Therefore, L. brevis MG5311 may be a useful probiotic candidate for ameliorating or preventing ALD.
1. Introduction
Alcoholic injury, characterized by multi-systemic and diverse pathophysiology, adversely affects a patient’s quality of life. Alcoholic liver disease (ALD) is caused by the chronic excessive consumption of alcohol. The number of ALD patients is rapidly increasing worldwide, and the associated high morbidity and mortality rates are emerging as serious issues [1,2]. ALD covers a broad spectrum of diseases, including early-stage fatty liver, steatohepatitis, liver fibrosis, cirrhosis, and liver cancer [3]. Metabolic disorders, such as obesity and diabetes, may exacerbate liver problems caused by the fatty liver disease along with ALD [4,5]. However, there is currently no approved drug or appropriate treatment other than abstinence or liver transplantation [6]. Therefore, there is an urgent need to develop new therapeutic agents to prevent or alleviate ALD.
Excessive alcohol consumption induces endotoxemia, alters the gut microbiome profile (dysbiosis), disrupts the gut barrier function, and increases intestinal permeability. Metabolites such as lipopolysaccharides (LPS) produced by gut bacteria leak into the hepatic portal vein, causing features of ALD, such as liver inflammation and fibrosis [7,8]. Alcohol metabolism in the liver promotes the production of acetaldehydes and reactive oxygen species (ROS) and weakens the antioxidant defense system. Hepatic oxidative stress results in alcohol-induced lipid peroxidation and tissue damage, including metabolic changes, inflammation, and cell membrane dysfunction [9].
The World Health Organization (WHO) defines probiotics as “living microorganisms that, when consumed in adequate amounts, confer a health benefit on the host,” and the most commonly used as probiotics are lactic acid bacteria (LAB) [10]. Some LAB strains provide various health benefits to the host, such as antimicrobial, anti-inflammatory, antioxidative, anti-allergic, and improving lipid metabolism and immune responses [11,12,13,14]. Recent studies have extensively demonstrated a close relationship between host health and gut microbiota [15]. The “gut-liver axis” has demonstrated that the imbalance of the gut microflora is an important factor exacerbating the progression of ALD [16]. LAB can prevent ALD by inhibiting hepatic oxidative stress and improving intestinal barrier function, thereby reducing endotoxemia in the gut-liver axis [16,17]. Segawa et al. (2008) reported that heat-killed Lactobacillus brevis SBC8803 inhibited gut-derived endotoxin migration into the liver of ethanol-containing diet-fed mice [18]. Lactobacillus rhamnosus GG ameliorated chronic alcohol-induced liver and intestinal barrier injury [19]. However, the mechanism by which LAB prevents ALD progression remains unclear.
In a previous study, we isolated and identified Levilactobacillus brevis MG5311 from fermented food. We confirmed that this strain protects against ethanol-induced HepG2 cell damage by increasing aldehyde dehydrogenase (ALDH) activity, regulating cytochrome P450 2E1 (CYP2E1), activating antioxidant enzymes, and modulating lipid metabolism. In addition, this strain has been confirmed to be safe and stable owing to its resistance to the gastrointestinal tract and adhesion ability in vitro [20]. Therefore, this study aimed to determine whether L. brevis MG5311 has a preventive effect on ethanol-induced liver injury in mice and to identify the mechanisms of action, thereby confirming its potential as a useful new probiotic candidate for preventing or improving ALD.
2. Materials and Methods
2.1. Materials
Lieber-DeCarli regular control liquid diets (D710027) and Lieber-DeCarli regular ethanol liquid diets (D710260) were purchased from Dyets Inc. (Bethlehem, PA, USA). Maltose dextrin was purchased from BioServ (Flemington, NJ, USA). NonidetTM P 40 substitute and 200-proof ethyl alcohol were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Antibody against Cytochrome P450 2E1 (CYP2E1) was purchased from Invitrogen (Waltham, MA, USA). Antibodies against superoxide dismutase (SOD), catalase (CAT), AMP-activated kinase (AMPK), and GAPDH were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against aldehyde dehydrogenase 2 (ALDH2), sirtuin 1 (SIRT1), sterol regulatory element-binding protein 1c (SREBP-1c), peroxisome proliferator-activated receptor α (PPARα), and glutathione peroxidase 1/2 (GPx 1/2) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Malondialdehyde (MDA, ab118970), GPx (ab102530), and triglyceride (TG, ab65336) assay kits were purchased from Abcam (Cambridge, UK).
2.2. L. brevis MG5311 Cultivation
L. brevis MG5311, isolated from food, was supplied by MEDIOGEN Co., Ltd. (Jecheon, Korea). The GenBank accession number for the 16S rRNA gene sequence of L. brevis MG5311 is MN720513.1, as assessed by the National Center of Biotechnology Information (NCBI). The strain was activated by culturing in de Man, Rogosa, and Sharpe broth (MRS, Difco, Detroit, MI, USA) medium at 37 °C for 18 h. Cells were collected by centrifugation (15,000× g, 15 min, 4 °C), and the harvested pellets were mixed with the cryoprotectant cocktail and then freeze-dried [21].
2.3. Animal Experimental Procedure
C57BL/6J mice (male, 7 weeks old, and 20–22 g) were obtained from the Central Lab. Animal Inc. (Seoul, Korea). Mice were housed in one mouse per cage in an air-conditioned room at 23 ± 1.0 °C and a relative humidity of 45 ± 5% under a 12 h light/dark cycle. Animal experiments were approved by the Institutional Animal Care and Use Committee of Duksung Women’s University (No. 2021-005-006). All efforts were made to minimize the pain in the mice.
