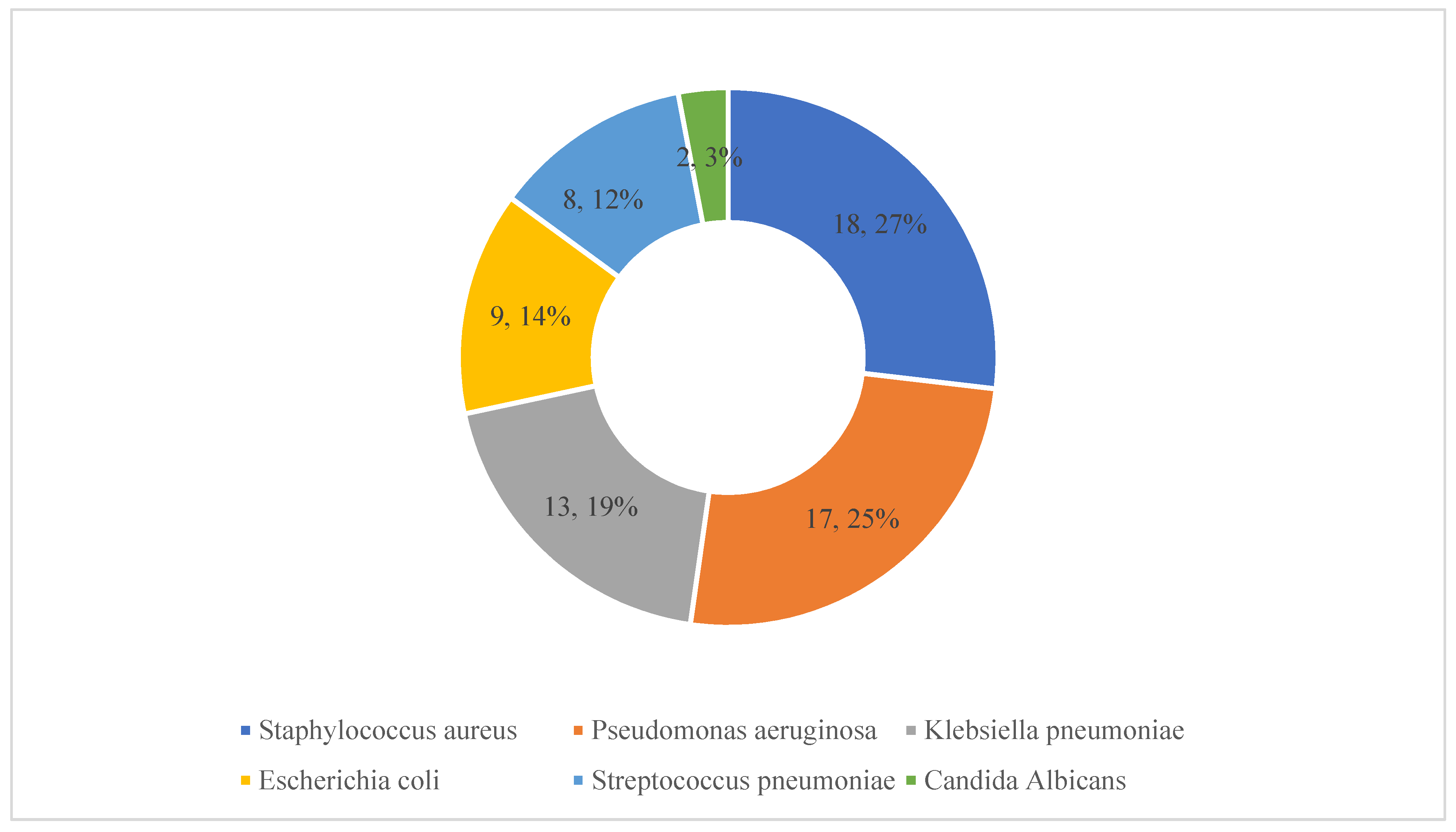Abstract
Healthcare-associated infections (HAIs) have a considerable impact on morbidity, mortality and costs. The COVID-19 pandemic resulted in an appreciable number of hospitalized patients being admitted to intensive care units (ICUs) globally with a greater risk of HAIs. Consequently, there is a need to evaluate predictors and outcomes of HAIs among COVID-19 patients admitted to ICUs. A retrospective study of patients with COVID-19 admitted to ICUs of three tertiary care hospitals in the Punjab province over a five-month period in 2021 was undertaken to ascertain predictors and outcomes of HAIs. Of the 4534 hospitalized COVID-19 patients, 678 were admitted to ICUs, of which 636 patients fulfilled the inclusion criteria. Overall, 67 HAIs were identified among the admitted patients. Ventilator-associated lower respiratory tract infections and catheter-related urinary tract infections were the most frequent HAIs. A significantly higher number of patients who developed HAIs were on anticoagulants (p = 0.003), antithrombotic agents (p < 0.001), antivirals (p < 0.001) and IL-6 inhibiting agents (p < 0.001). Secondary infections were significantly higher in patients who were on invasive mechanical ventilation (p < 0.001), had central venous access (p = 0.023), and urinary catheters (p < 0.001). The mortality rate was significantly higher in those with secondary infections (25.8% vs. 1.2%, p < 0.001). Our study concluded that COVID-19 patients admitted to ICUs have a high prevalence of HAIs associated with greater mortality. Key factors need to be addressed to reduce HAIs.
1. Introduction
Healthcare-associated infections (HAIs) are considered the most frequent adverse consequences of healthcare delivery. They represent a significant public health problem in terms of increasing hospital stay, morbidity and mortality as well as associated costs, including patients with coronavirus disease of 2019 (COVID-19) [1,2,3,4,5,6,7]. The prevalence of HAIs varies considerably across countries [6]. Among high-income countries, in the USA approximately 3 to 4% of patients will develop an HAI, with attributable costs estimated at $3384 ($885–$7717) per patient for vancomycin-resistant enterococci up to $39,787 ($20,813–$64,140) for MDR acinetobacter and $74,306 ($20,377–$128,235) for carbapenem-resistant (CR) acinetobacter [8,9,10]. Overall, it was estimated in 2016 that healthcare costs associated with HAIs in the US varied between $7.2 billion to $14.9 billion [11]. In China, the additional costs of treating antimicrobial-resistant HAIs were estimated at $15,557.25 per patient compared with non-HAI patients exacerbated by on average an extra 41 days in hospital [12].
Lower rates of HAIs were recently reported in Scotland at approximately 1% of acute care patients pre-COVID-19 [13]. Overall, 2,609,911 cases of HAIs were reported each year in the European Union and European Economic Area (EU/EEA) in 2011 to 2012, with an estimated 501 disability-adjusted life years (DALYs) per 100,000 general population [5]. The prevalence of HAIs in the African region ranges from 3% to 15% of patients with considerable under-reporting [14]. Rates for HAIs as low as 1.24% in China [15] compare favourably with those seen in Scotland and among some African countries [13,14]. However, other studies in China have reported higher rates, up to 26.1% of patients in adult intensive care units (ICUs) [16,17].
Generally, the burden of HAIs is higher in low- and middle-income countries (LMICs) compared with high-income countries. This arises because of typically suboptimal adherence to infection prevention and control guidelines, lack of effective policies to prevent HAIs, scarcity of strict regulations and their monitoring, inadequate hand hygiene protocols and a lack of antimicrobial stewardship programs (ASPs) [6,18,19,20,21,22].
