Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe
Abstract
1. Introduction
- -
- Assess the evolution of healthcare systems in CEE, i.e., examine the development of healthcare systems in CEE over the past five years, with a particular focus on patient access to OMPs;
- -
- Identify barriers and enablers, i.e., analyze both shared and country-specific challenges affecting OMP access, identifying key obstacles as well as factors that facilitate improvements;
- -
- Develop actionable recommendations, i.e., provide evidence-based strategies to optimize OMP availability across the region, addressing systemic inefficiencies and proposing targeted interventions.
2. Materials and Methods
2.1. Phase 1: Literature Review (September–October 2023)
2.2. Phase 2: Multi-Round Stakeholder Interviews (October–December 2023)
2.3. Phase 3: Advisory Board of HTA Experts (7 December 2023)
2.4. Phase 4: Scoping Review (November 2023–February 2024)
2.5. Phase 5: Data Integration and Analysis (March–October 2024)
2.6. Phase 6: Advisory Board’s Final Consensus on Barriers, Enablers, and Key Recommendations (October–December 2024)
3. Results
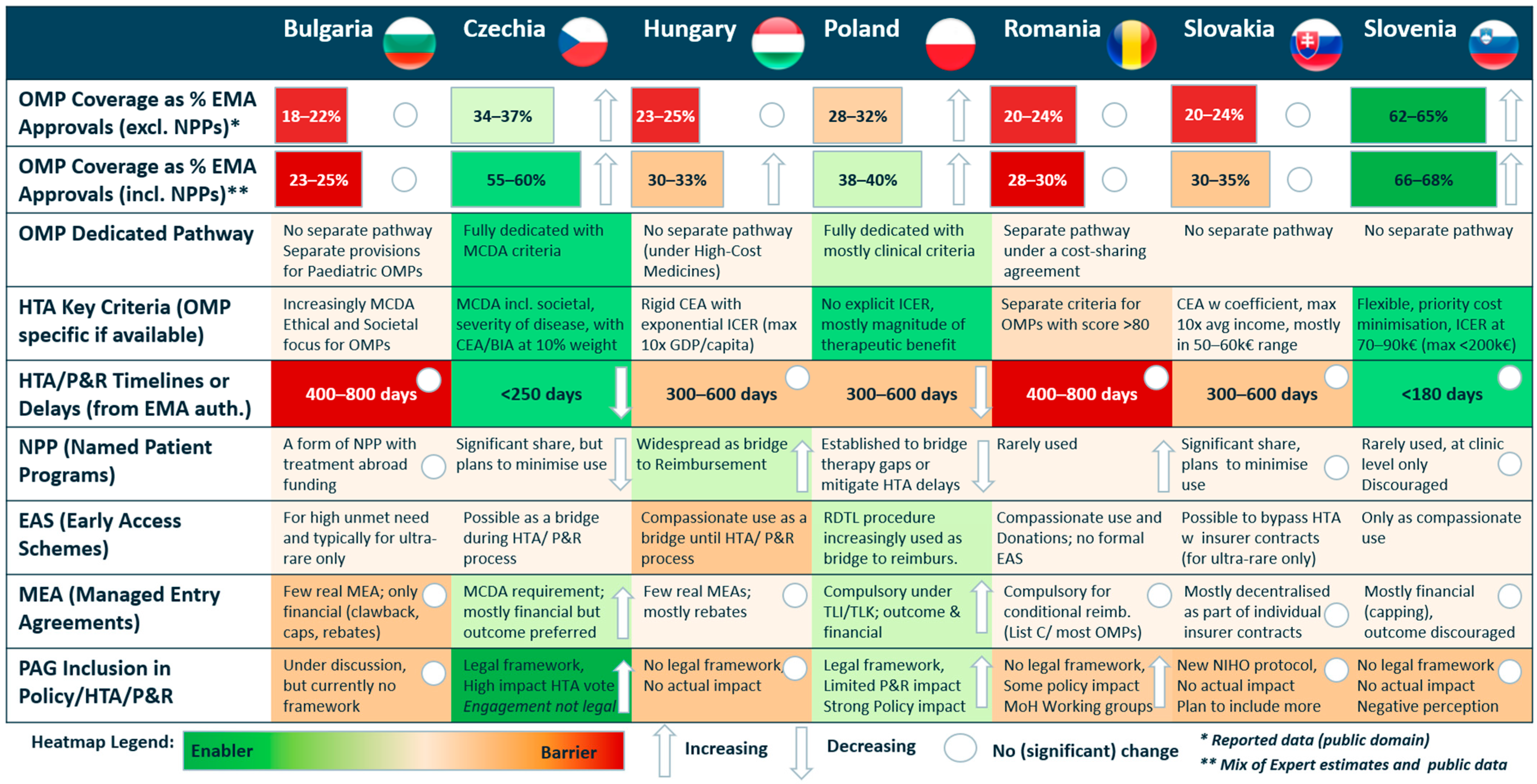
3.1. Overview of Patient Access to OMPs Across CEE
3.2. Advisory Board’s Consensus on Barriers, Enablers, and Key Recommendations for Facilitating Patient Access to OMPs Across CEE
4. Discussion
4.1. Healthcare System Evolution
4.2. Strengthening HTA Frameworks
4.3. Patient-Centric Policies
4.4. Sustainable Financing Mechanisms
