A Causal Inference Approach to Mediation Analysis in Vitreomacular Traction: How Much Does Traction Resolution Mediate Functional Outcomes?
Abstract
1. Introduction
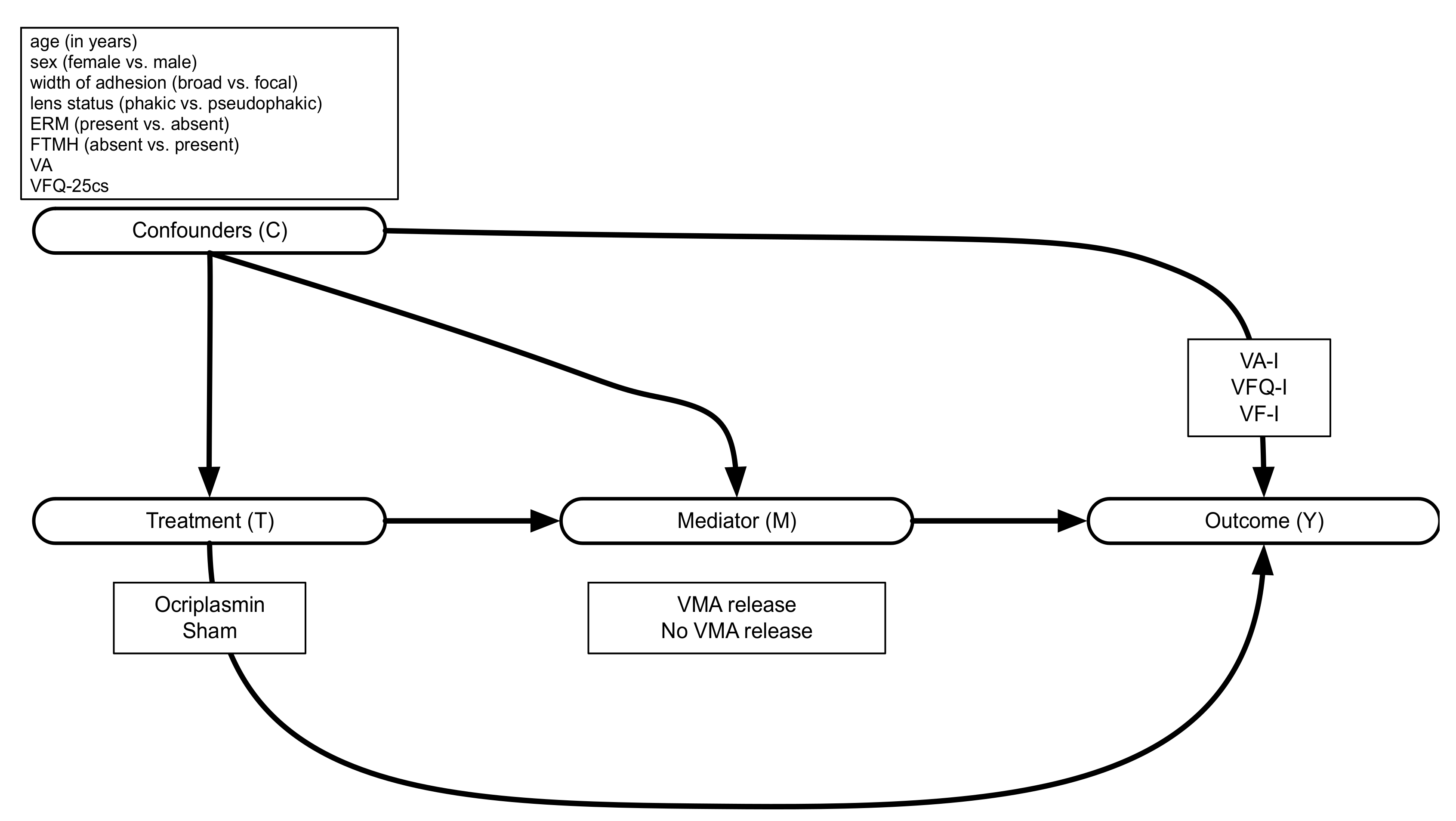
2. Materials and Methods
2.1. OASIS Variables for Mediation Analysis
2.2. Statistical Methods
2.2.1. Defining Mediated and Direct Effects in Mediation Analysis
2.2.2. Estimating the Mediated and Direct Effects
3. Results
3.1. Mediator and Outcome Models
3.2. Causal Mediation Effects
- Total population analysis
- 2.
