Covariables of Soil-Forming Factors and Their Influence on pH Distribution and Spatial Variability
Abstract
:1. Introduction
2. Materials and Methods
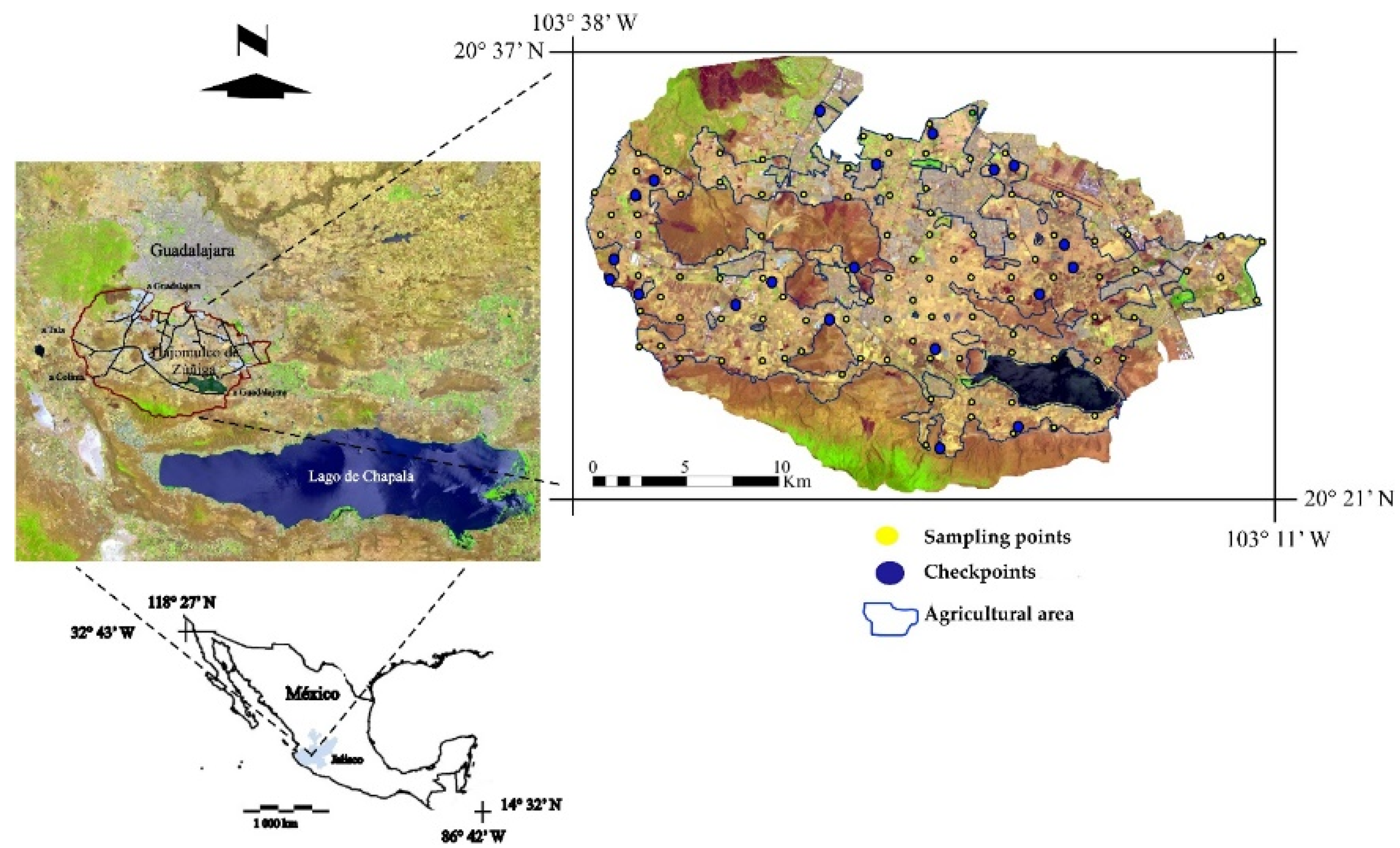
2.1. Location of Observation Points and Sample Collection
2.2. Physical and Chemical Determinations in the Laboratory
2.3. Determination of Environmental Covariables of the Soil-Forming Factors
2.4. Descriptive Statistics and Multivariate Analysis
2.5. Analysis of the Spatial Structure and Interpolation of the pH Data
2.6. Verification of the pH Map
3. Results
3.1. Multivariate Analysis of Environmental Covariables
3.2. Analysis of the Spatial Structure of pH
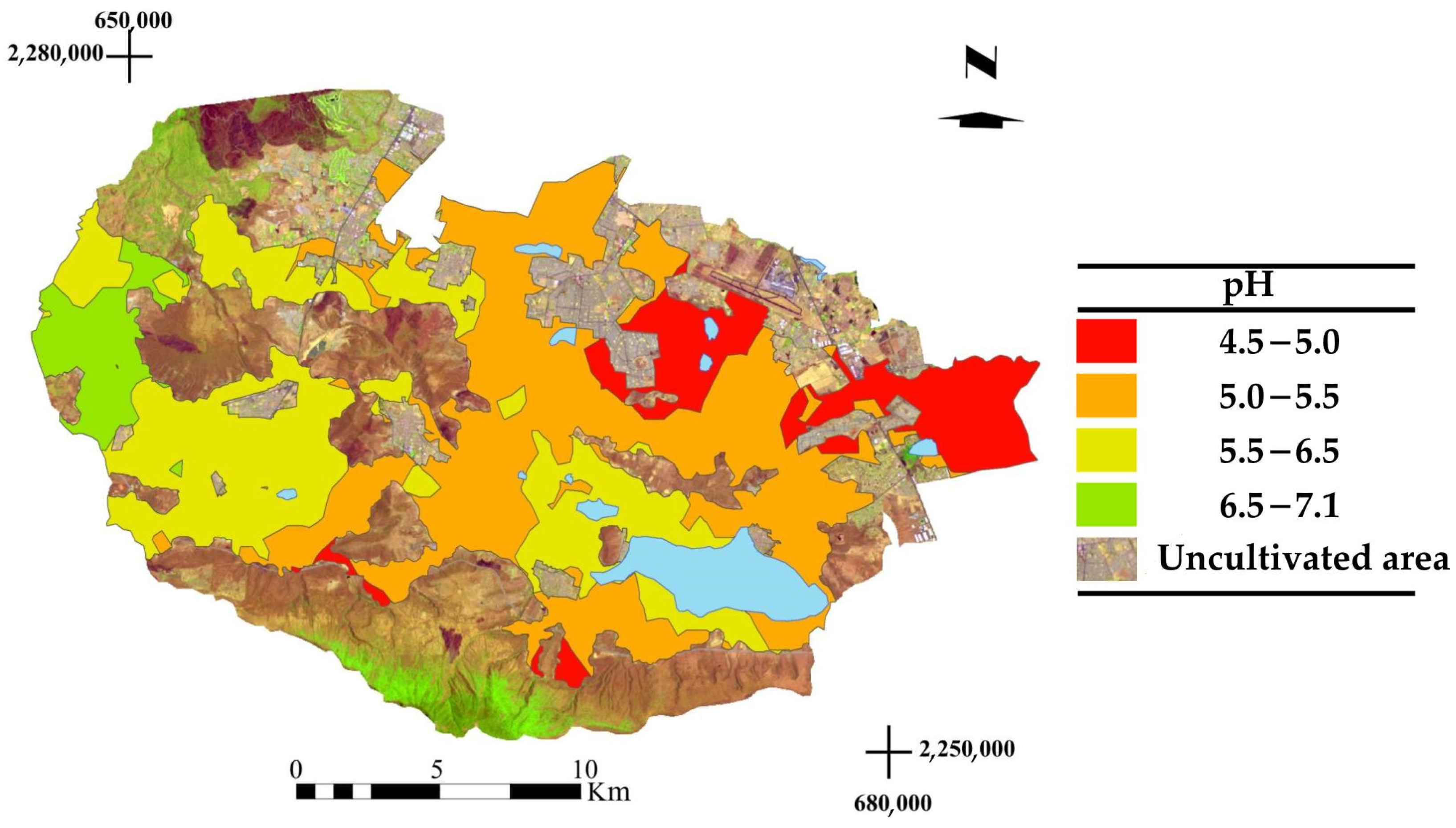
3.3. Spatial Distribution of pH
3.4. Verification of the pH Map
4. Discussion
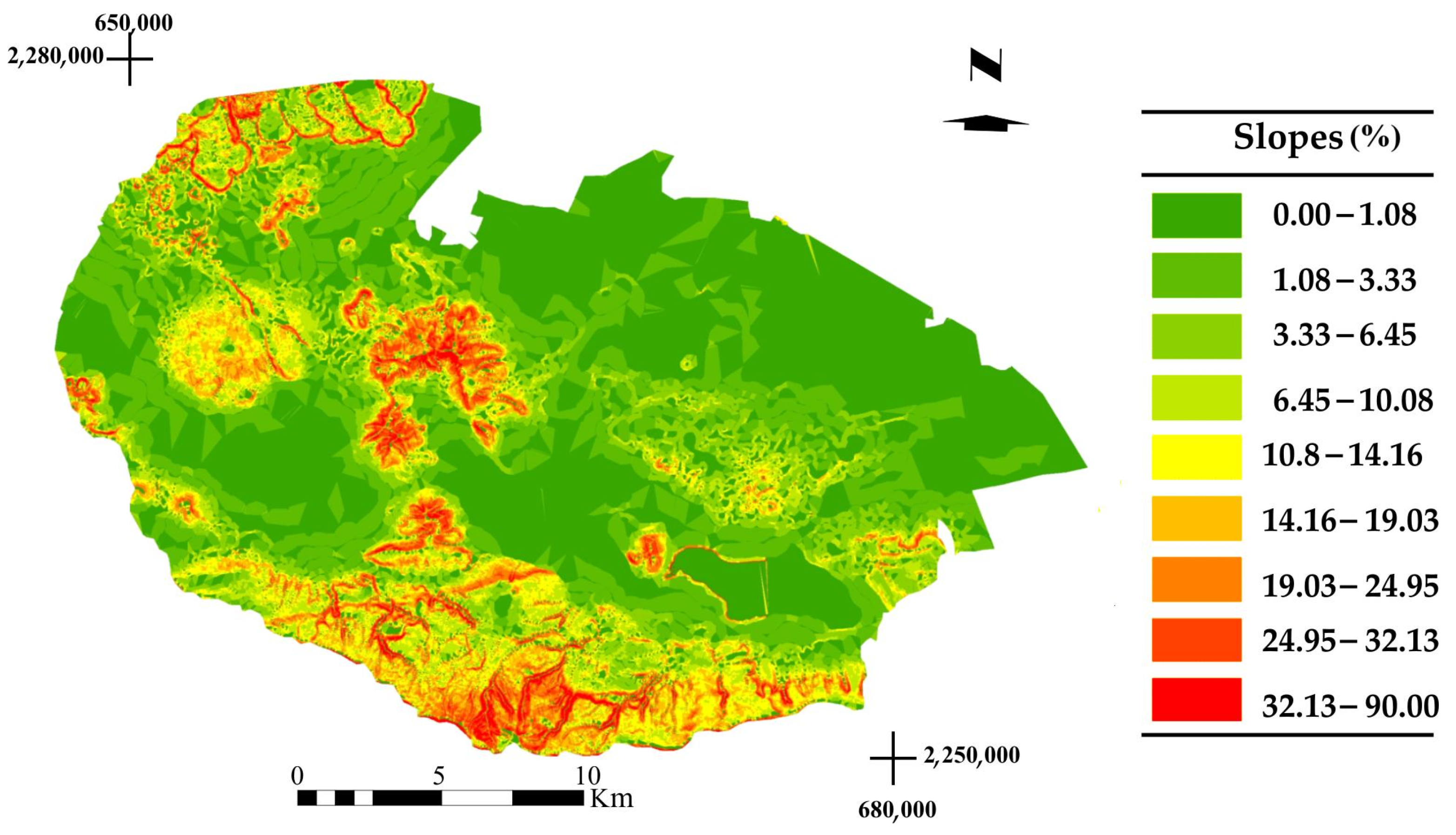
4.1. Behavior of the Environmental Covariables
4.2. Effect of the Covariables on pH
4.3. Spatial Structure of pH
4.4. Spatial Distribution of pH
4.5. Precision of the pH Map
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pahlavan-Rad, M.R.; Akbarimoghaddam, A. Spatial variability of soil texture fractions and pH in a flood plain (case study from eastern Iran). Catena 2018, 160, 275–281. [Google Scholar] [CrossRef]
- Saleh, A.M. Spatial Variability Mapping of Some Soil Properties in Jadwal Al_Amir Project/Babylon/Iraq. J. Indian Soc. Remote Sens. 2018, 46, 1481–1495. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Hu, W.; Bi, R.; Peak, D.; Si, B. Scale-and location-specific relationships between soil available micronutrients and environmental factors in the Fen River basin on the Chinese Loess Plateau. Catena 2016, 147, 764–772. [Google Scholar] [CrossRef]