4.5. Study Limitations
4.6. Enhancing Regional Collaboration
4.7. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEE | Central and Eastern Europe |
| HTA | health technology assessment |
| ICER | incremental cost-effectiveness ratio |
| MCDA | multi-criteria decision analysis |
| MEA | managed entry agreement |
| OMP | orphan medicinal product |
| PAG | patient advocacy group |
| PRO | patient-reported outcomes |
| R&D | research and development |
Appendix A. Case Studies from Czechia and Poland Illustrating the Implementation of OMP-Specific Pathways
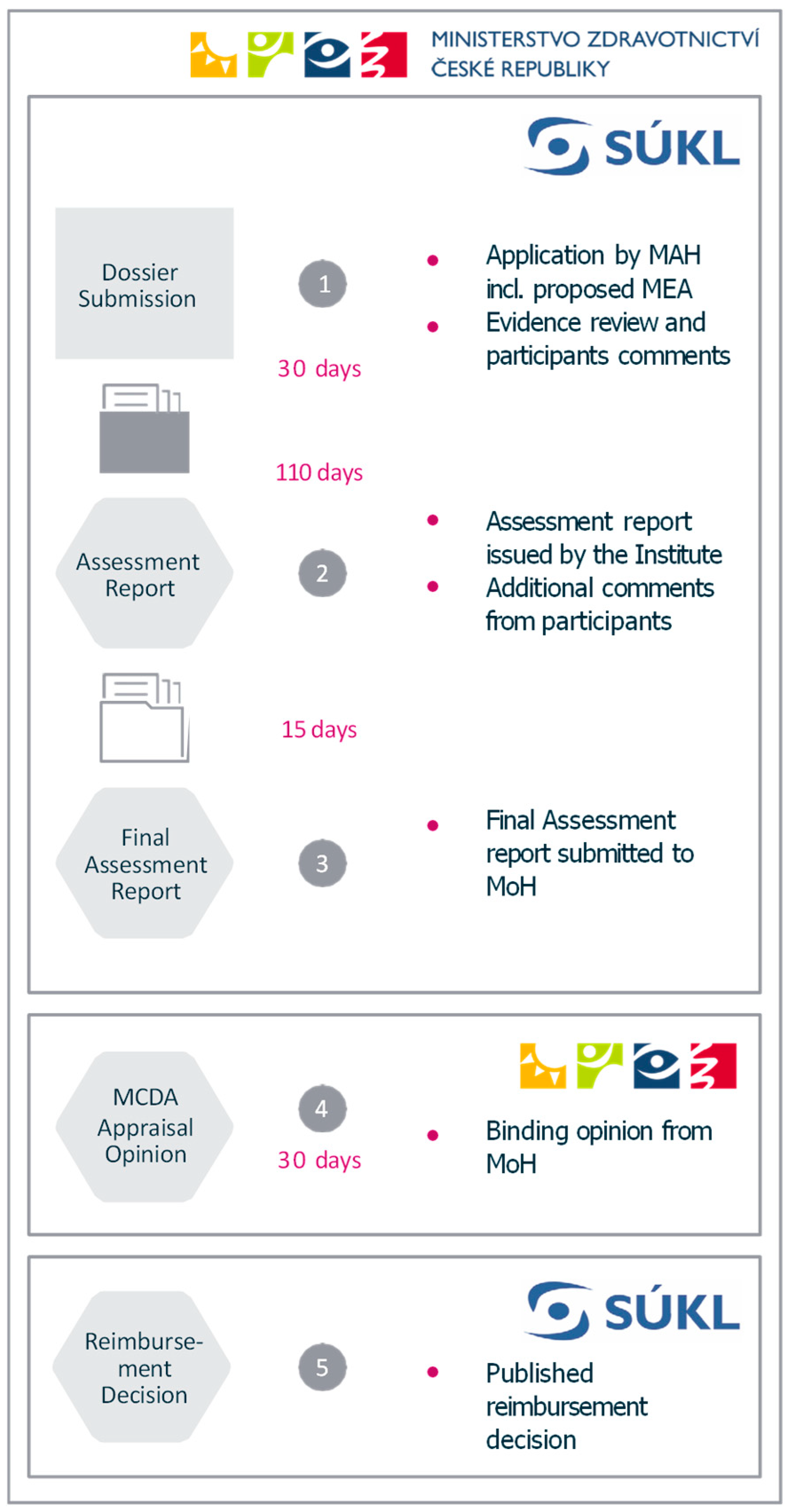
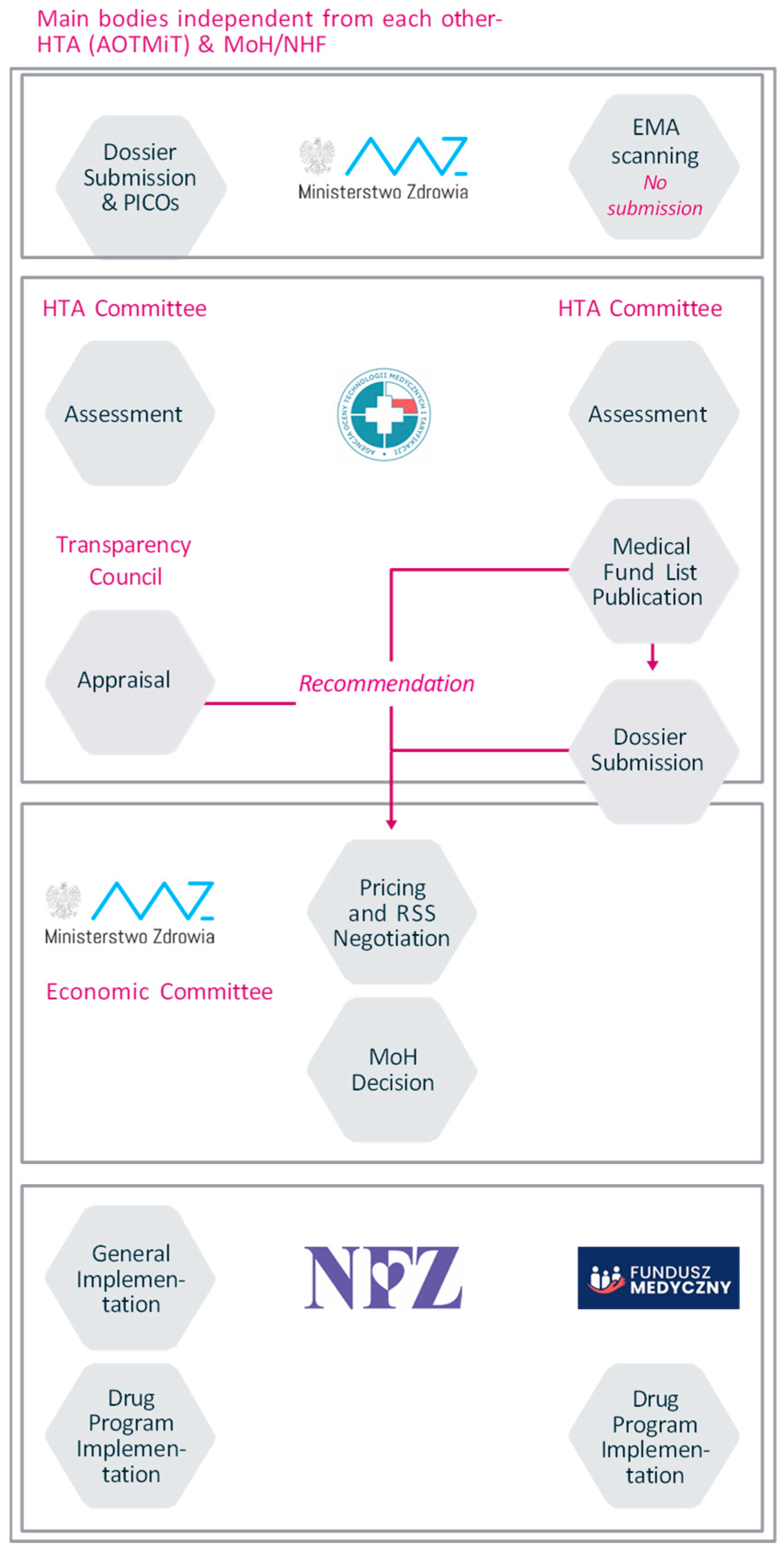
Appendix B. Case Study from Czechia Focusing on Patient Inclusion in Decision-Making Processes
References
- Klimova, B.; Storek, M.; Valis, M.; Kuca, K. Global View on Rare Diseases: A Mini Review. Curr. Med. Chem. 2017, 24, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Delaye, J.; Cacciatore, P.; Kole, A. Valuing the ‘Burden’ and Impact of Rare Diseases: A Scoping Review. Front. Pharmacol. 2022, 13, 914338. [Google Scholar] [CrossRef]
- Adachi, T.; El-Hattab, A.W.; Jain, R.; Crespo, K.A.N.; Lazo, C.I.Q.; Scarpa, M.; Summar, M.; Wattanasirichaigoon, D. Enhancing Equitable Access to Rare Disease Diagnosis and Treatment around the World: A Review of Evidence, Policies, and Challenges. Int. J. Environ. Res. Public Health 2023, 20, 4732. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S. Payer Policies to Support Innovation and Access to Medicines in the WHO European Region. Oslo Medicines Initiative Technical Report. World Health Organization Regional Office for Europe. 2022. Available online: https://iris.who.int/bitstream/handle/10665/361753/9789289058247-eng.pdf?sequence=1 (accessed on 21 January 2025).
- Korchagina, D.; Jaroslawski, S.; Jadot, G.; Toumi, M. Orphan Drugs in Oncology. In Regulatory and Economic Aspects in Oncology; Recent Results in Cancer Research; Walter, E., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 213, pp. 109–142. [Google Scholar] [CrossRef]
- Szegedi, M.; Zelei, T.; Arickx, F.; Bucsics, A.; Cohn-Zanchetta, E.; Fürst, J.; Kamusheva, M.; Kawalec, P.; Petrova, G.; Slaby, J.; et al. The European challenges of funding orphan medicinal products. Orphanet J. Rare Dis. 2018, 13, 184. [Google Scholar] [CrossRef]
- Mazzucato, M.; Minichiello, C.; Vianello, A.; Pozza, L.V.D.; Toto, E.; Facchin, P. Real-world use of orphan medicinal products (OMPs) in rare disease (RD) patients: A population-based registry study. Front. Pharmacol. 2022, 13, 940010. [Google Scholar] [CrossRef]
- Adkins, E.; Nicholson, L.; Floyd, D.; Ratcliffe, M.; Chevrou-Severac, H. Oncology drugs for orphan indications: How are HTA processes evolving for this specific drug category? ClinicoEcon. Outcomes Res. 2017, 9, 327–342. [Google Scholar] [CrossRef]
- Hren, R. Impact of the Pharma Economic Act on Diffusion of Innovation and Reduction of Costs in the Hungarian Prescription Drug Market (2007–2010). Value Health Reg. Issues 2013, 2, 290–299. [Google Scholar] [CrossRef]