Mice were randomly divided into three groups (n = 6/group). (1) normal diet (ND), (2) ethanol diet (EtOH), and (3) ethanol diet plus L. brevis MG5311 (MG5311). Ethanol-induced liver injury was induced in mice for 32 days. The gavage volume and concentration of binge feeding were determined according to the NIAAA protocol [22]. Briefly, all mice were acclimatized to a liquid diet for 4 days using the Lieber-DeCarli control diet. On day 5, the ethanol-diet-fed groups were fed a Lieber-DeCarli-ethanol diet containing 0.75% ethanol for 3 days. Afterward, mice were fed the diet containing 1.5% ethanol for 3 days and the diet containing 3.75% ethanol for 4 days sequentially. The mice were then fed a diet containing 5% ethanol for 22 days. The diets were served daily at 3 p.m. Finally, on day 33, 31.5% (v/v) ethanol solution was orally binged to the ethanol-diet-fed groups. The control diet group was fed a 45% (w/v) maltose dextrin solution instead of ethanol. MG5311 strain powder was dissolved in sterile phosphate-buffered saline (PBS) and orally administered once a day at 1 × 109 CFU/mouse during feeding of the ethanol-containing diet. Nine hours after binge feeding, all mice were anesthetized by CO2 inhalation and euthanized by cardiac puncture.
2.4. Measurement of Body and Liver Tissue Weight and Food Intake
After the liquid diet adaptation was completed, the mice were weighed every 3 days for the experimental period. Food intake was measured daily. Liver tissues dissected from mice were washed with PBS buffer and immediately weighed. Liver tissues were stored at −70 °C until further use. The weight ratio was expressed as the liver tissue weight compared to the last body weight measurement before the mice were sacrificed.
2.5. Biochemical Analysis
All blood samples were collected into EDTA-coated tubes and placed for 30 min at room temperature. The serum was obtained by centrifugation at 4000 rpm for 20 min at 4 °C. Serum alanine aminotransferase (ALT), aspartate transaminase (AST), and TG levels were measured using an auto-serum analyzer (FUJI, Tokyo, Japan).
2.6. Measurement of MDA Concentration
Liver tissue (10 mg) was homogenized in a lysis buffer with butylated hydroxytoluene (BHT). The tissue lysate was then centrifuged at 13,000× g for 10 min. The supernatant was then collected for further analysis. The MDA concentration in the liver tissue was measured using a commercial kit according to the manufacturer’s instructions [23].
2.7. Measurement of GPx Activity
Liver tissue (100 mg) was homogenized in 200 μL assay buffer. The homogenate was centrifuged at 10,000× g for 15 min at 4 °C. The supernatant was harvested, and 10-fold diluted samples were used for analysis. GPx activity in the liver tissue was measured using a commercial kit according to the manufacturer’s instructions [24].
2.8. Measurement of TG Contents
To measure TG content, liver tissue (100 mg) was homogenized in 1 mL of 5% Nonidet-P40 substitute in distilled water. [25]. The homogenate was heated at 90 °C for 3 min in a water bath and cooled at room temperature, which was repeated twice to solubilize all the TG. The homogenate was then centrifuged at 12,500 rpm for 2 min. The supernatant was harvested, and 20-fold diluted samples were used for analysis. TG content in the liver tissue was measured using a commercial kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. TG levels were quantified based on the protein concentration of the extract using the Bradford protein assay (Bio-Rad, Hercules, CA, USA).
2.9. Histopathological Examination
Liver tissues were fixed in 10% formalin solution for 24 h and embedded in paraffin to analyze histopathological changes. Subsequently, 4-μm liver sections were stained with hematoxylin and eosin (H&E) solution. Histopathological scores for hepatic steatosis and inflammation were quantified and expressed simply by the summation of 4 individual grades as follows: 0 (<5%), 1 (5–33%), 2 (33–66%), and 3 (>66%) [26]. A board-certified toxicological pathologist blindly performed all histological evaluation procedures.
2.10. Western Blot Analysis
The expression levels of signaling proteins involved in alcohol-induced mechanisms in liver tissue were determined using Western blot analysis. Briefly, the liver tissues were homogenized in PRO-PREP™ protein extraction solution (iNtRON Biotechnology Co., Seongnam, Korea) containing a protease and phosphatase inhibitor cocktail (GenDEPOT, Katy, TX, USA). Homogenized samples were centrifuged at 12,000 rpm for 20 min at 4 °C, and the supernatant was collected. Protein levels in the lysates were quantified using Bradford assay. The proteins (10 μg) were mixed with a loading buffer, electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide (SDS) gel, and transferred to a polyvinylidene difluoride (PVDF) membrane for 3 h at 70V. Then, the proteins were blocked with 10% skim milk in Tris-buffered saline containing Tween-20 (TBST) for 60 min, and the membranes were incubated overnight with primary antibodies against ALDH2, CYP2E1, SIRT1, SREBP-1c, PPARα, SOD, CAT, GPx 1/2, and GAPDH (1:1000) in TBST at 4 °C. The membranes were washed three times with TBST buffer. Membranes were then incubated with goat anti-rabbit IgG-HRP or goat anti-mouse IgG-HRP conjugated secondary antibodies (1:4000, diluted with 5% skim milk in TBST) for 60 min at room temperature and washed three times with TBST buffer. Proteins were visualized using an ECL detection kit (Amersham Pharmacia, Piscataway, NJ, USA) and quantified using Image J program 1.3 (National Institute of Health, Bethesda, MD, USA).
2.11. Statistical Analysis
All data are presented as the mean ± standard deviation. Statistical analyses were performed using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). One-way analysis of variance (ANOVA) was used to compare the mean value between groups. Significant differences between the groups were evaluated by Dunnett’s Multiple Comparison Test (p < 0.05).