- Subgroup analyses
- 3.
- Additional analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lescrauwaet, B.; Blot, K.; Jackson, T.L. Patient-reported outcomes of ocriplasmin for the treatment of vitreomacular traction: A systematic review and synthesis of the literature. Patient Relat. Outcome Meas. 2019, 10, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef]
- Jackson, T.L.; Donachie, P.H.J.; Johnston, R.L. Vitreomacular Traction Study Group Electronic Medical Record Database Study of Vitrectomy and Observation for Vitreomacular Traction. Retina 2016, 36, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. JETREA European Public Assessment Report 2013. Available online: https://www.ema.europa.eu/en/documents/assessment-report/jetrea-epar-public-assessment-report_en.pdf (accessed on 15 February 2024).
- Wickström, K.; Moseley, J. Biomarkers and Surrogate Endpoints in Drug Development: A European Regulatory View. Invest. Ophthalmol. Vis. Sci. 2017, 58, BIO27–BIO33. [Google Scholar] [CrossRef]
- Ciani, O.; Grigore, B.; Blommestein, H.; de Groot, S.; Möllenkamp, M.; Rabbe, S.; Daubner-Bendes, R.; Taylor, R.S. Validity of Surrogate Endpoints and Their Impact on Coverage Recommendations: A Retrospective Analysis across International Health Technology Assessment Agencies. Med. Decis. Mak. 2021, 41, 439–452. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Drug Approval Package_Jetrea (Ocriplasmin) BLA 125422. October 2012; pp. 1–2. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/125422_jetrea_toc.cfm (accessed on 15 February 2024).
- Stalmans, P.; Benz, M.S.; Gandorfer, A.; Kampik, A.; Girach, A.; Pakola, S.; Haller, J.A.; MIVI-TRUST Study Group. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N. Engl. J. Med. 2012, 367, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Haller, J.A.; Kaiser, P.K. Improvement in Patient-Reported Visual Function After Ocriplasmin for Vitreomacular Adhesion: Results of the Microplasmin for Intravitreous Injection-Traction Release Without Surgical Treatment (MIVI-TRUST) Trials. JAMA Ophthalmol. 2015, 133, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Gandorfer, A.; Benz, M.S.; Haller, J.A.; Stalmans, P.; Pakola, S.J.; Girach, A.; Kampik, A.; Toth, C.A.; Jaffe, G.J.; MIVI-TRUST Study Group. Association between anatomical resolution and functional outcomes in the mivi-trust studies using ocriplasmin to treat symptomatic vitreomacular adhesion/vitreomacular traction, including when associated with macular hole. Retina 2015, 35, 1151–1157. [Google Scholar] [CrossRef]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar] [CrossRef]
- Imai, K.; Tingley, D.; Yamamoto, T. Experimental Designs for Identifying Causal Mechanisms. J. R. Stat. Soc. Ser. Stat. Soc. 2013, 176, 5–51. [Google Scholar] [CrossRef]
- Keele, L.; Tingley, D.; Yamamoto, T. Identifying Mechanisms behind Policy Interventions via Causal mediation Analysis. J. Policy Anal. Manag. 2015, 34, 937–963. [Google Scholar] [CrossRef]
