INEAS’s Cost-Effectiveness Analysis of Vemurafenib: Paving the Way for Value-Based Pricing in Tunisia
Abstract
1. Introduction
2. Materials and Methods
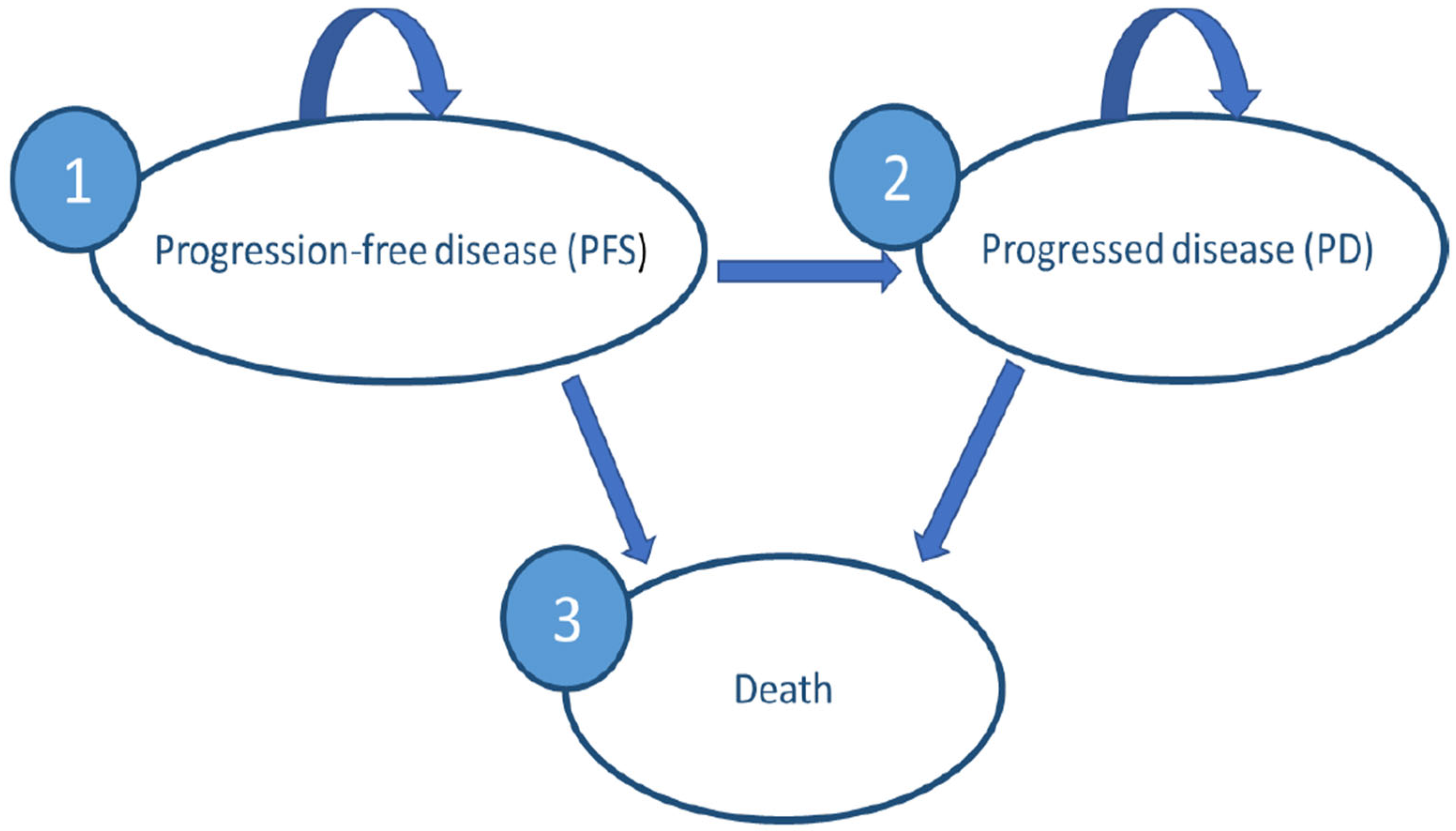
2.1. Selection of the Model Type and Structure
2.2. Decision Model
2.2.1. Model Parameters
- Transition probabilities