- Bloom, P.R.; Skyllberg, U. Soil pH and pH Buffering. In Handbook of Soil Science, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; Volume 1: Properties and processes; CRC Press (Taylor & Francis): Boca Raton, FL, USA, 2012; pp. 19.1–19.14. [Google Scholar]
- Tu, C.; He, T.; Lu, X.; Luo, Y.; Smith, P. Extent to which pH and topographic factors control soil organic carbon level in dry farming cropland soils of the mountainous region of Southwest China. Catena 2018, 163, 204–209. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education Limited: London, UK, 2017; 1104p. [Google Scholar]
- Lo Monaco, P.A.V.; Júnior, G.R.; Vieira, G.H.S.; Meneghelli, C.M.; Simon, C.P. Conchas de ostras e cascas de ovos moídas como corretivos da acidez do solo. Eng. Agric. 2016, 25, 584–590. [Google Scholar] [CrossRef]
- Acevedo-Sandoval, O.; Valera-Pérez, M.A.; Prieto-García, F. Propiedades físicas, químicas y mineralógicas de suelos forestales en Acaxochitlan, Hidalgo, México. Univ. Cienc. 2010, 26, 137–150. [Google Scholar]
- Hernández-Jiménez, A.; Llanes-Hernández, V.; Terry-Alfonso, E.; Carnero-Lazo, G. pH changes in brown soils of Cuba when eroded. Cult. Trop. 2020, 41, e04. [Google Scholar]
- Shiferaw, T.; Tadele, M. Review on Effect of Soil Acidity on Barley (Hordeum vulgare L.). J. Nat. Sci. Res. 2022, 13, 13–17. [Google Scholar]
- Getachew, A.; Chilot, Y.; Teklu, E. Soil Acidity Management; Ethiopian Institute of Agricultural Research (EIAR): Addis Ababa, Ethiopia, 2019; 56p. [Google Scholar]