- Imai, K.; Keele, L.; Tingley, D.; Yamamoto, T. Unpacking the Black Box of Causality: Learning about Causal Mechanisms from Experimental and Observational Studies. Am. Polit. Sci. Rev. 2011, 105, 765–789. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Keele, L.; Yamamoto, T. Identification, Inference and Sensitivity Analysis for Causal Mediation Effects. Stat. Sci. 2010, 25, 51–71. [Google Scholar] [CrossRef]
- Dugel, P.U.; Tolentino, M.; Feiner, L.; Kozma, P.; Leroy, A. Results of the 2-Year Ocriplasmin for Treatment for Symptomatic Vitreomacular Adhesion Including Macular Hole (OASIS) Randomized Trial. Ophthalmology 2016, 123, 2232–2247. [Google Scholar] [CrossRef]
- Jackson, T.L.; Verstraeten, T.; Duchateau, L.; Lescrauwaet, B. Visual function response to ocriplasmin for the treatment of vitreomacular traction and macular hole. Acta Ophthalmol. 2017, 95, e740–e745. [Google Scholar] [CrossRef]
- Lescrauwaet, B.; Duchateau, L.; Verstraeten, T.; Jackson, T.L. Visual Function Response to Ocriplasmin for the Treatment of Vitreomacular Traction and Macular Hole: The OASIS Study. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5842–5848. [Google Scholar] [CrossRef]
- Lee, H.; Cashin, A.G.; Lamb, S.E.; Hopewell, S.; Vansteelandt, S.; VanderWeele, T.J.; MacKinnon, D.P.; Mansell, G.; Collins, G.S.; Golub, R.M.; et al. A Guideline for Reporting Mediation Analyses of Randomized Trials and Observational Studies. JAMA 2021, 326, 1045–1056. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Vansteelandt, S. Odds ratios for mediation analysis for a dichotomous outcome. Am. J. Epidemiol. 2010, 172, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Keele, L.; Tingley, D. A General Approach to Causal Mediation Analysis. Psychol. Methods 2010, 15, 309–334. [Google Scholar] [CrossRef]
- Jackson, T.L.; Regillo, C.D.; Girach, A.; Dugel, P.U.; MIVI-TRUST Study Group. Baseline Predictors of Vitreomacular Adhesion/Traction Resolution Following an Intravitreal Injection of Ocriplasmin. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Stalmans, P.; Benz, M.S.; Gandorfer, A.; Pakola, S.J.; Girach, A.; Kampik, A.; Jaffe, G.J.; Toth, C.A.; MIVI-TRUST Study Group. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: Subgroup analyses from two randomized trials. Ophthalmology 2015, 122, 117–122. [Google Scholar] [CrossRef]
- Jackson, T.L.; Haller, J.; Blot, K.H.; Duchateau, L.; Lescrauwaet, B. Ocriplasmin for Treatment of Vitreomacular Traction and Macular Hole A Systematic Literature Review and Individual Participant Data Meta-Analysis of Randomized, Controlled, Double-Masked Trials. Surv. Ophthalmol. 2021, 67, 697–711. [Google Scholar] [CrossRef]
- Landau, S.; Emsley, R.; Dunn, G. Beyond total treatment effects in randomised controlled trials: Baseline measurement of intermediate outcomes needed to reduce confounding in mediation investigations. Clin. Trials. 2018, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Pearl, J. Direct and Indirect Effects. In Proceedings of the Seventh Conference on Uncertainty in Artificial Intelligence, San Francisco, CA, USA, 13–15 July 1991; Morgan Kaufmann: Cambridge, MA, USA, 2001; pp. 411–420. Available online: https://ftp.cs.ucla.edu/pub/stat_ser/R273-U.pdf (accessed on 15 February 2024).