- Tambor, M.; Klich, J.; Domagała, A. Financing Healthcare in Central and Eastern European Countries: How Far Are We from Universal Health Coverage? Int. J. Environ. Res. Public Health 2021, 18, 1382. [Google Scholar] [CrossRef]
- Lipska, I.; Di Bidino, R.; Niewada, M.; Nemeth, B.; Bochenek, T.; Kukla, M.; Więckowska, B.; Sobczak, A.; Iłowiecka, K.; Zemplenyi, A.; et al. Overcoming Barriers in Hospital-Based Health Technology Assessment (HB-HTA): International Expert Panel Consensus. Healthcare 2024, 12, 889. [Google Scholar] [CrossRef]
- Csanádi, M.; Kaló, Z.; Molken, M.R.-V.; Looman, W.; Huic, M.; Ercevic, D.; Atanasijevic, D.; Lorenzovici, L.; Petryszyn, P.; Pogány, G.; et al. Prioritization of implementation barriers related to integrated care models in Central and Eastern European countries. Health Policy 2022, 126, 1173–1179. [Google Scholar] [CrossRef]
- Godman, B.; Novakovic, T.; Tesic, D.; Oortwijn, W.; Martin, A.P.; Parker, M.; Haycox, A. Addressing challenges for sustainable healthcare in Central and Eastern Europe. Expert. Rev. Pharmacoecon. Outcomes Res. 2016, 16, 685–687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eurostat. Healthcare Expenditure Statistics by Function, Provider and Financing Scheme. November 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_expenditure_statistics_by_function,_provider_and_financing_scheme (accessed on 21 January 2025).