HAIs can develop in a hospital setting in patients receiving health care after 48 h or more after hospital admission [23,24]. Invasive devices, which include vascular devices, urinary catheters and ventilators employed in the medical care of patients, are associated with these HAIs [25,26,27]. The risk of HAI transmission is increased with the lack of awareness and training among health care providers especially in LMICs [28,29,30,31,32]. According to the Center for Diseases Control and Prevention (CDC), common HAIs include ventilator-associated pneumonia, central line-associated bloodstream infections, catheter-associated urinary tract infections and surgical site infections (SSIs) [33].
The COVID-19 pandemic considerably impacted on healthcare delivery systems, appreciably increasing hospitalizations, especially in ICUs [34,35,36,37]. The first case of COVID-19 was identified in Wuhan, China, in December 2019, and spread rapidly after that [38]. However, its impact on morbidity and mortality varied across countries depending on the rapidity and the extent with which lockdown and quarantining measures were introduced [39,40,41,42]. In any event, there was a prolongation of hospital stay associated with COVID-19 in the early stages of the pandemic with no effective vaccines or medicines available to reduce the severity of COVID-19 [42,43,44]. This increased the prevalence of HAIs especially amongst patients in critical care, including those in LMICs [45]. Moreover, the respiratory failure associated with severe or critical COVID-19 is most prevalent in those who are already compromised by age, obesity or comorbidities (including chronic lung disease, diabetes and immunosuppression) and leads to the use of immunosuppressive and immunomodulatory therapies along with mechanical ventilation, all of which further increase the risk of HAIs [4,45,46,47].
Pakistan is a low-middle income South Asian country where the COVID-19 pandemic has appreciably impacted morbidity and mortality, with five different disease waves reported since the first reported case on 26 February 2020 [48,49]. A previous study from Pakistan reported that most of the cases of patients with COVID-19 were mild to moderate; however, older patients with underlying chronic illness may develop severe disease leading to hospitalization as well as mechanical ventilation in intensive care units [50]. By the end of September 2022 over 30,000 deaths due to COVID-19 had been reported in Pakistan [51].
To date, there appears to be no study conducted in Pakistan to ascertain the prevalence of HAIs among COVID-19 patients admitted to ICUs. This is important given the increased morbidity and mortality among such patients [4,25,52]. Consequently, we sought to address this by conducting a retrospective, multicentre study among patients with COVID-19 admitted to the ICUs of hospitals designated for COVID-19 care in the province of Punjab, Pakistan. Punjab Province was chosen for this study as it currently accounts for more than half of the population of Pakistan [53]. In addition, previous research conducted in this province has shown a high consumption of antibiotics among patients with actual or suspected COVID-19 in successive waves [48,53]. This is a concern if such prescribing is inappropriate as this will increase antimicrobial resistance (AMR), with its subsequent impact on morbidity, mortality and costs [54,55,56,57]. There are already concerns with rising AMR rates in Pakistan, with ongoing initiatives to address this through the national action plan (NAP) [58,59]. However, there are ongoing challenges with implementing the NAP in Pakistan, which need to be addressed going forward [60].
2. Results
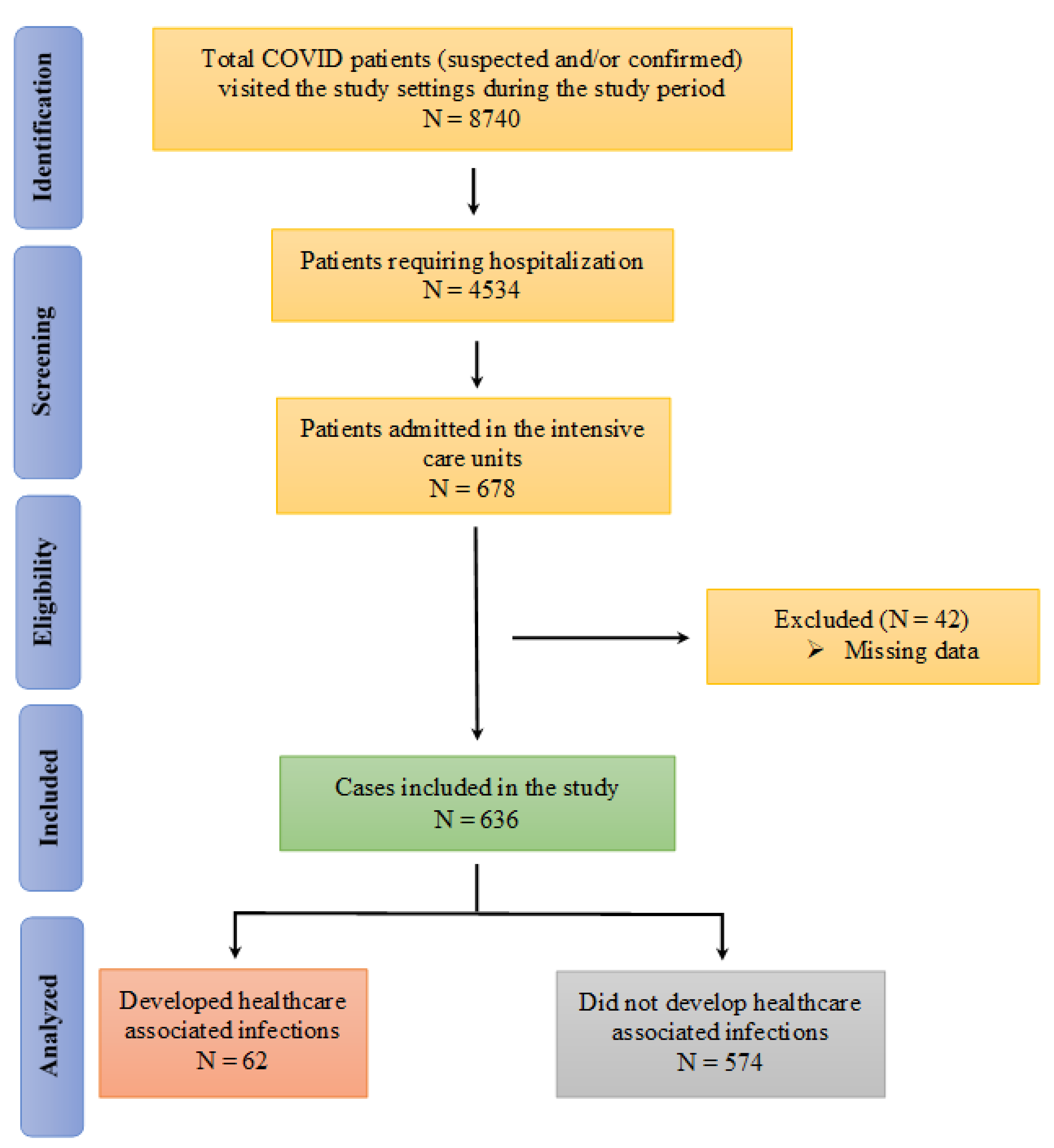
A total of 8740 suspected (results waiting) and/or confirmed (by RT-PCR) COVID-19 patients visited or were brought to the designated hospitals during the study period, with 4534 COVID-19 positive patients subsequently hospitalized. Of these, 678 patients were admitted to the ICUs. A total of 636 cases were subsequently included in the final analysis (Figure 1).