- Zhang, Y.-Y.; Wu, W.; Liu, H. Factors affecting variations of soil pH in different horizons in hilly regions. PLoS ONE 2019, 14, e0218563. [Google Scholar] [CrossRef] [Green Version]
- McBratney, A.B.; Mendonca-Santos, M.L.; Minasny, B. On digital soil mapping. Geoderma 2003, 117, 3–52. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Jianga, J.; Lua, H.; Honga, Z.; Qiana, W.; Xua, R.; Denga, K.; Guana, P. The mechanisms underlying the reduction in aluminum toxicity and improvements in the yield of sweet potato (Ipomoea batatas L.) after organic and inorganic amendment of an acidic ultisol. Agric. Ecosyst. Environ. 2020, 288, 106716. [Google Scholar] [CrossRef]
- Sellan, G.; Thomson, J.; Majalap, N.; Robert, R.; Brearley, F.Q. Impact of soil nitrogen availability and pH on tropical heath forest organic matter decomposition and decomposer activity. Pedobiologia J. Soil Ecol. 2020, 80, 150645. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Management of soil acidity of South American soils for sustainable crop production. Adv. Agron. 2014, 128, 221–275. [Google Scholar]
- Cruz-Macías, W.O.; Rodríguez-Larramendi, L.A.; Salas-Marina, M.A.; Hernández-García, V.; Campos-Saldaña, R.A.; Chávez-Hernández, M.H.; Gordillo-Curiel, A. Efecto de la materia orgánica y la capacidad de intercambio catiónico en la acidez de suelos cultivados con maíz en dos regiones de Chiapas, México. Terra Latinoam. 2020, 38, 475–480. [Google Scholar] [CrossRef]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 9, 155–188. [Google Scholar] [CrossRef] [Green Version]