3. Results
3.1. Effect of L. brevis MG5311 on Body and Liver Weight and Food Intake
The chronic binge ethanol-feeding animal model is easy to perform and closely reproduces acute-on-chronic liver injury in patients with ALD. However, extensive steatosis and elevated serum ALT or AST levels induced by multiple chronic binge drinking also increase animal mortality [27].
To induce alcoholic liver injury, mice were fed 0.75 to 5% ethanol for 32 days and received one binge dose of 31.5% (vol/vol) ethanol according to the NIAAA model [20]. The body weights of the EtOH-fed groups increased slightly throughout the experiment but were not significant. In contrast, the ND group showed a significant increase after 13 days (p < 0.05). However, no significant difference was observed between the EtOH and MG5311 groups (Figure 1A). In addition, there was no significant difference in food intake between the experimental groups (Figure 1B). However, the weight loss and difficulty in recovering in ethanol-fed mice may be due to inefficient energy use and reduced nutrient intake by chronic alcohol intake [28].
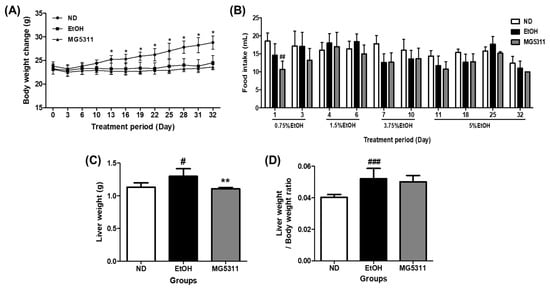
Figure 1.
Effect of L. brevis MG5311 on (A) body weight change, (B) food intake, (C) liver weight, and (D) liver/body weight ratio in chronic ethanol-fed mice. Mice were fed with ND or EtOH diets for 32 days and binge drinking once on day 33. EtOH-diet mice were treated with an experimental vehicle or MG5311 strain. Data are presented as mean ± SD (n = 6). Significance was analyzed by Dunnett’s test. # p < 0.05, ## p < 0.01, ### p < 0.001, vs. ND group. * p < 0.05, ** p < 0.01 vs. EtOH group. ND; normal diet, EtOH; ethanol diet, MG5311; ethanol diet-L. brevis MG5311.
In addition, the weight of liver tissue and liver/body weight ratio significantly increased in the EtOH-fed group compared to the ND group (Figure 1C,D).
3.2. Effect of L. brevis MG5311 on Changes in Serum Biomarkers
ALT and AST are important metabolic enzymes in hepatocytes and are generally present at low levels in plasma. ALT, AST, and TG leak into the blood in hepatocytes as liver damage caused by toxic substances progress [29]. In this study, serum obtained from all mice was analyzed to confirm the hematological changes caused by ethanol intake. The EtOH-fed group showed significantly increased AST and ALT levels compared to those in the ND group, indicating that alcohol can damage plasma and hepatocytes. In contrast, ALT and AST levels in the MG5311-treated group significantly decreased by 34.7% and 40.2%, respectively, compared to those in the EtOH-fed group (p < 0.05).
TG metabolism in liver tissue and blood TG levels are highly correlated [30]. In this study, the TG level in the serum of the MG5311 group (32.25 ± 5.91 mg/dL) was significantly lower than that in the EtOH-fed group (45.60 ± 10.43 mg/dL) (Figure 2). These results showed that MG5311 administration suppressed the elevation of AST, ALT, and TG levels by ethanol intake in mice.
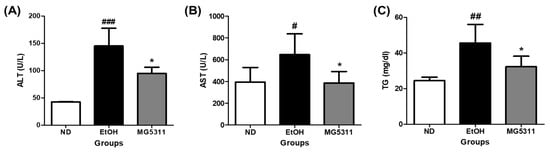
Figure 2.
Effect of L. brevis MG5311 on hepatic markers in the serum of ethanol-fed mice. (A) alanine transferase (ALT); (B) alanine aminotransferase (AST); and (C) triglyceride (TG). Data are presented as mean ± SD (n = 6). Significance was analyzed by Dunnett’s test. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. ND group. * p < 0.05 vs. EtOH group. ND; normal diet, EtOH; ethanol diet, MG5311; ethanol diet-L. brevis MG5311.
3.3. Effect of L. brevis MG5311 on the Protein Expression of ALDH2 and CYP2E1
The deleterious effects of alcohol metabolism primarily occur in hepatocytes [31]. Alcohol is primarily oxidized to acetic acid by the oxidative enzymes ADH and ALDH in the liver [32]. CYP2E1 is abundantly expressed in hepatocytes and is the most crucial enzyme in the hepatic microsomal alcohol oxidation system (MEOS) in binge drinking [33]. In this study, the protein expression of CYP2E1 and ALDH2 was evaluated to confirm the effect of MG5311 on alcohol metabolism in the liver using Western blotting. The EtOH-fed group showed significantly upregulated CYP2E1 expression compared to the ND group (p < 0.001). In contrast, CYP2E1 expression in the MG5311 group was 0.5-fold lower than in the EtOH group (p < 0.001) (Figure 3A,B). However, there was no significant difference in the expression level of ALDH2 between the groups (Figure 3A,C).
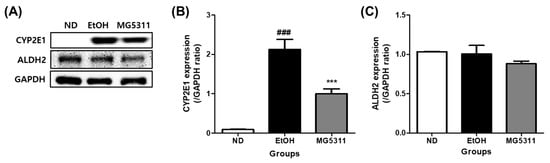
Figure 3.