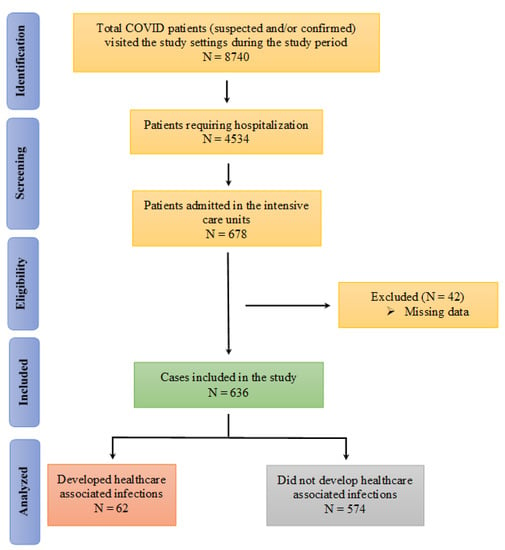
Figure 1.
Flow diagram of the identification of the patients for inclusion in the study.
The demographic details and clinical features of the COVID-19 patients admitted to the ICUs with or without HAIs are shown in Table 1. The majority of the study population were male (62.6%), aged above 50 years (76.3%) and had multiple symptoms including a fever, cough, sore throat and body ache. Two thirds (69.3%) of patients had severe COVID-19 while 23.9% were critical. Abnormal chest X-ray findings were present in 92.9% of the patients in the ICUs, whereas white blood cells and C-reactive protein elevations were seen in 78.1% and 72.6% of patients, respectively. Additionally, abnormalities in serum ferritin and D-dimer were present in 50.3% and 67.9% of patients, respectively. The development of HAIs was more common in those >50 years (p = 0.039) and patients who presented with critical diseases.

Table 1.
Demographics and clinical features of COVID-19 patients with and without healthcare-associated infections (HAIs).
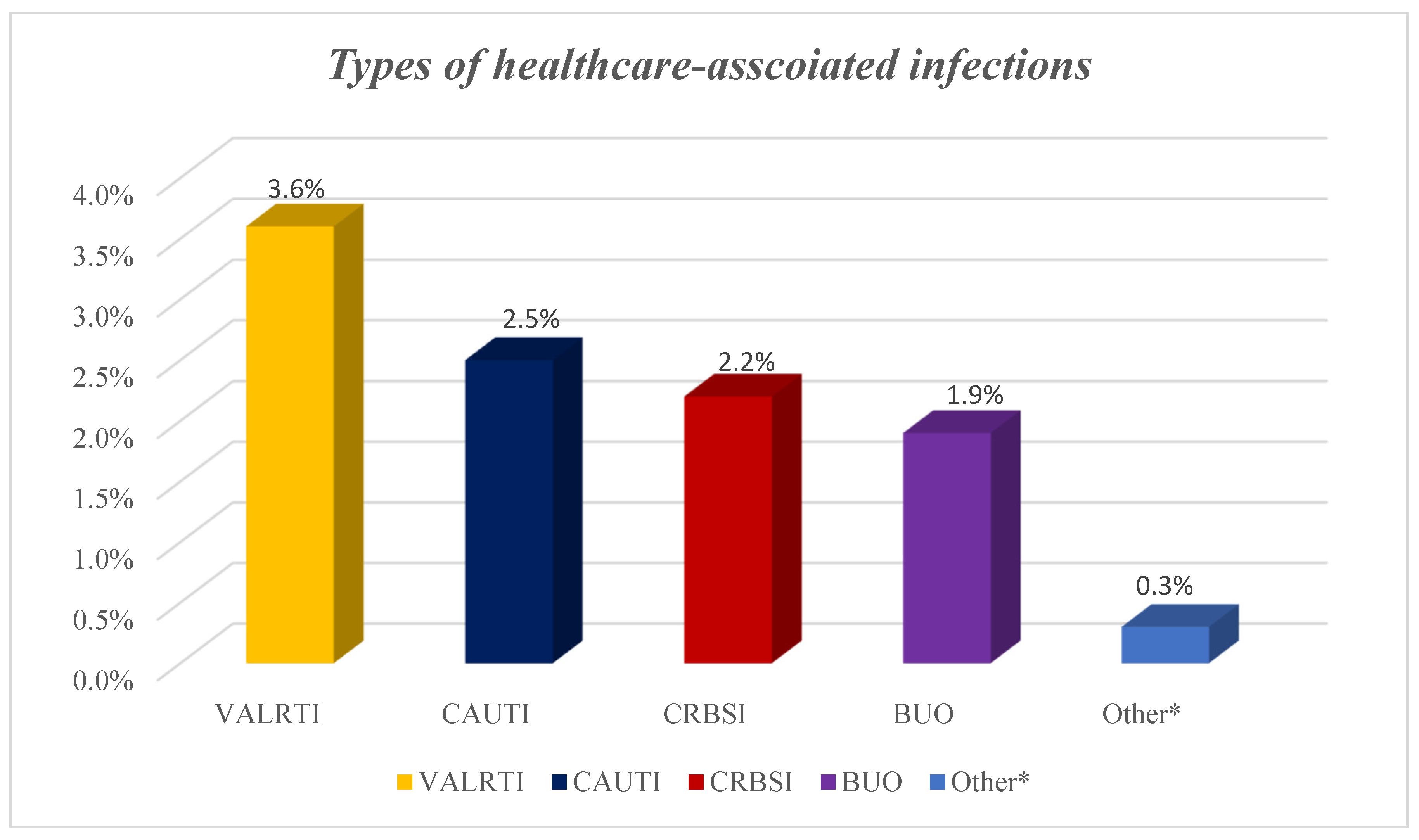
A total of 67 HAIs from different microorganisms developed among the 62 patients with HAIs on the ICU giving a prevalence of 9.7%. Out of the 67 HAIs, 23 were ventilator-associated lower respiratory tract infections, 16 catheter-related urinary tract infections, 14 catheter-related blood stream infections, 12 blood infections of unknown origin and two were fungal infections (Figure 2).
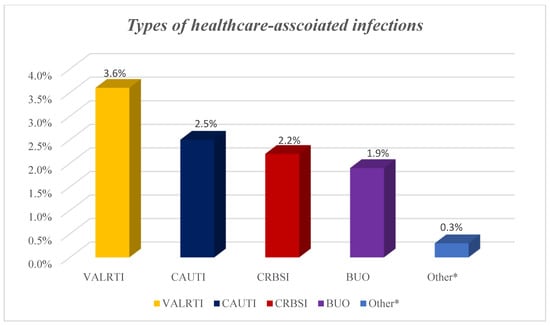
Figure 2.
Types of healthcare-associated infections among COVID-19 patients. NB: BUO: bloodstream infections of unknown origin; CAUTI: catheter-associated urinary tract infection; CRBSI: catheter-related blood stream infection; HAI: healthcare-associated infections; VALRTI: ventilator-associated lower respiratory tract infections; Others* included fungal infections.
Details of the pathogens causing these HAIs are presented in Figure 3. Staphylococcus aureus, pseudomonas aeruginosa and Klebsiella pneumoniae were identified in 18, 17 and 13 patients, respectively.
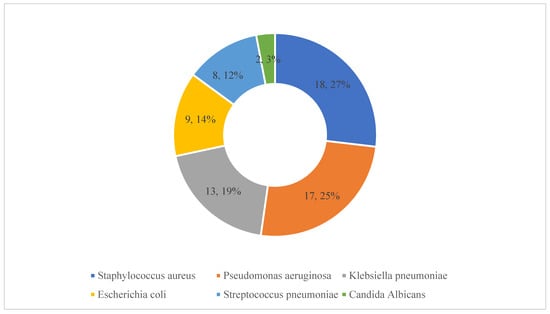
Figure 3.