- Djodjic, F.; Bieroza, M.; Bergström, L. Land use, geology and soil properties control nutrient concentrations in headwater streams. Sci. Total Environ. 2021, 772, 145108. [Google Scholar] [CrossRef]
- Alemán-Montes, B.; Búcaro-González, A.; Henríquez-Henríquez, C.; Largaespada-Zelaya, K. Mapeo digital de suelos agrícolas en la región occidental del Valle Central de Costa Rica. Agron. Costarricense 2019, 43, 157–166. [Google Scholar] [CrossRef]
- Yescas-Coronado, P.; Álvarez-Reyna, V.P.; Segura-Castruita, M.A.; García-Carrillo, M.; Hernández-Hernández, V.; González-Cervantes, G. Variabilidad Espacial del Carbono Orgánico e Inorgánico del Suelo en la Comarca Lagunera, México. Bol. Soc. Geol. Mex. 2018, 70, 591–610. [Google Scholar] [CrossRef]
- Malone, B.; Minasny, B.; McBratney, A. Using R for Digital Soil Mapping; Springer: Cham, Switzerland, 2017; 262p. [Google Scholar]
- Colín García, G.; Fernández-Reynoso, D.S.; Martínez-Menez, M.R.; Ríos-Berber, J.D.; Sánchez-Guzmán, P.; Rubio-Granados, E.; Ibáñez-Castillo, L.A. Clasificación digital de suelos a través de covariables ambientales de la cuenca del río Mixteco. Terra Latinoam. 2017, 35, 281–291. [Google Scholar]
- Gobierno del Estado de Jalisco. Actualización del Atlas Municipal de Riesgos por Amenazas Naturales y Antrópicas en el Municipio de Tlajomulco de Zúñiga, Jalisco. Mapa Geológico. 2018. Available online: https://tlajomulco.gob.mx/ProteccionCivil/1.1.7%20MAPA%20GEOL%C3%93GICO.pdf (accessed on 20 December 2020).