Effect of L. brevis MG5311 on protein expression of ethanol metabolism enzymes in liver tissues of ethanol-fed mice. (A) representative Western blot; (B) cytochrome P450 2E1 (CYP2E1); and (C) aldehyde dehydrogenase 2 (ALDH2). Data are presented as mean ± SD (n = 3). Significance was analyzed by Dunnett’s test. ### p < 0.001 vs. ND group. *** p < 0.001 vs. EtOH group. ND; normal diet, EtOH; ethanol diet, MG5311; ethanol diet-L. brevis MG5311.
3.4. Effect of L. brevis MG5311 on MDA Content and GPx Activity in Liver Tissue
Lipid peroxidation mediated by alcohol exposure increases dramatically within liver tissue and is a cause of liver injury [31]. We measured the levels of MDA and the activity of the endogenous scavenger GPx in hepatic tissues to determine the effect of L. brevis MG5311 on inhibiting lipid peroxidation. MDA levels were significantly increased in EtOH-fed groups compared to the ND group (p < 0.001), indicating that lipid peroxidation was induced by ethanol administration. However, the MDA level in the MG5311 group was 0.78-fold lower than that in the EtOH group (p < 0.001) (Figure 4A).
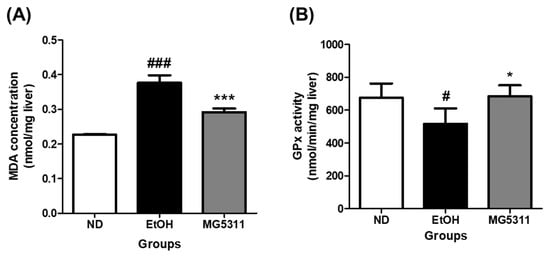
Figure 4.
Effect of L. brevis MG5311 on (A) malondialdehyde (MDA) concentration; and (B) glutathione peroxidase (GPx) activity in liver tissue of ethanol-fed mice. Data are presented as mean ± SD (n = 3). Significance was analyzed by Dunnett’s test. # p < 0.05, ### p < 0.001 vs. ND group. * p < 0.05, *** p < 0.001 vs. EtOH group. ND, normal diet group; EtOH, ethanol diet group; MG5311, ethanol diet-L. brevis MG5311 administered group.
GPx is a family of enzymes that constitute the primary antioxidant defense system [34]. In this study, the GPx activity of the EtOH-fed group decreased by 23.4% compared with that of the ND group (p < 0.05). On the other hand, GPx activity in the MG5311 group increased by 32.4% compared to that in the EtOH group (p < 0.05) (Figure 4B). Therefore, these results confirmed that MG5311 administration plays a role in ameliorating ethanol-induced liver damage by lowering MDA levels and increasing GPx activity as a defense system in liver tissues.
3.5. Effect of L. brevis MG5311 on the Protein Expression of Antioxidant Enzymes
Alcohol causes hepatic oxidative stress and weakens antioxidant enzyme activity [35]. Therefore, restoring the activity of antioxidant enzymes such as SOD, CAT, and GPx-1 alleviates ALD. We measured the protein expression levels of antioxidant enzymes in the liver tissues. The expression levels of both Cu/Zn-SOD (SOD1) and CAT were significantly lowered in the EtOH-fed group than in the ND group (p < 0.001). However, the expression levels of both SOD1 and CAT in the MG5311 administered group was 3.1-fold (p < 0.001) and 1.3-fold (p < 0.001) higher than those in the EtOH group (Figure 5). In addition, the GPx 1/2 expression levels were significantly higher than those in the EtOH group (p < 0.05).
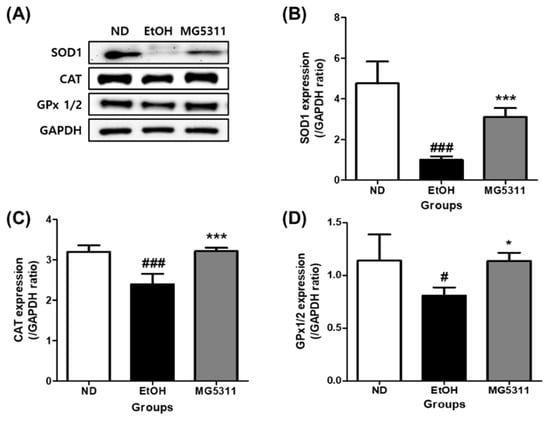
Figure 5.
Effect of L. brevis MG5311 on protein expression of antioxidant enzymes in the liver tissue of ethanol-fed mice. (A) representative Western blot; (B) superoxide dismutase 1 (SOD1); (C) catalase (CAT); and (D) glutathione peroxidase 1/2 (GPx 1/2). Data are presented as mean ± SD (n = 3). Significance was analyzed by Dunnett’s test. # p < 0.05, ### p < 0.001 vs. ND group. * p < 0.05, *** p < 0.001 vs. EtOH group. ND, normal diet group; EtOH, ethanol diet group; MG5311, ethanol diet-L. brevis MG5311 administered group.
3.6. Effect of L. brevis MG5311 on TG Contents and Histological Changes in Liver Tissue
Hepatic steatosis is the first consequence of alcohol abuse and is characterized by the excessive accumulation of TG in hepatocytes due to impaired lipid metabolism [31]. The histopathological features of the liver tissues were assessed using H&E staining. The ND group showed an increase in body weight during the experiment, but no steatosis was observed in the liver tissues. Micro-vesicular steatosis and inflammation were significantly higher in the EtOH-fed group than in the ND group (p < 0.001). However, the MG5311 group showed lower steatosis and inflammation levels than the EtOH group (p < 0.05) (Figure 6A,C).