Details of microorganisms responsible for healthcare-associated infections. Note: The numbers refer to the number of patients with these isolates along with the percentages (n = 67).
The use of antipyretics, corticosteroids and antibiotics was universal (100%) in the studied cases (Table 1). Nearly 80% of the patients received anticoagulants, whereas 18.2% received antithrombotic agents. The use of remdesivir and tocilizumab was seen in 57.2% and 11.5% of patients, respectively. A significantly higher number of patients who developed HAIs were on anticoagulants (p = 0.003), antithrombotic agents (p < 0.001), antivirals (p < 0.001) and IL-6 inhibiting agents (p < 0.001) compared with the non-HAI group. In the case of anticoagulants, it was not possible to differentiate their prescribing for either prophylaxis or treatment. The rate of secondary infection was significantly higher in patients who were on invasive mechanical ventilation (p < 0.001), had central venous access (p = 0.023), or had a urinary catheter placed (p < 0.001).
The predictors of HAI development are shown in Table 2. In our multivariable regression model, the prescribing of tocilizumab, and the presence of urinary catheters were found to be independent predictors of HAIs (Table 2).

Table 2.
Factors associated with the development of healthcare-associated infections (HAIs) in COVID-19 patients.
As shown in Table 3, patients who developed a secondary infection had a significantly longer length of stay in the ICU (p < 0.001). Overall, 96% of patients admitted to the ICU with COVID-19 had a complete recovery and were discharged from the hospital, whereas 3.6% (n = 23) died. The mortality rate though was significantly higher in patients who developed HAIs (25.8% vs. 1.2%, p < 0.001).

Table 3.
Outcomes of COVID-19 patients with healthcare-associated infections (HAIs) in ICUs.
In our multivariable-adjusted model using covariates that were significantly associated with mortality in the univariate analysis, e.g., COVID-19 severity (moderate-severe vs. critical), underlying heart disease, invasive mechanical ventilation, presence of a urinary catheter, and HAI development, HAIs (OR 11.18, 95% CI 3.65–34.28, p < 0.001) and heart disease (OR 3.67, 95% CI 1.23–10.99, p = 0.020) were the independent predictors of mortality.
3. Discussion
We believe this is one of the first studies conducted among COVID-19 patients admitted to ICUs in Pakistan to evaluate the predictors and outcomes of HAIs. The prevalence of HAIs among patients with COVID-19 admitted to the ICUs in our study was 9.7%. This compares with the findings of He et al. (2020) just after the emergence of the COVID-19 pandemic, who indicated the prevalence of HAIs among all patients admitted with COVID-19 was 7.1% in a single centre in Wuhan, China [61]. In a study reported from Singapore, the incidence of HAIs among hospitalized patients with COVID-19 treated in ICUs was 14.3% [62], with similarly low rates (6.1%) seen in the UK for bacterial co-infections among patients in ICU with COVID-19 [63]. This contrasts with the study of Grasselli et al. (2021), who reported appreciably higher prevalence rates of HAIs among COVID-19 patients admitted to the ICUs among participating Italian hospitals at 46% [4]. Similarly, Falcone et al. (2020) reported high rates at 71.6% among patients with COVID-19 admitted to ICUs in participating Italian hospitals [64]. Our findings also contrast with those of Ghali et al. (2021) in Tunisia where HAIs were seen in 35.1% of patients in ICU with COVID-19 [24], and with de Hesselle et al. (2022) among patients critically ill in ICUs enrolled into the Lean European Open Survey on SARS-CoV-2-Infected Patients (LEOSS) study [27]. Secondary bacterial infections were documented in the critical phase in 40.4% of cases in this study, and secondary fungal infections in 14.6% of cases [27]. Other studies though have recorded appreciably higher rates with up to 68% of patients in ICUs acquiring secondary bacterial infections during their stay, principally secondary pneumonia [65]. These observed differences may reflect the indications for admission to ICUs and the severity of admitted patients. Some patients in the ICUs in our study did not require oxygen therapy, and there were also relatively low numbers of patients requiring central venous catheters (Table 1). There has also been improved knowledge regarding managing patients with COVID-19 admitted to hospital during successive waves of the pandemic, including the use of steroids in patients requiring respiratory support [66], which may also influence the findings between studies.
Our study highlighted that the most common HAIs reported among patients with COVID-19 admitted to the ICU were ventilator-associated lower respiratory tract infections, i.e., ventilator-associated pneumonia, followed by catheter-associated urinary tract infections and catheter-related blood stream infections. These findings are similar to those of Grasselli et al. (2021) [4], with high rates of HAIs seen among ventilated patients in the studies of de Hesselle et al. (2022) [27], Bardi et al. (2021) [52] and Falcone (2020) et al. [64]. There findings are also similar to a study from Tunisia where frequent HAIs reported among hospitalized COVID-19 patients admitted to ICU were pneumonia/ lower respiratory tract infections followed by urinary tract infections [24]. Following guidelines, including following recommendations regarding catheter placements and care, can help to reduce these HAIs [3]. This reflects increasing recognition of adherence to standard guidelines among key stakeholder groups as a measure of the quality of care provided [67,68,69,70].
The most frequently isolated microorganisms associated with HAIs in our study were Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae, which is similar to other studies; however, others have reported differences [71,72,73]. Our findings are also similar to those of a previous study from the USA [74], with a study from Italy highlighting that Acinetobacter baumannii were most frequent cause of HAIs among critically ill hospitalized COVID-19 patients [4]. Differences in reported studies among patients in ICUs may reflect differences in endemic bacteria and local antibiotic prescribing practices as well the case mix differences between the various studies, with more mild and moderate cases in some studies along with a smaller number of ventilated patients.
Our study also revealed that HAIs among ICU patients developed more often among those aged above 50 years and with comorbidities including diabetes mellitus and hypertension, as well as with COVID-19 severity, abnormal chest X-rays and laboratory investigations including C-reactive proteins and white blood cells. This is similar to studies from Belgium, China, Italy and Spain as well as among a number of LMICs [45,52,61,64,65]. Others risk factors involved with HAI development among COVID-19 patients admitted to ICU in our study were those prescribed anticoagulants, antithrombotic, antivirals and IL-6 inhibitors including tocilizumab, with tocilizumab typically prescribed to severe or critical patients. In these circumstances though, it is not possible to attribute the greater risk of HAI to the recognised immunosuppressant effects of tocilizumab. In contrast to the findings from our study, a study from the USA found that key risk factors for HAIs for patients admitted to hospital with COVID-19 were dexamethasone and antibiotic exposure at the time of hospital admission [75]. However, this was for all patients and not just those admitted to ICUs. Ascertaining the effect of both steroids and prior or current antibiotic use on HAI risk was not possible in our study due to the universal use of both in the ICU population.
Similar to other studies [4,45,52], we observed higher mortality amongst COVID-19 patients who developed HAIs compared with those who did not develop HAIs. However, the influence of other factors such as disease severity and need for mechanical ventilation were not controlled.