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Instituto de Geografía-Universidad Nacional Autónoma de México: México city, Mexico, 2004; 98p. [Google Scholar]
- SMN (Servicio Meteorológico Nacional). Normales Climatológicas Periodos 1951–2010 y 1981–2000. Servicio Meteorológico Nacional, México, 2015. Available online: http://smn.cna.gob.mx (accessed on 20 December 2020).
- IIEG-Jalisco (Instituto de Información Estadística y Geografía de Jalisco). Recursos Cartográficos/Suelos, Vegetación y Uso del Suelo. 2018. Available online: https://iieg.gob.mx/contenido/Municipios/TlajomulcodeZuniga.pdf (accessed on 18 December 2020).
- INEGI (Instituto Nacional de Estadística y Geografía). Sistema de Descarga de Productos Digitales. 2019. Available online: http://www.beta.inegi.org.mx/temas/mapas/topografico/ (accessed on 5 December 2020).
- NOM-021-RECNAT-2000S; Norma Oficial Mexicana que establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreos y Análisis. Diario Oficial de la Federación: México, Mexico, 2002; 85p.
- Böhner, J.; Antoniç, O. Land-Surface parameters specific to topo-climatology. In Geomorphometry. Concepts, Software, Applications; Hengl, T., Reuter, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 195–213. [Google Scholar]
- Figueroa-Jáuregui, M.L.; Martínez-Menez, M.R.; Ortiz-Solorio, C.A.; Fernández-Reynoso, D. Influencia de los factores formadores en las propiedades de los suelos en la Mixteca, Oaxaca, México. Terra Latinoam. 2018, 36, 287–299. [Google Scholar] [CrossRef]
- Vani, V.; Mandla, V.R. Comparative study of NDVI and SAVI vegetation indices in Anantapur district semi-arid areas. Int. J. Civ. Eng. Technol. 2017, 8, 559–566. [Google Scholar]
- ESRI (Environmental Systems Research Institute). ArcGis 10.3. Recorrido Rápido por la ArcGIS Spatial Analyst Extension. 2014. Available online: https://desktop.arcgis.com/es/arcmap/10.3/guide-books/extensions/spatial-analyst/a-quick-tour-of-spatial-analyst.htm (accessed on 18 January 2020).
- Pike, R.J.; Evans, I.S.; Hengl, T. Geomorphometry: A brief Guide. Dev. Soil Sci. 2009, 33, 3–30. [Google Scholar]
- INEGI (Instituto Nacional de Estadística y Geografía). Continuo de Elevaciones Mexicano. INEGI. Aguascalientes, México. 2015. Available online: http://www.inegi.org.mx/ (accessed on 5 December 2020).
- Minitab Inc. Minitab® State College. Minitab Inc. Pennsylvania, EEUU. 2013. Available online: https://www.minitab.com/es-mx/ (accessed on 15 August 2021).