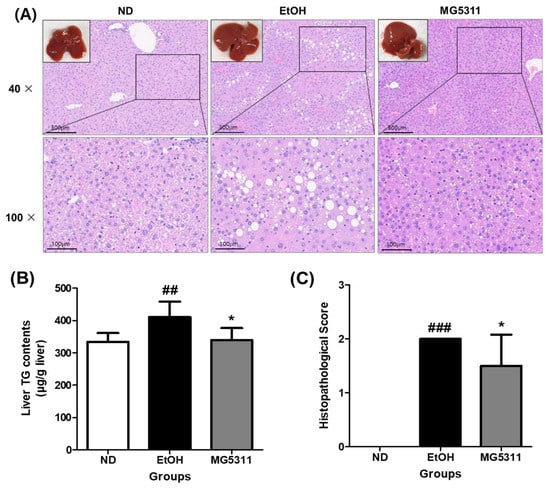
Figure 6.
Effect of L. brevis MG5311 on histological changes in liver tissues of ethanol-fed mice. (A) representative images of liver tissue and H&E-stained liver tissue (40× and 100× magnification); (B) hepatic TG content; and (C) histopathological score. Data are presented as mean ± SD (n = 6). Significance was analyzed by Dunnett’s test. ## p < 0.01, ### p < 0.001 vs. ND group. * p < 0.05 vs. EtOH group. ND, normal diet group; EtOH, ethanol diet group; MG5311, ethanol diet-L. brevis MG5311 administered group.
The TG content in the liver tissues was quantified using a commercial kit. In the EtOH-fed group (410.45 ± 47.76 μg/g liver), the TG content was significantly higher than that in the ND group (p < 0.01). In contrast, the TG levels in the MG5311 group (339.06 ± 37.49 μg/g liver) were considerably lower than those in the EtOH group (p < 0.05) (Figure 6B).
3.7. Effect of L. brevis MG5311 on Protein Expression of Hepatic Steatosis-Related Genes
Alcohol consumption increases the fat synthesis and reduces fatty acid oxidation, leading to metabolic disorders and excessive lipid accumulation [36]. We investigated the protein expression levels of hepatic steatosis-related genes in ethanol-fed mice. The EtOH-fed group showed significantly downregulated SIRT1 and PPARα expression compared with the ND group. However, the expression levels of both genes in the MG5311 group were 1.8-fold (p < 0.001) and 1.2-fold (p < 0.01) higher than those in the EtOH group (Figure 7B,D).
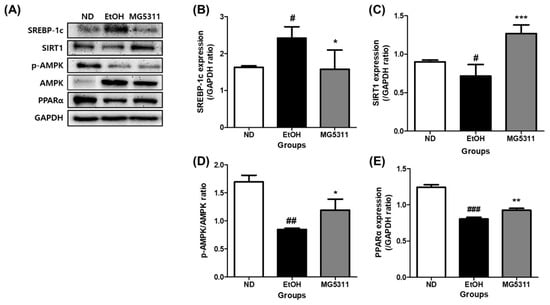
Figure 7.
Effect of L. brevis MG5311 on hepatic steatosis-related gene expression in liver tissues of ethanol-fed mice. (A) representative Western blot; (B) sterol regulatory element-binding transcription factor 1c (SREBP-1c); (C) sirtuin 1 (SIRT1); (D) phospho-AMP-activated protein kinase/AMP-activated protein kinase (p-AMPK/AMPK) (E) peroxisome proliferator-activated receptor α (PPARα). Data are presented as mean ± SD (n = 3). Significance was analyzed by Dunnett’s test. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. ND group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. EtOH group. ND, normal diet group; EtOH, ethanol diet group; MG5311, ethanol diet-L. brevis MG5311 administered group.
In addition, the expression level of SREBP-1c was higher in the EtOH group than in the ND group (p < 0.05) (Figure 7C). However, MG5311 administration significantly lowered SREBP-1c expression (p < 0.05). AMPK phosphorylation was not observed in EtOH-fed groups.
4. Discussion
ALD is a complex disease that involves several pathogenic metabolic mechanisms. ALD is caused by oxidative stress induced by alcohol metabolism, mutagenic acetaldehyde formation, and pro-inflammatory cytokine production [37]. Therefore, functional supplements that exhibit antioxidant activity and modulate lipid metabolism in the liver may serve as therapeutic agents to prevent ALD. Studies have demonstrated that probiotics alleviate alcohol-induced liver damage via antioxidant and anti-inflammatory activity [38,39,40,41]. Therefore, the present study investigated the hepatoprotective effects and mechanisms of the probiotic candidate L. brevis MG5311 administration in chronic and binge ethanol-fed mice.
Alcohol metabolism increases the production of acetaldehyde, which causes protein inactivation and DNA damage [32]. ALDH plays an essential role in converting the highly toxic acetaldehyde decomposed by ADH into acetic acid and oxidizing acetic acid to carbon dioxide and water. In addition, the conversion of aldehyde to innocuous acetic acid by ALDH suggests a role in primary protection against ROS [42]. ALDH2 is the main enzyme involved in alcohol-derived acetaldehyde metabolism [43]. In a previous study, L. plantarum HFY09 improved ALDH levels in the liver of ethanol-fed mice [44]. L. brevis HY7410 lowers blood alcohol levels by enhancing ADH and ALDH activity [45]. Our previous in vitro study confirmed that cell-free extracts of MG5311 increase ALDH activity in ethanol-induced HepG2 cells [18]. However, it was not confirmed that MG5311 administration did not affect ALDH2 protein expression in the liver of ethanol-treated mice.