Finally, we are aware that 100% of COVID-19 patients in the ICU were prescribed antibiotics irrespective of whether they had an HAI or not. This likely reflects clinical uncertainty and concern for bacterial co-infection in patients who are severely unwell and vulnerable to acute deterioration. High antibiotic prescribing among patients admitted to hospital with COVID-19 has been observed widely in Pakistan and across countries and is in direct contrast with the low rates of bacterial co-infection observed in numerous studies [48,53,76,77,78]. High rates of unnecessary antimicrobial prescribing risk enhancing AMR and contribute further to HAI risk [48,53,54,57,75,76]. This is of particular concern in Pakistan where there are already high rates of AMR [53,54,59], and should be the focus of further studies alongside antimicrobial stewardship interventions. We will be following this up in future studies.
We are aware that there are a number of limitations with our study. Firstly, we collected data from only three public sector tertiary care hospitals. Consequently, we cannot generalize our findings to the whole of Pakistan. Secondly, we did not collect information from private sector hospitals. However, this was deliberate as patients admitted to ICUs in tertiary public hospitals are likely to be more critically ill than those in private or lower-level public hospitals. Despite these limitations, we believe our findings will facilitate clinicians, public health experts and policy makers regarding potential measures to reduce HAIs among hospitalized patients particularly those with COVID-19. This information can subsequently lead to the development of possible quality improvement programmes as part of ASPs among ICUs in Pakistan to reduce future HAI rates.
4. Materials and Methods
4.1. Study Design and Setting
A retrospective review of medical records was conducted among patients admitted to the ICU due to COVID-19, in three purposely selected public sector tertiary care hospitals, designated for COVID-19 in the Punjab province. Data was collected for all COVID-19 ICU patients over a five-month period from April to August 2021, which was during the third and fourth COVID-19 waves in Pakistan. This methodology involving a retrospective review of patient records is similar to other studies involving the co-authors [48,53,79,80,81,82,83].
All three hospitals were equipped with the necessary equipment and devices, including mechanical ventilators, laboratory facilities and medicines to treat patients with COVID-19.
4.2. The Data Collection Form
A data collection form was designed based on published studies, as well as current guidelines [21,23,26,84,85], to collect data of ICU patients admitted due to COVID-19. The following variables were included in the data collection form: (1) Demographic characteristics: age of the patient in years, their gender and residence; (2) Total number of days in ICU, presence of any comorbidities, clinical signs and symptoms, and oxygen use. The presence of co-morbidities is important since we were aware that some patients were admitted to ICU without the need for oxygen therapy; however they had comorbidities including pre-existing respiratory illness, were immunocompromised, or had underlying cardiac diseases or diabetes mellitus, whilst potentially waiting for laboratory results; (3) COVID-19 severity: Assessed as asymptomatic, mild, moderate, severe or critical as per the guidelines issued by the Ministry of Health or as per guidelines issued by National Health Services, Regulation and Coordination, Government of Pakistan [84]. Those patients who had oxygen saturation below 94% but above 90%, and chest X-rays with infiltrates involving <50% of the total lung fields, were declared as moderate cases. Severe cases were those who had a fever and cough along with respiratory rate < 30, severe respiratory distress, chest X-ray with infiltrates involving <50% of the total lung fields, and oxygen saturation ≤ 90 on room air. The presence of acute respiratory distress syndrome (ARDS) or worsening of respiratory symptoms, bilateral opacities or lung collapse in chest X-rays or CT scans and respiratory or cardiac failure were considered as critical cases. Moderate, severe and critical COVID-19 patients were admitted to the ICU depending upon other disease manifestations; (4) Laboratory findings including chest X-rays, white blood cell (WBCs) counts, C-reactive protein (CRP) levels, D-dimer and serum ferritin levels were also documented. The X-ray findings were reviewed by medical doctors and the treating physicians were consulted in case of any confusion. Normal ranges/presence or absence of WBCs and CRP, D-dimer and serum ferritin were taken from the references mentioned on the testing kits; (5) Medicines prescribed at the time of admission to the ICU were also documented including antipyretics, antihistamines, anticoagulants, antithrombotic medicines and antibiotics; (6) Devices used in the ICU to treat patients, including any invasive mechanical ventilation, central venous catheters, urinary catheters and any orotracheal tubes; (7) Presence of HAIs. HAIs were defined as an infection appearing ≥48 h after hospital admission unless patients had been discharged from the hospital [86,87]. The type or incidence of HAIs were confirmed using the methodology of the European Centers for Diseases Control and Prevention (ECDC) [5,85,88,89]. If HAIs were present, the type of HAI along with the microorganism associated with the HAI [85,89,90]; (8) Outcomes: Whether the patient was discharged from hospital or died.
The draft data collection form was tested in a pilot study involving 30 patients to see if the developed forms were able to capture the necessary data sets for the study. These patients were subsequently excluded from the full study. Based on the pilot study results, no modification to the data collection form was necessary.
4.3. The Data Collection Procedure
The team of investigators (including medical doctors and pharmacists) visited the three hospitals after permission was granted by the hospital administration. They were provided with full training by the principal investigator (ZUM) before commencing the data collection process. The medical records of patients were accessed and reviewed thoroughly to obtain the required information. All the records were in a paper-based format and in case of any uncertainties, healthcare professions were requested for subsequent interpretation.
4.4. Inclusion and Exclusion Criteria
The inclusion criteria were any patient with confirmed COVID-19 (confirmed via RT-PCR testing) admitted to the ICU between April and August 2021, among the three selected hospitals, who had a complete medical record available in the record room. All other patients admitted to the hospitals with COVID-19, but who were not admitted to the ICU, those admitted to the ICU with COVID-19 but outside the time period for the study, and those with incomplete medical records, were excluded from this study.
4.5. Data Analysis
The data were entered onto a Microsoft Excel® sheet, cleaned and after coding imported into the Statistical Package for the Social Sciences (SPSS Inc., version 22, IBM, Chicago, IL, USA). The mean and standard deviation were calculated for continuous variables whereas frequency/number (N) and percentage (%) were calculated for categorical variables. Demographic data, clinical features of COVID-19 patients, and outcomes were compared between patients with HAIs and those with no HAI using χ2 test and Fischer’s exact test as appropriate. We performed binary logistic regression to determine the factors associated with the development of HAIs among COVID-19 patients as well as for the predictors of mortality. A p < 0.05 (two-sided) was considered statistically significant.
4.6. Ethical Considerations
Ethical approval was obtained from the Office of Research, Innovation and Commercialization (ORIC), Lahore College for Women University (LCWU), Lahore, Pakistan (Ref. no. ORIC/LCWU/395). Ethical approval of this study was also obtained from the ethics committees of the participating hospitals.
Since all data was obtained from medical records and drug prescription charts without patient contact, written consent was not required. This is similar to other retrospective and point prevalence surveys conducted by the co-authors [53,80,91,92,93,94,95]. Furthermore, no personal data was recorded, and any patient data recorded was de-identified. Participants’ data were subsequently coded and stored in a password-protected Microsoft Excel® sheet accessible only to the researchers in order to ensure patient confidentiality.
5. Conclusions and Recommendations
There is a high prevalence of HAIs among COVID-19 patients admitted to ICUs in Pakistan. This is associated with higher mortality, although mortality rates are lower than seen in a number of other countries potentially reflecting lower rates of mechanical ventilation and central venous catheters in the cohort studied. Independent factors associated with an increase in HAIs were the use of urinary catheters and mechanical ventilation. Tocilizumab was also independently associated with higher risk of HAI; however, this may reflect disease severity and longer hospital stay and requires further study. By adopting core elements of infection prevention and control, the prevalence as well as the mortality associated with HAIs can be minimized. This includes reviewing key issues and approaches surrounding the use of catheters alongside mechanical ventilation. Moreover, a multisectoral approach under a targeted antimicrobial stewardship programme (ASP) can help address inappropriate antimicrobial use among critically ill patients admitted to the ICU. We will now be working with key personnel in these hospitals to seek ways to reduce HAIs among ICUs in Pakistan through instigating appropriate ASPs and will be following this up in future studies.