- Cuadras, C.M. Nuevos Métodos de Análisis Multivariante; CMC: Barcelona, Spain, 2020; 304p. [Google Scholar]
- Jaramillo, D.F. Variabilidad espacial del suelo, bases para su estudio. Rev. Fac. Cienc. 2012, 1, 73–87. [Google Scholar]
- Gallardo, A. Geostadística. Ecosistemas 2006, 15, 48–58. [Google Scholar]
- Elbasiouny, H.; Abowaly, M.; Alkheirb, A.A.; Gad, A.A. Spatial variation of soil carbon and nitrogen pools by using ordinary Kriging method in an area of north Nile Delta, Egypt. Catena 2014, 113, 70–78. [Google Scholar] [CrossRef]
- Douglas, E.M.; Jacobs, J.M.; Sumner, D.M.; Ray, R.L. A comparison of models for estimating potential evapotranspiration for Florida land cover types. J. Hydrol. 2009, 373, 366–376. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Parking, T.B.; Karlen, D.L.; Novak, J.M.; Turco, R.F.; Konopka, A.E. Field-scale variability of soil properties in central Iowa soils. Soil Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- López-Acevedo, R.M.; Poch, C.R.M.; Porta, C.J. Edafología: Uso y Protección de Suelos; Cuarta, Ed.; Mundi-Prensa Libro: México city, Mexico, 2019; 624p. [Google Scholar]
- Zúñiga, F.; Buenaño, M.; Risco, D. Caracterización física y química de suelos de origen volcánico con actividad agrícola, próximos al volcán Tungurahua. Rev. Ecuat. Investig. Agropecu. 2016, 1, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Mishra, U.; Ussiri, D.A.; Lal, R. Tillage effects on soil organic carbon storage and dynamics in Corn Belt of Ohio USA. Soil Till. Res. 2010, 107, 88–96. [Google Scholar] [CrossRef]
- Torres, E.; Linares, G.; Tenorio, M.G.; Peña, R.; Castelán, R.; Rodríguez, A. Índices de vegetación y Uso de Suelo en la Región Terrestre Prioritaria 105: Cuetzalan, México. Rev. Iberoam. Cienc. 2014, 1, 112–120. [Google Scholar]
- Ruiz-Corral, J.A.; Valdez-Díaz, L.E.; Flores-López, H.E.; Medina-García, G.; Ramírez- Díaz, J.L.; Pérez-Domínguez, J.F.; Aceves-Rodríguez, J.J.; González-Ávila, A.; Soltero-Díaz, L.; Medina-Ocegueda, S.; et al. Potencial productivo agrícola de la región centro de Jalisco; Centro de Investigación Regional del Pacifico Centro, INIFAP: México city, Mexico, 2005; 84p. [Google Scholar]
- Wang, Y.; Zhang, X.C.; Zhang, J.L.; Li, S.J. 2009. Spatial variability of soil organic carbon in a watershed on the loess plateau. Pedosphere 2009, 19, 486–495. [Google Scholar] [CrossRef]
- Camacho, R.; Salazar, S.; González, L.; Pacheco, H.; Suárez, C. Caracterización geomorfológica de las dunas longitudinales del Istmo de Médanos, estado Falcón, Venezuela. Investig. Geográf. 2011, 76, 7–19. [Google Scholar] [CrossRef]
- Panico, S.C.; Ceccherini, M.T.; Memoli, V.; Maisto, G.; Pietramellara, G.; Barile, R.; De Marco, A. Effects of different vegetation types on burnt soil properties and microbial communities. Int. J. Wildland Fire. 2020, 29, 628–636. [Google Scholar] [CrossRef]
- Ibarra-Castillo, D.; Ruiz-Corral, J.A.; González-Eguiarte, D.R.; Flores-Garnica, J.G.; Díaz-Padilla, G. Distribución espacial del ph de los suelos agrícolas de Zapopan, Jalisco, México. Agric. Téc. Méx. 2009, 35, 267–276. [Google Scholar]