Binge drinking metabolism involves the induction of CYP2E1 in the microsomal system and is a major cause of ALD [33]. CYP2E1 in alcohol metabolism generates ROS, which causes various types of tissue damage, such as oxidative stress, metabolic changes, and membrane dysfunction of intracellular organelles [46]. Upregulated intestinal CYP2E1 increases nitroxidative stress and promotes gut leakage, which may lead to inflammatory liver damage and endotoxemia [47]. L. rhamnosus GG supplementation decreased CYP2E1 expression and TNFα production [41]. This study confirmed that L. brevis MG5311 administration protects against ALD by suppressing CYP2E1 expression.
ALD patients appear to exhibit oxidative stress [33]. ROS increase during alcohol metabolism causes lipid peroxidation, upregulates MDA levels, and damages proteins and DNA, ultimately impairing cell function [48]. MDA is a by-product of lipid peroxidation, and the amount of MDA indirectly reflects the extent of liver damage. In a previous study, the administration of L. plantarum B7 and a probiotic mixture (L. rhamnosus L34 and L. casei L39) reduced MDA levels in rats with alcohol-induced liver injury [49]. L. brevis MG5311 administration also reduced MDA levels in the liver tissues of ethanol-fed mice.
Alcohol promotes the increased production of ROS in liver metabolism and weakens antioxidant defenses, leading to oxidative stress in the liver. ROS levels are reduced by the activation and expression of antioxidant enzymes, including SOD, CAT, and GPx. SOD is an endogenous antioxidant enzyme that catalyzes the transformation of two molecules of superoxide anions into hydrogen peroxide and oxygen. CAT catalyzes the decomposition or reduction of hydrogen peroxide to water and oxygen. GPx protects cells from oxidative stress by inhibiting lipid peroxidation in the lipid peroxidation process [50,51]. Therefore, increasing the activity of these antioxidant enzymes may be a potent strategy against ALD. Administration of a probiotic mixture (L. plantarum KLDS1.0344 and L. acidophilus KLDS1.0901) can protect against liver injury induced by 4% ethanol for 6 weeks in mice by improving the levels of SOD1, CAT, and GPx-1 [36]. MG5311 promoted SOD1 and CAT protein expression in the liver tissues in this study. It also increases the activity and expression of GPx 1/2. Thus, we have demonstrated that MG5311 administration could improve ROS-induced liver damage during alcohol metabolism by increasing the activity and expression of antioxidant enzymes.
Excessive alcohol exposure causes excessive TG accumulation in hepatocytes, interfering with liver metabolic function [31]. Representative transcription factors that regulate lipid metabolism in hepatocytes include SREBP-1c and PPARα [52,53]. SREBP-1c is a lipid synthesis transcription factor involved in cholesterol and fatty acid synthesis, and it is implicated in the pathogenesis of alcohol-induced steatosis. SREBP-1c expression is inhibited by AMPK and SIRT [54]. Activation of SIRT1 and AMPK improves hepatic steatosis by enhancing carnitine palmitoyltransferase 1 (CPT-1) expression and promoting fatty acid β-oxidation for lipolysis [55,56]. In particular, SIRT1, an NAD+-dependent deacetylase, can directly interact with and deacetylates SREBP-1c in an AMPK pathway-dependent or independent manner in ALD [56]. Alcohol consumption induces fatty liver by downregulating AMPK and SIRT1, increasing the acetylated form of SREBP-1c, and inhibiting fatty acid oxidation. Overexpression of SIRT1 alleviated AFLD by reducing alcohol-induced SREBP-1c hyperacetylation and activity [57]. Therefore, regulation of the SIRT1-SREBP-1c axis is one of the major mechanisms linking the pathogenesis of alcohol-induced hepatic steatosis. In this study, MG5311 administration increased SIRT1 expression and decreased SREBP-1c expression without affecting AMPK activity. Therefore, MG5311 administration inhibited fatty accumulation in the liver through SIRT1/SREBP-1c regulation in ethanol-fed mice.
Excessive alcohol exposure impairs fatty acid catabolism by inhibiting mitochondrial β-oxidation, which is the most crucial contributor to alcohol-induced fatty liver disease [52]. PPARα is a master regulator of lipid metabolism (fatty acid β-oxidation) and regulates peroxisomal catalase and cellular NAD+ levels during alcohol metabolism [52]. Alcohol inhibits PPARα to delay fatty acid oxidation. PPARα activity can be indirectly inhibited by the upregulation of CYP2E1-derived oxidative stress [58]. In contrast, SIRT1 positively modulates PPARα to regulate lipid homeostasis [53]. In the present study, MG5311 administration increased PPARα protein expression. These results confirmed that MG5311 improved lipid peroxidation in the liver by regulating the SIRT1/PPARα pathway.
The gut is directly linked to the liver through the portal vein (gut-liver axis). Gut microbiota dysbiosis can cause a variety of liver disorders [16,17]. Recent studies have shown that probiotics and their metabolites could improve ALD by reducing the alcohol-induced gut microbial imbalance, gut permeability, bacterial translocation, and endotoxemia, thereby restoring a balanced gut microbial community and strengthening the intestinal barrier [59,60]. This study confirmed the ameliorative effect of MG5311 on alcohol-induced oxidative stress and lipid metabolism in an ALD mouse model. Therefore, further studies are needed to confirm the direct effect of MG5311 on gut microflora and intestinal permeability.
5. Conclusions
This study was conducted to evaluate whether oral administration of MG5311 has a hepatoprotective effect in ALD mice subjected to chronic plus once binge drinking. MG5311 administration to mice improved the elevated serum ALT, AST, and TG levels, which were increased by the ethanol diet. MG5311 reduced the protein expression levels of CYP2E1 and increased the protein expression levels of the antioxidant enzymes SOD1, CAT, and GPx 1/2. Moreover, MG5311 suppressed hepatic steatosis by regulating SREBP-1c and PPARα expression via upregulating the SIRT1 pathways. Taken together, it was confirmed that MG5311 administration inhibits alcohol-induced oxidative stress and promotes lipid β-oxidation in the liver (Figure 8). Therefore, MG5311 may be a potential functional probiotic candidate for preventing ALD. Further studies on the effect of MG5311 on changes in gut microbiota and intestinal permeability will be conducted.