Author Contributions
Conceptualization, Z.U.M., S.T., B.G. and R.A.S.; methodology, Z.U.M., J.C.M., B.G. and R.A.S.; validation, Z.U.M., S.T., Z.I., M.S., Y.H.K. and T.H.M.; formal analysis, Z.U.M., M.S., J.C.M., B.G. and R.A.S.; investigation, Z.U.M., S.T., Z.I., M.S., Y.H.K. and T.H.M.; resources, Z.U.M.; data curation, Z.U.M., S.T., Z.I., J.C.M., M.S., Y.H.K., T.H.M., B.G. and R.A.S.; writing—original draft preparation, Z.U.M., M.S. and B.G.; writing—review and editing, Z.U.M., S.T., Z.I., J.C.M., M.S., Y.H.K., T.H.M., B.G. and R.A.S.; visualization, Z.U.M. and S.T.; supervision, Z.U.M.; project administration, Z.U.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was obtained from the ORIC, LCWU, Lahore, Pakistan (Ref. no. ORIC/LCWU/395). Ethical approval of this study was also obtained from the ethics committees of the participating hospitals.
Informed Consent Statement
This was not obtained since all data was obtained from medical records and drug prescription charts without any patient contact. This is similar to other retrospective and point prevalence surveys conducted by the co-authors [46,75,86,87,88,89,90]. Furthermore, no personal data was recorded, and any patient data recorded was deidentified.
Data Availability Statement
Further data is available from the co-authors on reasonable request.
Acknowledgments
We thank personnel in the participating hospitals for their help with collating patients’ records ready for review and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pouwels, K.B.; Vansteelandt, S.; Batra, R.; Edgeworth, J.; Wordsworth, S.; Robotham, J.V. Estimating the Effect of Healthcare-Associated Infections on Excess Length of Hospital Stay Using Inverse Probability-Weighted Survival Curves. Clin. Infect. Dis. 2020, 71, e415–e420. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.M.; Rupp, M.E.; Cawcutt, K.A. Prevention of Central-Line Associated Bloodstream Infections: 2021 Update. Infect. Dis. Clin. N. Am. 2021, 35, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Godman, B.; Hassali, M.A.; Hashmi, F.K.; Azhar, F.; Rehman, I.U. Point prevalence surveys of health-care-associated infections: A systematic review. Pathog. Glob. Health 2019, 113, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Birhane, M.; Bekele, S.; Kibru, G.; Teshager, L.; Yilma, Y.; Ahmed, Y.; Fentahun, N.; Assefa, H.; Gashaw, M.; et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: Longitudinal study. Antimicrob. Resist. Infect. Control. 2018, 7, 2. [Google Scholar] [CrossRef]
- Nelson, R.E.; Schweizer, M.; Jones, M.; Stevens, V.W.; Khader, K.; Perencevich, E.; Rubin, M.; Samore, M. The Cost and Mortality Burden of Hospital-Onset Antimicrobial-Resistant Healthcare-Associated Infections in the USA. Open Forum Infect. Dis. 2017, 4 (Suppl. 1), S177–S178. [Google Scholar] [CrossRef][Green Version]
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Al Jernigan, J.; Baggs, J. National Estimates of Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72 (Suppl. 1), S17–S26. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J. Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Forrester, J.D.; Maggio, P.M.; Tennakoon, L. Cost of Health Care-Associated Infections in the United States. J. Patient Saf. 2022, 18, e477–e479. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, D.; Li, H.; Wang, Q.; Mao, Z.; Fang, L.; Ren, N.; Sun, J. Direct medical burden of antimicrobial-resistant healthcare-associated infections: Empirical evidence from China. J. Hosp. Infect. 2020, 105, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Dancer, S.; Haahr, L.; Manoukian, S.; Mason, H.; Kavanagh, K.; Cook, B.; et al. Epidemiology of healthcare-associated infection reported from a hospital-wide incidence study: Considerations for infection prevention and control planning. J. Hosp. Infect. 2021, 114, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.L.; Mwatondo, A.; Alimi, Y.H.; Varma, J.K.; Vilas, V. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: A review. Int. J. Infect. Dis. 2021, 103, 469–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Z.-F.; Chen, S.-X.; Zhou, D.-R.; Li, Z.-K.; Meng, Y.; Zhou, J.-F.; Hou, T.-Y. Prevalence of healthcare-associated infections and antimicrobial use in China: Results from the 2018 point prevalence survey in 189 hospitals in Guangdong Province. Int. J. Infect. Dis. 2019, 89, 179–184. [Google Scholar] [CrossRef]
- Sun, J.; Qin, W.; Jia, L.; Sun, Z.; Xu, H.; Hui, Y.; Gu, A.; Li, W. Analysis of Continuous Prevalence Survey of Healthcare-Associated Infections Based on the Real-Time Monitoring System in 2018 in Shandong in China. BioMed Res. Int. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Tartari, E.; Huang, J.; Harbarth, S.; Pittet, D.; Zingg, W. The Prevalence of Healthcare-Associated Infections in Mainland China: A Systematic Review and Meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 701–709. [Google Scholar] [CrossRef]
- Weinshel, K.; Dramowski, A.; Hajdu, Á.; Jacob, S.; Khanal, B.; Zoltán, M.; Mougkou, K.; Phukan, C.; Staneloni, M.I.; Singh, N. Gap analysis of infection control practices in low- and middle-income countries. Infect. Control Hosp. Epidemiol. 2015, 36, 1208–1214. [Google Scholar] [CrossRef]
- Sengupta, S.; Barman, P.; Lo, J. Opportunities to Overcome Implementation Challenges of Infection Prevention and Control in Low-Middle Income Countries. Curr. Treat. Options Infect. Dis. 2019, 11, 267–280. [Google Scholar] [CrossRef]
- Salman, M.; Raza, M.H.; Mustafa, Z.U.; Shrestha, S.; Ali, M.; Faham, H.; Asif, N.; Shehzadi, N.; Hussain, K. Knowledge, attitudes and practices of hand hygiene among Pakistani health professionals: A cross-sectional study. J. Infect. Dev. Ctries. 2018, 12, 063–066. [Google Scholar] [CrossRef]
- Shahida, S.I.A.; Dey, B.; Islam, F.; Venkatesh, K.; Goodman, A. Hospital Acquired Infections in Low and Middle Income Countries: Root Cause Analysis and the Development of Infection Control Practices in Bangladesh. Open J. Obstet. Gynecol. 2016, 6, 28–39. [Google Scholar] [CrossRef]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug. Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Ghali, H.; Ben Cheikh, A.; Bhiri, S.; Khefacha, S.; Latiri, H.S.; Ben Rejeb, M. Trends of Healthcare-associated Infections in a Tuinisian University Hospital and Impact of COVID-19 Pandemic. Inquiry 2021, 58, 469580211067930. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Mularoni, A.; Giacobbe, D.R.; Castaldo, N.; Vena, A. New Antibiotics for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Semin Respir. Crit. Care Med. 2022, 43, 280–294. [Google Scholar] [CrossRef]