- Latos-Bieleńska, A. Plan dla chorób rzadkich (Rare Diseases Plan). November 2024. Available online: https://chorobyrzadkie.gov.pl/pl/choroby-rzadkie/plan-dla-chorob-rzadkich (accessed on 21 January 2025). (In Polish)
- Czech, M.; Baran-Kooiker, A.; Atikeler, K.; Demirtshyan, M.; Gaitova, K.; Holownia-Voloskova, M.; Turcu-Stiolica, A.; Kooiker, C.; Piniazhko, O.; Konstandyan, N.; et al. A Review of Rare Disease Policies and Orphan Drug Reimbursement Systems in 12 Eurasian Countries. Front. Public Health 2020, 7, 416. [Google Scholar] [CrossRef] [PubMed]
- Zelei, T.; Molnár, M.J.; Szegedi, M.; Kaló, Z. Systematic review on the evaluation criteria of orphan medicines in Central and Eastern European countries. Orphanet J. Rare Dis. 2016, 11, 72. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Toleman, M.; Rowling, S.; Frederiks, A.; Andersen, N. Social Science Research: Principles, Methods and Practices; University of Southern Queensland: Darling Heights, Australia, 2019. [Google Scholar] [CrossRef]
- Hussein, A. The use of Triangulation in Social Sciences Research: Can qualitative and quantitative methods be combined? J. Comp. Soc. Work. 2009, 4, 106–117. [Google Scholar] [CrossRef]
- GAP. The GAP (GEARING UP ACCESS PROPOSAL FOR V4) Tool. Available online: https://gapv4.eu/gapv4/ (accessed on 21 January 2025).
- Newton, M.; Stoddart, K.; Travaglio, M.; Troein, P. EFPIA Patients W.A.I.T. Indicator 2023 Survey. June 2024. Available online: https://rtl.ee/wp-content/uploads/2024/06/EFPIA-2023-Patient-W.A.I.T.-Indicator-Final12.06.2024.pdf (accessed on 21 January 2025).
- Medicinal Products for Rare Diseases in Europe. Orphanet Report Series, Orphan Drugs Collection. April 2024. Available online: https://www.orpha.net/pdfs/orphacom/cahiers/docs/GB/Medicinal_products_for_rare_diseases_in_Europe.pdf (accessed on 21 January 2025).
- Rais, C.; Kaló, Z.; Csanádi, M.; Negulescu, V. Current and future perspectives for the implementation of health technology assessment in Romania. Health Policy Technol. 2020, 9, 45–52. [Google Scholar] [CrossRef]
- Ivanova, H.; Macaulay, R. PMU74 Health Technology Assassment in Bulgaria—3 Year Report Card. Value Health 2019, 22, S262. [Google Scholar] [CrossRef]
- Decker, B.; Mlcoch, T.; Pustovalova, A.; Dolezal, T. Novel approach to decision making for orphan drugs. Int. J. Technol. Assess. Health Care 2023, 39, e10. [Google Scholar] [CrossRef]
- HAS Press Department. Early Access Authorisation: A Positive Initial Report and Refined Assessment Methods. 20 May 2022. Available online: https://www.has-sante.fr/jcms/p_3340090/en/early-access-authorisation-a-positive-initial-report-and- (accessed on 21 January 2025).