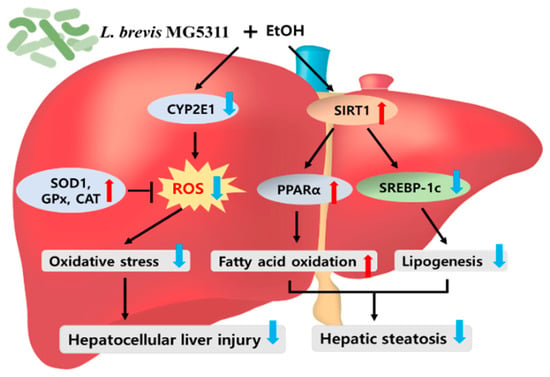
Figure 8.
L. brevis MG5311 exerts hepatoprotective effects by regulating hepatic oxidative stress and lipid metabolism. CYP2E1, Cytochrome P450 2E1; SIRT1, Sirtuin 1; SOD1, superoxide dismutase 1; GPx, glutathione peroxidase; CAT, catalase; PPARα, peroxisome proliferator-activated receptor α; SREBP-1c, sterol regulatory element-binding protein 1c.
Author Contributions
Conceptualization and methodology, S.-I.C. and C.-H.K.; investigation, H.J. and S.Y.; writing—original draft, H.J.; writing—review and editing, S.-I.C. and G.-H.K.; project administration, C.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Toshikuni, N.; Tsutsumi, M.; Arisawa, T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 8393–8406. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.; Maor, Y. The interplay between alcoholic liver disease, obesity, and the metabolic syndrome. Visc. Med. 2020, 36, 198–205. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Y.; Wang, Y.; Sun, H.; You, Y.; Piao, C.; Liu, J.; Wang, Y. Lactobacillus rhamnosus granules dose-dependently balance intestinal microbiome disorders and ameliorate chronic alcohol-induced liver injury. J. Med. Food. 2020, 23, 114–124. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef]
- Almeida, J.I.; Tenreiro, M.F.; Martinez-Santamaria, L.; Guerrero-Aspizua, S.; Gisbert, J.P.; Alves, P.M.; Serra, M.; Baptista, P.M. Hallmarks of the human intestinal microbiome on liver maturation and function. J. Hepatol. 2022, 76, 694–725. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver disease. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group; Food and Agriculture Organization of the United Nations and World Health Organization: London, UK; Ottawa, ON, Canada, 2002. [Google Scholar]
- Jang, H.J.; Lee, N.K.; Paik, H.D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 1521–1528. [Google Scholar] [CrossRef]
- Stankovic, M.; Veljovic, K.; Popovic, N.; Kojic, S.; Dunjic Manevski, S.; Radojkovic, D.; Golic, N. Lactobacillus brevis BGZLS10-17 and Lb. plantarum BGPKM22 exhibit anti-inflammatory effect by attenuation of NF-κB and MAPK signaling in human bronchial epithelial cells. Int. J. Mol. Sci. 2022, 23, 5547. [Google Scholar] [CrossRef]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, J.H.; Lee, J.S.; Kang, S.D.; Shim, S.; Jung, M.Y.; Yang, H.; Byun, S.; Lee, K.W. Heat-killed Lactobacillus brevis enhances phagocytic activity and generates immune-stimulatory effects through activating the TAK1 pathway. J. Microbiol. Biotechnol. 2020, 30, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: A review of the literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, Y.; Hu, S.; You, Y.; Wen, J.; Li, W.; Wang, Y. Probiotics for alleviating alcoholic liver injury. Gastroenterol. Res. Pract. 2019, 2019, 9097276. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.; Wakita, Y.; Hirata, H.; Watari, J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int. J. Food. Microbiol. 2008, 128, 371–377. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, H.; Xu, L.; Zhang, W.; Lv, J.; Chen, Y. The amelioration of alcohol-induced liver and intestinal barrier injury by Lactobacillus rhamnosus Gorbach-Goldin (LGG) is dependent on Interleukin 22 (IL-22) expression. Bioengineered 2022, 13, 12650–12660. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.; Jeong, Y.; Kang, C.H. Lactic acid bacteria exert a hepatoprotective effect against ethanol-induced liver injury in HepG2 cells. Microorganisms 2021, 9, 1844. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, Y.; Kim, J.S.; Jeong, Y.; Park, H.M.; Kim, J.W.; Kim, J.E.; Kim, H.; Paek, N.S.; Kang, C.H. Evaluating the cryoprotective encapsulation of the lactic acid bacteria in simulated gastrointestinal conditions. Biotechnol. Bioprocess Eng. 2020, 25, 287–292. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding. Nat. Protoc. 2013, 8, 627. [Google Scholar] [CrossRef]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Simoes, I.C.M.; Karkucinska-Wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western diet causes obesity-induced nonalcoholic fatty liver disease development by differentially compromising the autophagic response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Viswanathan, S.; Adami, E.; Singh, B.K.; Chothani, S.P.; Ng, B.; Lim, W.W.; Zhou, J.; Tripathi, M.; Ko, N.S.J.; et al. Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat. Commun. 2021, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Vázquez, M.T.; Canals, S.; del Moral, M.G.; Martínez-Naves, E.; Nevzorova, Y.A.; Cubero, F.J. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063–5075. [Google Scholar] [CrossRef]
- Kamran, U.; Towey, J.; Khanna, A.; Chauhan, A.; Rajoriya, N.; Holt, A. Nutrition in alcohol-related liver disease: Physiopathology and management. World J. Gastroenterol. 2020, 26, 2916–2930. [Google Scholar] [CrossRef]
- Pratt, D.S.; Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar]