- Bennett, E.E.; VanBuren, J.; Holubkov, R.; Bratton, S.L. Presence of Invasive Devices and Risks of Healthcare-Associated Infections and Sepsis. J. Pediatr. Intensive Care 2018, 7, 188–195. [Google Scholar]
- de Hesselle, M.L.; Borgmann, S.; Rieg, S.; Vehreshild, J.J.; Spinner, C.D.; Koll, C.E.M.; Hower, M.; Stecher, M.; Ebert, D.; Hanses, F.; et al. Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients. J. Clin. Med. 2022, 11, 5239. [Google Scholar] [CrossRef]
- Onyedibe, K.; Shehu, N.; Igbanugo, J.; Okolo, M.; Gomerep, S.; Isa, S.; Egah, D. Hand hygiene knowledge, training and practice: A cross-sectional study in a tertiary health institution, North-central Nigeria. Niger. J. Clin. Pr. 2019, 22, 1008–1013. [Google Scholar] [CrossRef]
- Sarani, H.; Balouchi, A.; Masinaeinezhad, N.; Ebrahimitabas, E. Knowledge, Attitude and Practice of Nurses about Standard Precautions for Hospital-Acquired Infection in Teaching Hospitals Affiliated to Zabol University of Medical Sciences (2014). Glob. J. Health Sci. 2015, 8, 193–198. [Google Scholar] [CrossRef]
- Sharif, I.; Rashid, Z.; Tariq, N.; Mashhadi, F.; Din, M.; Wazir, L.; Dogar, M.A.; Asif, Y.; Jadoon, A. Gaps in knowledge and practices regarding nosocomial infections among nursing staff of a tertiary care hospital of Rawalpindi. PAFMJ 2019, 69, 1210–1225. [Google Scholar]
- Asfaw, N. Knowledge and practice of nurses towards prevention of hospital acquired infections and its associated factors. Int. J. Afr. Nurs. Sci. 2021, 15, 100333. [Google Scholar] [CrossRef]
- Zaidi, N.J.N.; Naz, S.; Mumtaz, A. Gaps in Knowledge and Practices About Health Care Associated Infections Among Health Care Workers at a Tertiary Care Hospital. J. Islamabad Med. Dent. Coll. (JIMDC). 2016, 5, 84–87. [Google Scholar]
- CDC. Types of Healthcare-Associated Infections. 2014. Available online: https://www.cdc.gov/hai/infectiontypes.html (accessed on 2 September 2022).
- Simpson, C.R.; Robertson, C.; Vasileiou, E.; Moore, E.; McCowan, C.; Agrawal, U.; Stagg, H.R.; Docherty, A.; Mulholland, R.; Murray, J.L.; et al. Temporal trends and forecasting of COVID-19 hospitalisations and deaths in Scotland using a national real-time patient-level data platform: A statistical modelling study. Lancet Digit. Health 2021, 3, e517–e525. [Google Scholar] [CrossRef]
- Sapiano, M.R.P.; Dudeck, M.A.; Soe, M.; Edwards, J.R.; O’Leary, E.N.; Wu, H.; Allen-Bridson, K.; Amor, A.; Arcement, R.; Tejedor, S.C.; et al. Impact of coronavirus disease 2019 (COVID-19) on US Hospitals and Patients, April-July 2020. Infect. Control Hosp. Epidemiol. 2022, 43, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Delmonte, D.; Scognamiglio, T.; Signorelli, C. COVID-19 deaths in Lombardy, Italy: Data in context. Lancet Public Health 2020, 5, e310. [Google Scholar] [CrossRef]
- Peters, A.W.; Chawla, K.S.; Turnbull, Z.A. Transforming ORs into ICUs. N. Engl. J. Med. 2020, 382, e52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mazzucchelli, R.; Agudo Dieguez, A.; Dieguez Costa, E.M.; Crespí Villarías, N. Democracy and COVID-19 mortality in Europe. Rev. Esp. Salud. Publica. 2020, 94, e202006073. [Google Scholar]
- Ng, Y.; Li, Z.; Chua, Y.X.; Chaw, W.L.; Zhao, Z.; Er, B.; Pung, R.; Chiew, C.J.; Lye, D.; Heng, D.; et al. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 307–311. [Google Scholar] [CrossRef]
- Thai, P.Q.; Rabaa, M.A.; Luong, D.H.; Tan, D.Q.; Quang, T.D.; Quach, H.-L.; Thi, H.N.-A.; Dinh, P.C.; Nghia, N.D.; Tu, T.A.; et al. The First 100 Days of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Control in Vietnam. Clin. Infect. Dis. 2021, 72, e334–e342. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wamboga, J.; Miljković, N.; Mwita, J.C.; Rwegerera, G.M.; et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, O.O.; Godman, B.; Fadare, J.O.; Mudenda, S.; Adeoti, A.O.; Yinka-Ogunleye, A.F.; Ogundele, S.O.; Oyawole, M.R.; Schönfeldt, M.; Rashed, W.M.; et al. Coronavirus Disease 2019 (COVID-19) Pandemic across Africa: Current Status of Vaccinations and Implications for the Future. Vaccines 2022, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Myatra, S.N.; Divatia, J.V.; Biswas, S.; Shrivastava, A.; Al-Ruzzieh, M.A.; Ayaad, O.; Bat-Erdene, A.; Bat-Erdene, I.; Narankhuu, B.; et al. The impact of COVID-19 on health care-associated infections in intensive care units in low- and middle-income countries: International Nosocomial In-fection Control Consortium (INICC) findings. Int. J. Infect. Dis. 2022, 118, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.P.M.; Borges, I.C.; Buss, L.; Machado, A.; Bassetti, B.R.; Cocentino, B.; Bicalho, C.S.; Carrilho, C.M.; Rodrigues, C.; Neto, E.A.S.; et al. Healthcare-associated infections on the intensive care unit in 21 Brazilian hospitals during the early months of the coronavirus disease 2019 (COVID-19) pandemic: An ecological study. Infect. Control. Hosp. Epidemiology 2022, 1–7. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Alessandri, F.; Mazzalai, E.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control. 2021, 10, 87. [Google Scholar] [CrossRef]
- Ramzan, K.; Shafiq, S.; Raees, I.; Mustafa, Z.U.; Salman, M.; Khan, A.H.; Meyer, J.C.; Godman, B. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics 2022, 11, 789. [Google Scholar] [CrossRef]
- Salman, M.; Mustafa, Z.U.; Khan, T.M.; Shehzadi, N.; Hussain, K. How Prepared Was Pakistan for the COVID-19 Outbreak? Disaster Med. Public Health Prep. 2020, 14, e44–e45. [Google Scholar] [CrossRef]
- Kamran, S.H.; Ul Mustafa, Z.; Rao, A.Z.; Hasan, S.S.; Zahoor, F.; Sarwar, M.U.; Khan, S.; Butt, S.; Rameez, M.; Abbas, M.A. SARS-CoV-2 infection pattern, transmission and treatment: Multi-center study in low to middle-income districts hospitals in Punjab, Pakistan. Pak. J. Pharm. Sci. 2021, 34, 1135–1142. [Google Scholar]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 2 September 2022).
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Saleem, M.S.; Ikram, M.N.; Salman, M.; Butt, S.A.; Khan, S.; Godman, B.; Seaton, R.A. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: Findings from a multicenter, point prevalence survey. Pathog. Glob. Health 2021, 116, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Naghavi, M. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Bank Group. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance. 2019. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32552/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf?sequence=1&isAllowed=y (accessed on 2 September 2022).