- Wilson, M. Weathering of the primary rock-forming minerals: Processes, products and rates. Clay Minerals 2004, 39, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Churchman, G.J.; Lowe, D.J. Alteration, formation and occurrence of minerals in soils. In Handbook of Soli Science, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; Volume 1: Properties and processes; CRC Press (Taylor & Francis): Boca Raton, FL, USA, 2012; pp. 20.1–20.72. [Google Scholar]
- Sidari, M.; Ronzello, G.; Vecchio, G.; Muscolo, A. Influence of slope aspects on soil chemical and biochemical properties in a Pinus laricio forest ecosystem of Aspromonte (Southern Italy). Eur. J. Soil Biol. 2008, 44, 364–372. [Google Scholar] [CrossRef]
- Sreenivas, K.; Dadhwal, K.V.; Kumar, S.; Harsha, G.S.; Mitran, T.; Sujatha, G.; Janaki, G.R.S.; Fyzee, M.A.; Ravisankar, T. Digital mapping of soil organic and inorganic carbon status in India. Geoderma 2016, 269, 160–173. [Google Scholar] [CrossRef]
- Henríquez, C.; Méndez, J.C.; Masís, R. Interpolación de variables de fertilidad de suelo mediante el análisis kriging y su validación. Agron. Costarric. 2013, 37, 71–82. [Google Scholar] [CrossRef]
- Villatoro, M.; Henríquez, C.; Sancho, F. Comparación de los interpoladores IDW y Kriging en la variación espacial de pH, Ca, CICE y P del suelo. Agron. Costarric. 2008, 32, 95–105. [Google Scholar]
- Yamamoto, J.K.; Landim, P.M.B. Geoestatística: Conceitos e Aplicações; Oficina de Textos: São Paulo, Brazil, 2013; 215p. [Google Scholar]
- Pingguo, Y.; Byrne, J.M.; Yang, M. Spatial variability of soil magnetic susceptibility, organic carbon and total nitrogen from farmland in northern China. Catena 2016, 145, 92–98. [Google Scholar] [CrossRef]
- Zang, H.; Wang, J.; Kuzyakova, Y. N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl. Soil Ecol. 2016, 108, 47–53. [Google Scholar] [CrossRef]
- Soto-Bravo, F.; González-Lutz, M.I. Análisis de métodos estadísticos para evaluar el desempeño de modelos de simulación en cultivos hortícolas. Agron. Mesoam. 2018, 30, 517–534. [Google Scholar] [CrossRef]

| Var | N † | Σ | ± σ | Min. | Median | Max. | Asy | Kur | |
|---|---|---|---|---|---|---|---|---|---|
| pH | 101 | 5.34 | 0.75 | 4.59–6.09 | 4.00 | 5.24 | 7.15 | 0.56 | −0.51 |
| OM | 101 | 2.16 | 0.81 | 1.35–2.97 | 0.14 | 2.22 | 3.93 | −0.31 | 0.14 |
| CLA | 101 | 66.67 | 10.37 | 56.30–77.04 | 40.00 | 69.50 | 82.00 | −0.55 | −0.61 |
| SILT | 101 | 16.40 | 5.12 | 11.28–21.52 | 7.00 | 16.00 | 32.00 | 0.35 | −0.16 |
| SAN | 101 | 16.93 | 8.02 | 8.91–24.95 | 6.00 | 13.50 | 38.00 | 1.05 | 0.21 |
| NDVI | 101 | 0.22 | 0.082 | 0.14–0.30 | 0.04 | 0.22 | 0.68 | 2.04 | 11.07 |
| SAVI | 101 | 0.16 | 0.056 | 0.10–0.21 | 0.03 | 0.16 | 0.45 | 1.61 | 8.29 |
| SP | 101 | 11.66 | 10.17 | 1.49–21.83 | 0.17 | 5.55 | 80.59 | 2.51 | 6.16 |
| TEM | 101 | 19.50 | 0.78 | 18.74–20.28 | 14.90 | 19.70 | 20.30 | −3.76 | 18.69 |
| PP | 101 | 839.40 | 33.32 | 806.08–872.72 | 742.00 | 830.00 | 919.00 | 0.38 | −1.01 |
| DAT | 101 | 3.55 | 1.92 | 1.63–5.19 | 0.01 | 3.75 | 5.00 | −1.16 | −0.30 |
| HUM | 101 | 254.44 | 55.71 | 198.73–310.15 | 150.00 | 250.00 | 350.00 | −0.20 | −0.71 |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 | PC11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eigen value | 2.377 | 2.057 | 1.369 | 1.264 | 1.094 | 0.901 | 0.720 | 0.672 | 0.496 | 0.047 | 0.000 |
| Proportion | 0.216 | 0.187 | 0.124 | 0.115 | 0.099 | 0.082 | 0.065 | 0.061 | 0.045 | 0.004 | 0.000 |