- Ádám, I.; Callenbach, M.; Németh, B.; Vreman, R.A.; Tollin, C.; Pontén, J.; Dawoud, D.; Elvidge, J.; Crabb, N.; Doorn-Khosrovani, S.B.v.W.v.; et al. Outcome-based reimbursement in Central-Eastern Europe and Middle-East. Front. Med. 2022, 9, 940886. [Google Scholar] [CrossRef]
- Dimitrova, M.; Jakab, I.; Mitkova, Z.; Kamusheva, M.; Tachkov, K.; Nemeth, B.; Zemplenyi, A.; Dawoud, D.; Delnoij, D.M.J.; Houýez, F.; et al. Potential Barriers of Patient Involvement in Health Technology Assessment in Central and Eastern European Countries. Front. Public Health 2022, 10, 922708. [Google Scholar] [CrossRef]
- Jakab, I.; Dimitrova, M.; Houÿez, F.; Bereczky, T.; Fövényes, M.; Maravic, Z.; Belina, I.; Andriciuc, C.; Tóth, K.; Piniazhko, O.; et al. Recommendations for patient involvement in health technology assessment in Central and Eastern European countries. Front. Public Health 2023, 11, 1176200. [Google Scholar] [CrossRef]
- Mela, A.; Lis, D.; Rdzanek, E.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Drop, B.; Blicharski, T.; Niewada, M. AOTMiT reimbursement recommendations compared to other HTA agencies. Eur. J. Health Econ. 2024, 25, 1291–1310. [Google Scholar] [CrossRef] [PubMed]
- Fontrier, A.-M.; Kamphuis, B.; Kanavos, P. How can health technology assessment be improved to optimise access to medicines? Results from a Delphi study in Europe: Better access to medicines through HTA. Eur. J. Health Econ. 2024, 25, 935–950. [Google Scholar] [CrossRef]
- Pitter, J.G.; Nagy, L.; Nagy, B.; Hren, R. Development Perspectives for Curative Technologies in Primary Demyelinating Disorders of the Central Nervous System with Neuromyelitis Optica Spectrum Disorder (NMOSD) and Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD) at the Forefront. J. Pers. Med. 2024, 14, 599. [Google Scholar] [CrossRef]
- Andreu, P.; Karam, J.; Child, C.; Chiesi, G.; Cioffi, G. Rare Disease Burden of Care and the Economic Impact on Citizens in Germany, France and Italy. 2024. Available online: https://chiesirarediseases.com/assets/pdf/rare-disease-burden-of-care-and-the-economic-impact-on-citizens.pdf (accessed on 21 January 2025).

| Country | Round 1: Policymakers and HTA Professionals | Round 2: Clinicians, Payers, Health Economists, and Industry Representatives | Round 3: Patient Advocacy Groups and Academic Researchers |
|---|---|---|---|
| DineBulgaria | Former Deputy Health Minister | Head of local ISPOR chapter, member of industry association ARPharM | Former Minister of Health and current patient ombudsman |
| Czechia | Current Deputy Health Minister | Senior employee of public insurance company VZP, head of industry association AIFP | Head of patient council (MoH advisory body) |
| Hungary | Former deputy head of OGYEI (HTA body) | Senior health economics expert, Syreon Institute | Managing partner of local HTA consulting company, former senior advisor to rare diseases patient organization |
| Poland | Former Deputy Health Minister | CEO of local HTA consulting company, head of local ISPOR chapter | Head of “umbrella” rare diseases patient organization, national consultant in neurology/lead for Polish rare diseases plan |
| Romania | Former head of National Agency for Medicines and Medical Devices (HTA body) | Former head of ARPIM industry association | Board member of EURORDIS and Head of “umbrella” rare diseases patient organization |
| Slovakia | Former deputy head of NIH (HTA body) | Head of local ISPOR chapter | Scientific advisor to rare diseases patient organization |
| Slovenia | Former senior expert from JAZMP (pricing body) | Senior health economics expert, Syreon Institute | Head of “umbrella” rare diseases patient organization |
| Dimension | |
|---|---|
| 1 | Dedicated OMP HTA pathways: The existence and implementation of pathways tailored to OMPs. |
| 2 | Tailoring of HTA criteria to OMPs: Adaptations in assessment frameworks specific to the unique challenges of OMPs. |
| 3 | Use of Named Patient Programs (NPPs): Interim mechanisms for providing access during HTA evaluations. |
| 4 | Use of Early Access Schemes (EASs): Systems enabling OMP availability prior to formal reimbursement decisions. |
| 5 | Managed entry agreements (MEAs): Financial and outcome-based models designed to mitigate high-cost barriers. |
| 6 | Patient inclusion in HTA and reimbursement processes: The integration of patient perspectives in decision-making frameworks. |
| Barrier | |
| 1 | Low awareness: Low awareness of rare diseases and OMPs’ specificity, in terms of disease burden, unmet needs, and net costs, leading to limited societal solidarity and low priority in drug policy and financing. |