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N.; et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics 2021, 11, 35. [Google Scholar] [CrossRef]
- Ministry of National Health Services Regulations; Coordination Government of Pakistan. National AMR Action Plan for Pakistan. 2017. Available online: https://www.nih.org.pk/wp-content/uploads/2018/08/AMR-National-Action-Plan-Pakistan.pdf (accessed on 2 September 2022).
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K. Pakistan’s national action plan for antimicrobial resistance: Translating ideas into reality. Lancet Infect Dis. 2018, 18, 1066–1067. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti-Infect. Ther. 2022, 20, 71–93. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Wang, Z.; Chen, H.; Tian, L.; Liu, D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect. Control Hosp. Epidemiol. 2020, 41, 982–983. [Google Scholar] [CrossRef]
- Ong, C.C.H.; Farhanah, S.; Linn, K.Z.; Tang, Y.W.; Poon, C.Y.; Lim, A.Y.; Tan, H.R.; Hamed, N.H.B.; Huan, X.; Puah, S.H.; et al. Nosocomial infections among COVID-19 patients: An analysis of intensive care unit surveillance data. Antimicrob. Resist. Infect. Control. 2021, 10, 119. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2020, 76, 1078–1084. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; Dubois, J. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Ihsan, B.; Malik, I.; Ahmad, N.; Saleem, Z.; Sehar, A.; Baber, Z.U. Antibiotic stewardship program in Pakistan: A multicenter qualitative study exploring medical doctors’ knowledge, perception and practices. BMC Infect. Dis. 2021, 21, 374. [Google Scholar] [CrossRef]
- Niaz, Q.; Godman, B.; Campbell, S.; Kibuule, D. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int. J. Clin. Pharm. 2020, 42, 1227–1236. [Google Scholar] [CrossRef]
- Campbell, S.M.; Meyer, J.; Godman, B. Why Compliance to National Prescribing Guidelines is Important Especially across Sub-Saharan Africa and Suggestions for the Future. Biomed. Pharm. Sci. 2021, 4, 1–7. [Google Scholar]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.S.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care. 2021, 25, 25. [Google Scholar] [CrossRef]
- Russo, A.; Olivadese, V.; Trecarichi, E.M.; Torti, C. Bacterial Ventilator-Associated Pneumonia in COVID-19 Patients: Data from the Second and Third Waves of the Pandemic. J. Clin. Med. 2022, 11, 2279. [Google Scholar] [CrossRef]
- Kumar, G.; Adams, A.; Hererra, M.; Rojas, E.R.; Singh, V.; Sakhuja, A.; Meersman, M.; Dalton, D.; Kethireddy, S.; Nanchal, R.; et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int. J. Infect. Dis. 2020, 104, 287–292. [Google Scholar] [CrossRef]
- Smith, L.; Karaba, S.M.; Amoah, J.; Jones, G.; Avery, R.K.; Dzintars, K.; Helsel, T.; Cosgrove, S.E.; Fabre, V.; Hesel, T.; et al. Hospital-acquired infections among adult patients admitted for coronavirus disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2022, 43, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE. 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; I de Silva, T.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Haque, M.; Shetty, A.; Acharya, J.; Kumar, M.; Sinha, V.; Manohar, B.; Gowere, M.; Godman, B. Current management of children with COVID-19 in hospitals in India; Pilot study and findings. Adv. Hum. Biol. 2022, 12, 16–21. [Google Scholar]
- Chowdhury, K.; Haque, M.; Nusrat, N.; Adnan, N.; Islam, S.; Lutfor, A.B.; Begum, D.; Rabbany, A.; Karim, E.; Malek, A.; et al. Management of Children Admitted to Hospitals across Bangladesh with Suspected or Confirmed COVID-19 and the Implications for the Future: A Nationwide Cross-Sectional Study. Antibiotics 2022, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, N.; Haque, M.; Chowdhury, K.; Adnan, N.; Lutfor, A.B.; Karim, E.; Hassan, M.; Rabbany, A.; Begum, D.; Hasan, M.N.; et al. Pilot Study on the Current Management of Children with COVID-19 In Hospitals in Bangladesh; Findings and Implications. Bangladesh J. Med Sci. 2021, 188–198. [Google Scholar] [CrossRef]
- Kumar, S.; Haque, M.; Shetty, A.; Choudhary, S.; Bhatt, R.; Sinha, V.; Manohar, B.; Chowdhury, K.; Nusrat, N.; Jahan, N.; et al. Characteristics and Management of Children With Suspected COVID-19 Admitted to Hospitals in India: Implications for Future Care. Cureus 2022, 14, e27230. [Google Scholar] [CrossRef]
- Paramadhas, B.D.A.; Tiroyakgosi, C.; Mpinda-Joseph, P.; Morokotso, M.; Matome, M.; Sinkala, F.; Gaolebe, M.; Malone, B.; Molosiwa, E.; Shanmugam, M.G.; et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert. Rev. Anti. Infect Ther. 2019, 17, 535–546. [Google Scholar] [CrossRef]
- Government of Pakistan; Ministry of National Health Services, Regulations and Coordination. Clinical Management Guidelines for COVID-19 Infections. 2020. Available online: https://nhsrc.gov.pk/SiteImage/Misc/files/20200704%20Clinical%20Management%20Guidelines%20for%20COVID-19%20infections_1203.pdf (accessed on 3 September 2022).
- ECDC. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial use in European Acute Care Hospitals—ECDC PPS Validation Protocol Version 3.1.2. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/PPS-HAI-AMR-protocol.pdf (accessed on 3 September 2022).
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Boev, C.; Kiss, E. Hospital-Acquired Infections: Current Trends and Prevention. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65. [Google Scholar] [CrossRef]
- ECDC. Healthcare-Associated Infections. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 3 September 2022).
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 1988, 16, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Momanyi, L.; Opanga, S.; Nyamu, D.; Oluka, M.; Kurdi, A.; Godman, B. Antibiotic Prescribing Patterns at a Leading Referral Hospital in Kenya: A Point Prevalence Survey. J. Res. Pharm. Pract. 2019, 8, 149–154. [Google Scholar] [PubMed]
- Mustafa, Z.U.; Salman, M.; Yasir, M.; Godman, B.; Majeed, H.A.; Kanwal, M.; Iqbal, M.; Riaz, M.B.; Hayat, K.; Hasan, S.S. Antibiotic consumption among hospitalized neonates and children in Punjab province, Pakistan. Expert. Rev. Anti. Infect Ther. 2022, 20, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Z.U.; Salman, M.; Aslam, N.; Asif, N.; Hussain, K.; Shehzadi, N.; Hayat, K. Antibiotic use among hospitalized children with lower respiratory tract infections: A multicenter, retrospective study from Punjab, Pakistan. Expert. Rev. Anti. Infect Ther. 2022, 20, 131–136. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Hashmi, F.K.; Saleem, F. A multicenter point prevalence survey of healthcare-associated infections in Pakistan: Findings and implications. Am. J. Infect. Control. 2019, 47, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Skosana, P.P.; Schellack, N.; Godman, B.; Kurdi, A.; Bennie, M.; Kruger, D.; Mayer, J.C. A national, multicentre web-based point prevalence survey of antimicrobial use in community healthcare centres across South Africa and the implications. Hosp. Pract. 2022, 50, 306–317. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).