- Robins, J.M. Semantics of Causal DAG Models and the Identification of Direct and Indirect Effects. Highly Structured Stochastic Systems; Oxford University Press: New York, NY, USA, 2003; pp. 70–82. [Google Scholar] [CrossRef]
- Pearl, J. The Causal Mediation Formula—A Guide to the Assessment of Pathways and Mechanisms. Prev. Sci. 2012, 13, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.; Tingley, D. Causal Mediation Analysis. Stata. J. 2012, 11, 605–619. [Google Scholar] [CrossRef]
- Mein, C.; Dugel, P.U.; Feiner, L.; Drenser, K.; Miller, D.; Benz, M.; Meunier, E.; Moro, L.; Fineman, M.S. Patient-reported visual function from the ocriplasmin for treatment for symptomatic vitreomacular adhesion, including macular hole (oasis) study. Retina 2020, 40, 1331–1338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steel, D.H.W.; Lotery, A.J. Idiopathic vitreomacular traction and macular hole: A comprehensive review of pathophysiology, diagnosis, and treatment. Eye 2013, 27 (Suppl. 1), S1–S21. [Google Scholar] [CrossRef]
- Petrillo, J.; Bressler, N.M.; Lamoureux, E.; Ferreira, A.; Cano, S. Development of a new Rasch-based scoring algorithm for the National Eye Institute Visual Functioning Questionnaire to improve its interpretability. Health Qual Life Outcomes 2017, 15, 157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collett, D. Modelling Binary Data, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Grigore, B.; Ciani, O.; Dams, F.; Federici, C.; de Groot, S.; Möllenkamp, M.; Rabbe, S.; Shatrov, K.; Zemplenyi, A.; Taylor, R.S. Surrogate Endpoints in Health Technology Assessment: An International Review of Methodological Guidelines. PharmacoEconomics 2020, 38, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.J.; Taylor, R.S. Informed decision-making: Statistical methodology for surrogacy evaluation and its role in licensing and reimbursement assessments. Pharm. Stat. 2022, 21, 740–756. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH-Biomarker-Working-Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Food and Drug Administration (US); National Institutes of Health (US): Silver Spring, MD, USA; Bethesda, MD, USA, 2016.
- Bandello, F.; Blot, K.; Lescrauwaet, B. Natural history of vitreomacular traction. In Proceedings of the 5th San Rafaele OCT & Retina Forum, Milan, Italy, 29–30 March 2019. [Google Scholar]
- Pearl, J.; Mackenzie, D. The Book of Why: The New Science of Cause and Effect; Basic Books, Hachette Book Group: New York, NY, USA, 2018. [Google Scholar]
- Baghban, R.; Farajnia, S.; Ghasemi, Y.; Mortazavi, M.; Ghasemali, S.; Zakariazadeh, M.; Zarghami, N.; Samadi, N. Engineering of Ocriplasmin Variants by Bioinformatics Methods for the Reduction of Proteolytic and Autolytic Activities. Iran. J. Med. Sci. 2021, 46, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, S.; Vansteelandt, S.; Loeys, T. Boosting the precision of mediation analyses of randomised experiments through covariate adjustment. Stat. Med. 2017, 36, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Reiter, G.S.; Riedl, S.; Seeböck, P.; Vogl, W.D.; Blodi, B.A.; Domalpally, A.; Fawzi, A.; Jia, Y.; Sarraf, D.; et al. AI-based monitoring of retinal fluid in disease activity and under therapy. Prog. Retin. Eye Res. 2021, 86, 100972. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Zahid, R.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Meng, X.; Reese, J.; Le, T.K.; Lunasco, L.; Hu, M.; et al. Longitudinal Assessment of Ellipsoid Zone Integrity, Subretinal Hyperreflective Material, and Subretinal Pigment Epithelium Disease in Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2021, 5, 1204–1213. [Google Scholar] [CrossRef]
- Dugel, P.U.; Jhaveri, C.D.; Chakravarthy, U.; Wykoff, C.C.; Singh, R.P.; Hamilton, R.; Weissgerber, G.; Mulyukov, Z.; Holz, F.G. Effect of Retinal Thickness Variability on Visual Outcomes and Fluid Persistence in Neovascular Age-Related Macular Degeneration: A Post Hoc Analysis of the HAWK and HARRIER Studies. Retina 2022, 42, 511–518. [Google Scholar] [CrossRef]
- Chaudhary, V.; Matonti, F.; Zarranz-Ventura, J.; Stewart, M.W. Impact of Fluid Compartments on Functional Outcomes for Patients with Neovascular Age-Related Macular Degeneration. Retina 2022, 42, 589–606. [Google Scholar] [CrossRef]
- Riedl, S.; Vogl, W.D.; Waldstein, S.M.; Schmidt-Erfurth, U.; Bogunović, H. Impact of intra- and subretinal fluid on vision based on volume quantification in the HARBOR trial. Ophthalmol. Retin. 2022, 6, 291–297. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH E9 (R1) Addendum on Estimands and Sensitivity Analysis in Clinical Trials to the Guideline on Statistical Principles for Clinical Trials.pdf. February 2020. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical-principles_en.pdf (accessed on 15 February 2024).