| 2 | Inadequate HTA: Methodologies are unadjusted to the unique nature of OMPs, requiring unattainable or partially unattainable evidence, often unable to appropriately assess the societal perspective, and use overly restrictive decision criteria, such as ICER thresholds. |
| 3 | Insufficient and inefficient financing models: Models are not delineated for OMPs and are often grouped with funds for other technologies and services, increasing competition for resources. |
| 4 | Sub-optimal use of novel access schemes: Schemes such as outcome-based MEAs or systematic Early Access Schemes (EASs) for potential breakthrough innovations are under-utilized, instead applying ‘cost-myopic’ financial and cost-containment measures. |
| 5 | Fragmented patient engagement: There is infrequent or unsystematized patient participation in HTA and reimbursement processes, with often inadmissible patient data inputs. |
| Enabler | |
| 1 | Rare disease policy frameworks: Setting out comprehensive and long-term guidance for patients, clinicians, payers, and policymakers, assigning clear priorities, proposing systemic solutions to existing barriers, and establishing multi-stakeholder collaboration platforms (plans for rare diseases and rare disease programs). |
| 2 | OMP-specific HTA: Using broad societal perspective, beyond cost effectiveness and budget impact, to appraise the true value of OMPs, either with MCDA or a MCDA “decision tree” process. |
| 3 | Patient empowerment and education: Developing legal frameworks, practical solutions, and a stepwise approach towards meaningful engagement and inclusion of patients and patient organizations in key HTA and reimbursement processes. |
| Key Recommendation | |
| 1 | Raising awareness: High urgency to raise awareness of the actual burden of rare diseases and to systematize possible patient access solutions based on the existing and novel best practices from CEE and broader EU. |
| 2 | Integrating rare disease care: Mapping and optimizing patient pathways by involving all medical specialists to improve care, diagnostic accuracy, treatment effectiveness, and overall patient support. |
| 3 | Regional collaborations: Exchange best practices, co-create solutions, create data networks and regional Centers of Excellence (CoEs), and implement policy frameworks for joint HTA and novel payment mechanisms. |
| 4 | Including and amplifying voice of patients: Intensify efforts to map, educate and empower PAGs to be optimally engaged in HTA and decision-making, utilizing the value of patient data (PRO, preferences, and patient experience). |
| 5 | Adopting best practices: Identify and map best practices, primarily from across the CEE and from wider Europe, to inform policy changes, financing models, and other immediately transferrable solutions (see key recommendation 3 above). |
| 6 | Promoting health equity: Through a series of papers/publications showcasing equity as an integral part of value-based healthcare and future-proofing CEE healthcare systems. |
| 7 | Incentivizing OMP R&D: Both at the EU level and specifically at the CEE level, by participating actively in the implementation of the EU Pharmaceutical Package, by addressing local barriers and by continuously creating local/regional initiatives. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluszczynski, T.; Nemeth, B.; Władysiuk, M.; Czech, M.; Kamusheva, M.; Fotin, N.; Rose, S.; Doležal, T.; Hren, R. Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe. J. Mark. Access Health Policy 2025, 13, 24. https://doi.org/10.3390/jmahp13020024
Kluszczynski T, Nemeth B, Władysiuk M, Czech M, Kamusheva M, Fotin N, Rose S, Doležal T, Hren R. Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe. Journal of Market Access & Health Policy. 2025; 13(2):24. https://doi.org/10.3390/jmahp13020024
Chicago/Turabian StyleKluszczynski, Tomasz, Bertalan Nemeth, Magdalena Władysiuk, Marcin Czech, Maria Kamusheva, Nicolae Fotin, Sandra Rose, Tomáš Doležal, and Rok Hren. 2025. "Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe" Journal of Market Access & Health Policy 13, no. 2: 24. https://doi.org/10.3390/jmahp13020024
APA StyleKluszczynski, T., Nemeth, B., Władysiuk, M., Czech, M., Kamusheva, M., Fotin, N., Rose, S., Doležal, T., & Hren, R. (2025). Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe. Journal of Market Access & Health Policy, 13(2), 24. https://doi.org/10.3390/jmahp13020024