| Accumulated | 0.216 | 0.403 | 0.528 | 0.643 | 0.742 | 0.824 | 0.889 | 0.951 | 0.996 | 1.000 | 1.000 |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| OM | 0.184 | 0.164 | 0.053 | 0.171 | 0.718 |
| CLA | −0.624 | −0.111 | −0.094 | −0.061 | 0.024 |
| SILT | 0.395 | −0.129 | 0.247 | 0.183 | 0.112 |
| SAN | 0.555 | −0.061 | −0.037 | −0.038 | −0.102 |
| NDVI | −0.071 | −0.643 | −0.209 | 0.167 | 0.075 |
| SAVI | −0.091 | −0.656 | −0.163 | 0.105 | 0.113 |
| SP | 0.085 | 0.019 | 0.201 | 0.403 | −0.589 |
| TEM | 0.168 | 0.178 | −0.589 | −0.171 | 0.107 |
| PP | −0.249 | 0.009 | 0.455 | 0.300 | 0.234 |
| DAT | 0.028 | −0.197 | 0.188 | −0.656 | −0.109 |
| HUM | −0.029 | 0.158 | −0.479 | 0.425 | −0.132 |
| N | UTM | † pHp | pHm | RMSE | MEB | |
|---|---|---|---|---|---|---|
| X | Y | |||||
| 1 | 664,920 | 2,271,661 | 5.1 | 5.0 | 0.01 | 0.10 |
| 2 | 676,133 | 2,266,878 | 4.8 | 4.9 | 0.01 | −0.10 |
| 3 | 650,569 | 2,263,984 | 5.8 | 5.6 | 0.04 | 0.20 |
| 4 | 673,606 | 2,256,021 | 5.4 | 5.3 | 0.01 | 0.10 |
| 5 | 663,437 | 2,265,538 | 5.2 | 5.1 | 0.01 | 0.10 |
| 6 | 658,798 | 2,264,621 | 5.2 | 5.3 | 0.01 | −0.10 |
| 7 | 672,989 | 2,271,684 | 4.1 | 4.0 | 0.01 | 0.10 |
| 8 | 662,276 | 2,262,246 | 5.2 | 5.2 | 0.00 | 0.00 |
| 9 | 661,783 | 2,275,120 | 5.1 | 5.4 | 0.09 | −0.30 |
| 10 | 668,505 | 2,273,387 | 5.0 | 5.0 | 0.00 | 0.00 |
| 11 | 672,275 | 2,271,658 | 5.1 | 5.2 | 0.01 | −0.10 |
| 12 | 651,688 | 2,270,674 | 6.1 | 6.2 | 0.01 | −0.10 |
| 13 | 668,691 | 2,254,534 | 5.3 | 5.2 | 0.01 | 0.10 |
| 14 | 649,245 | 2,266,025 | 5.8 | 5.8 | 0.00 | 0.00 |
| 15 | 668,604 | 2,260,668 | 5.3 | 5.2 | 0.01 | 0.10 |
| 16 | 649,088 | 2,264,952 | 5.9 | 5.8 | 0.01 | 0.10 |
| 17 | 656,660 | 2,263,242 | 5.5 | 5.4 | 0.01 | 0.10 |
| 18 | 650,581 | 2,269,980 | 5.7 | 5.7 | 0.00 | 0.00 |
| 19 | 674,992 | 2,264,123 | 4.8 | 4.8 | 0.00 | 0.00 |
| 20 | 676,930 | 2,265,449 | 4.9 | 4.4 | 0.01 | 0.10 |
| 0.158 | 0.020 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yescas-Coronado, P.; Segura-Castruita, M.Á.; Chávez-Rodríguez, A.M.; Gómez-Leyva, J.F.; Martínez-Sifuentes, A.R.; Amador-Camacho, O.; González-Medina, R. Covariables of Soil-Forming Factors and Their Influence on pH Distribution and Spatial Variability. Agriculture 2022, 12, 2132. https://doi.org/10.3390/agriculture12122132
Yescas-Coronado P, Segura-Castruita MÁ, Chávez-Rodríguez AM, Gómez-Leyva JF, Martínez-Sifuentes AR, Amador-Camacho O, González-Medina R. Covariables of Soil-Forming Factors and Their Influence on pH Distribution and Spatial Variability. Agriculture. 2022; 12(12):2132. https://doi.org/10.3390/agriculture12122132
Chicago/Turabian StyleYescas-Coronado, Pedro, Miguel Ángel Segura-Castruita, Arturo Moisés Chávez-Rodríguez, Juan Florencio Gómez-Leyva, Aldo Rafael Martínez-Sifuentes, Osvaldo Amador-Camacho, and Raúl González-Medina. 2022. "Covariables of Soil-Forming Factors and Their Influence on pH Distribution and Spatial Variability" Agriculture 12, no. 12: 2132. https://doi.org/10.3390/agriculture12122132
APA StyleYescas-Coronado, P., Segura-Castruita, M. Á., Chávez-Rodríguez, A. M., Gómez-Leyva, J. F., Martínez-Sifuentes, A. R., Amador-Camacho, O., & González-Medina, R. (2022). Covariables of Soil-Forming Factors and Their Influence on pH Distribution and Spatial Variability. Agriculture, 12(12), 2132. https://doi.org/10.3390/agriculture12122132