| VFQ-I (%) * | VA-I (%) * | VF-I (%) * | |
|---|---|---|---|
| (1) † [95% CI] | 5.7 ‡ [1.16, 10.86] | 11.8 ‡ [4.99, 19.41] | 5.2 [−0.31, 11.31] |
| (0) † [95% CI] | 8.3 [−3.30, 19.53] | 12.1 ‡ [1.53, 22.95] | 24.1 ‡ [11.58, 36.56] |
[95% CI] | 13.9 ‡ [2.61, 24.23] | 23.9 ‡ [12.15, 34.97] | 29.3 ‡ [17.81, 40.20] |
| Proportion Mediated | 40.0 [22.75, 167.28] | 48.9 [33.67, 96.77] | 17.7 [12.98, 29.28] |
| VFQ-I (%) * | VA-I (%) * | VF-I (%) * | |
|---|---|---|---|
| (1) † [95% CI] | 7.5 ‡ [2.19, 13.21] | 10.7 ‡ [3.71, 18.42] | 6.5 ‡ [0.14, 14.03] |
| (0) † [95% CI] | 5.15 [−11.18, 21.29] | 3.37 [−13.49, 21.15] | 24.9 ‡ [8.90, 40.68] |
[95% CI] | 12.6 [−6.50, 29.12] | 14.1 [−2.10, 29.77] | 31.5 ‡ [16.07, 45.63] |
| Proportion Mediated | 49.5 [−414.38, 496.92] | 71.7 [−387.39, 497.93] | 20.7 [14.37, 40.81] |
| VFQ-I (%) * | VA-I (%) * | VF-I (%) * | |
|---|---|---|---|
| (1) † [95% CI] | 4.3 [−0.80, 9.76] | 10.5 ‡ [3.64, 18.16] | 5.4 ‡ [1.40, 10.39] |
| (0) † [95% CI] | 10.7 [−0.98, 22.06] | 14.4 ‡ [3.20, 25.57] | 23.1 ‡ [10.56, 35.21] |
[95% CI] | 15.0 ‡ [3.99, 24.96] | 24.9 ‡ [13.19, 35.86] | 28.5 ‡ [15.50, 40.30] |
| Proportion Mediated | 28.3 [16.85, 99.1] | 42.0 [29.26, 79.57] | 18.8 [13.43, 34.93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lescrauwaet, B.; Vansteelandt, S.; Jackson, T.L.; Sadda, S.R.; Duchateau, L. A Causal Inference Approach to Mediation Analysis in Vitreomacular Traction: How Much Does Traction Resolution Mediate Functional Outcomes? J. Mark. Access Health Policy 2024, 12, 280-293. https://doi.org/10.3390/jmahp12040022
Lescrauwaet B, Vansteelandt S, Jackson TL, Sadda SR, Duchateau L. A Causal Inference Approach to Mediation Analysis in Vitreomacular Traction: How Much Does Traction Resolution Mediate Functional Outcomes? Journal of Market Access & Health Policy. 2024; 12(4):280-293. https://doi.org/10.3390/jmahp12040022
Chicago/Turabian StyleLescrauwaet, Benedicte, Stijn Vansteelandt, Timothy L. Jackson, SriniVas R. Sadda, and Luc Duchateau. 2024. "A Causal Inference Approach to Mediation Analysis in Vitreomacular Traction: How Much Does Traction Resolution Mediate Functional Outcomes?" Journal of Market Access & Health Policy 12, no. 4: 280-293. https://doi.org/10.3390/jmahp12040022
APA StyleLescrauwaet, B., Vansteelandt, S., Jackson, T. L., Sadda, S. R., & Duchateau, L. (2024). A Causal Inference Approach to Mediation Analysis in Vitreomacular Traction: How Much Does Traction Resolution Mediate Functional Outcomes? Journal of Market Access & Health Policy, 12(4), 280-293. https://doi.org/10.3390/jmahp12040022