- Seth, D.; Haber, P.S.; Syn, W.K.; Diehl, A.M.; Day, C.P. Pathogenesis of alcohol-induced liver disease: Classical concepts and recent advances. J. Gastroenterol. Hepatol. 2011, 26, 1089–1105. [Google Scholar] [CrossRef]
- Edenberg, H.J.; McClintick, J.N. Alcohol dehydrogenases, aldehyde dehydrogenase and alcohol use disorder: A critical review. Alcohol Clin. Exp. Res. 2018, 42, 2281–2297. [Google Scholar] [CrossRef]
- French, S.W.; Morimoto, M.; Reitz, R.C.; Koop, D.; Klopfenstein, B.; Estes, K.; Clot, P.; Ingelman-Sundberg, M.; Albano, E. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J. Nutr. 1997, 127, 907S–911S. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.W.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver disease. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Centi, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef]
- Wang, Y.h.; Gao, J.; Zhang, J.; Yao-hui, H.U. Lactobacillus rhamnosus B10 treatment ameliorates ethanol-induced mouse liver injury by antioxidant pathways. Food Sci. 2012, 33, 270–274. [Google Scholar]
- Gan, Y.; Chen, X.; Yi, R.; Zhao, X. Antioxidative and anti-inflammatory effect of Lactobacillus plantarum ZS62 on alcohol-induced subacute hepatic damage. Oxid. Med. Cell Longev. 2021, 2021, 7337988. [Google Scholar] [CrossRef]
- Fang, T.J.; Guo, J.T.; Lin, M.K.; Lee, M.S.; Chen, Y.L.; Lin, W.H. Protective effects of Lactobacillus plantarum against chronic alcohol-induced liver injury in the murine model. Appl. Microbiol. Biotechnol. 2019, 103, 8597–8608. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Kirpich, I.; Ma, Z.; Wang, C.; Zhang, M.; Suttles, J.; McClain, C.; Feng, W. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J. Nutr. Biochem. 2013, 24, 1609–1615. [Google Scholar] [CrossRef]
- Singh, S.; Brocker, C.; Koppaka, V.; Ying, C.; Jackson, B.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Xu, H.; Gao, Y. Aldehyde dehydrogenase, liver disease and cancer. Int. J. Biol. Sci. 2020, 16, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Tong, J.; Zhou, X.; Long, X.; Pan, Y.; Liu, W.; Zhao, X. Hepatoprotective effect of Lactobacillus plantarum HFY09 on ethanol-induced liver injury in mice. Front. Nutr. 2021, 24, 684588. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.T.; Kim, Y.H.; Bae, J.S.; Lim, K.S.; Huh, C.S.; Yang, W.Y.; Kim, H.S.; Baek, Y.J. Effect of Lactobacillus brevis HY7401 intake on the serum ethanol concentration in rats. Korean J. Food Sci. Technol. 2004, 36, 604–608. [Google Scholar]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Ha, S.K.; Choi, Y.; Akbar, M.; Song, B.J. Role of CYP2E1 in mitochondrial dysfunction and hepatic tissue injury in alcoholic and non-alcoholic diseases. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef]
- Han, K.H.; Hashimoto, N.; Fukushima, M. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J. Gastroenterol. 2016, 22, 37–49. [Google Scholar] [CrossRef]
- Chayanupatkul, M.; Somanawat, K.; Chuaypen, N.; Klaikeaw, N.; Wanpiyarat, N.; Siriviriyakul, P.; Tumwasorn, S.; Werawatganon, D. Probiotics and their beneficial effects on alcohol-induced liver injury in a rat model: The role of fecal microbiota. BMC Complement. Altern. Med. 2022, 22, 168. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First-line defense antioxidants-superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 mixture prevents chronic alcoholic liver injury in mice by protecting the intestinal barrier and regulating gut microbiota and liver-related pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef]
- Ji, C.; Chan, C.; Kaplowitz, N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J. Hepatol. 2006, 45, 717–724. [Google Scholar] [CrossRef]
- Yue, R.; Chen, G.; Xie, G.; Hao, L.; Guo, W.; Sun, X.; Jia, W.; Zhang, Q.; Zhou, Z.; Zhong, W. Activation of PPARα-catalase pathway reverses alcoholic liver injury via upregulating NAD synthesis and accelerating alcohol clearance. Free Radic. Biol. Med. 2021, 174, 249–263. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef]
- Ponugoti, B.; Kim, D.H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.; Chiang, C.; Veenstra, T.D.; Kemper, J.K. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808. [Google Scholar] [CrossRef]
- You, M.; Liang, X.; Ajmo, J.M.; Ness, G.C. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G892–G898. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhuge, J.; Wang, X.; Bai, J.; Cederbaum, A.I. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 2008, 47, 1483–1494. [Google Scholar] [CrossRef]
- Chen, P.; Torralba, M.; Tan, J.; Embree, M.; Zengler, K.; Stärkel, P.; van Pijkeren, J.P.; DePew, J.; Loomba, R.; Ho, S.B.; et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 2015, 148, 203–214. [Google Scholar] [CrossRef]
- Hong, M.; Han, D.H.; Hong, J.; Kim, D.J.; Suk, K.T. Are probiotics effective in targeting alcoholic liver diseases? Probiotics Antimicrob. Proteins 2019, 11, 335–347. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).