- Health-related quality of life
- Costs
- ○
- The source of unit costs differs depending on the perspective: The CNAM Perspective
- ○
- The public health facilities (PHFs) perspective
2.2.2. Validation of the Model
2.2.3. Sensitivity Analyses
3. Results
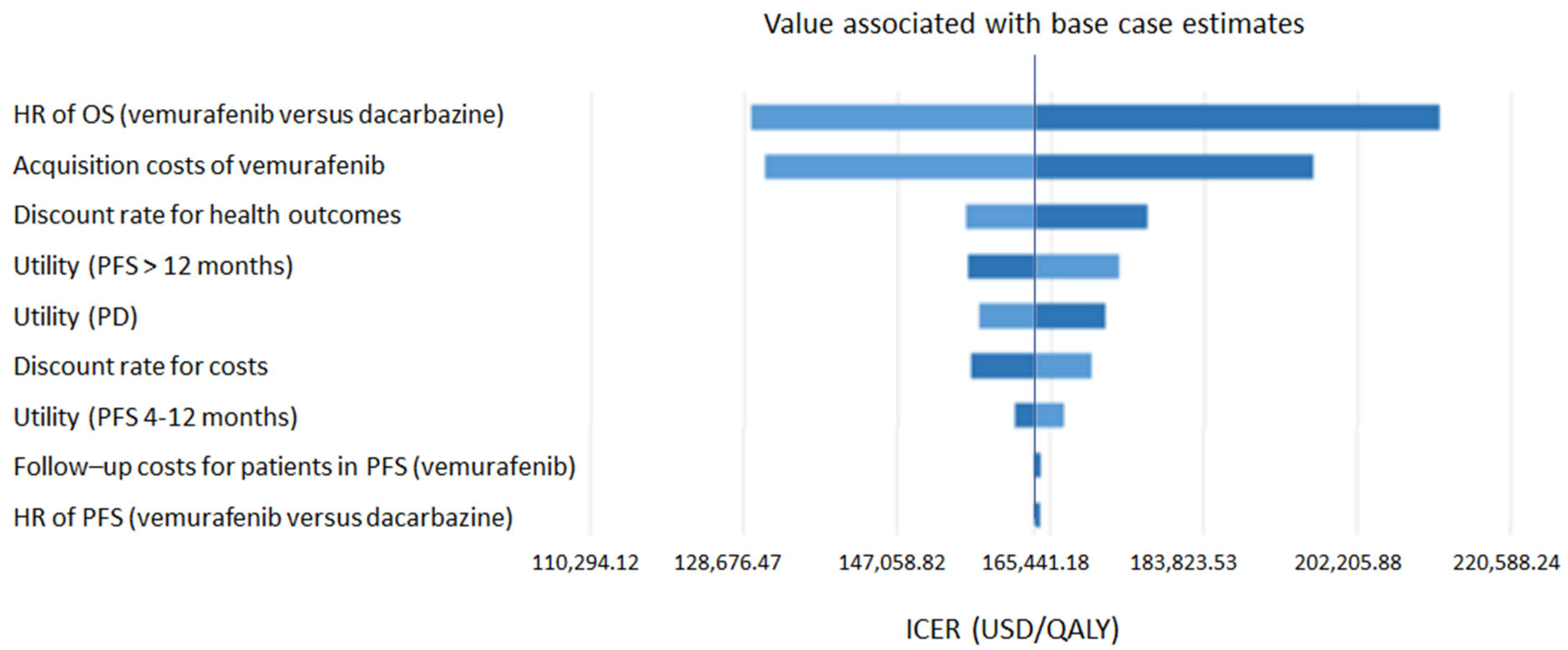
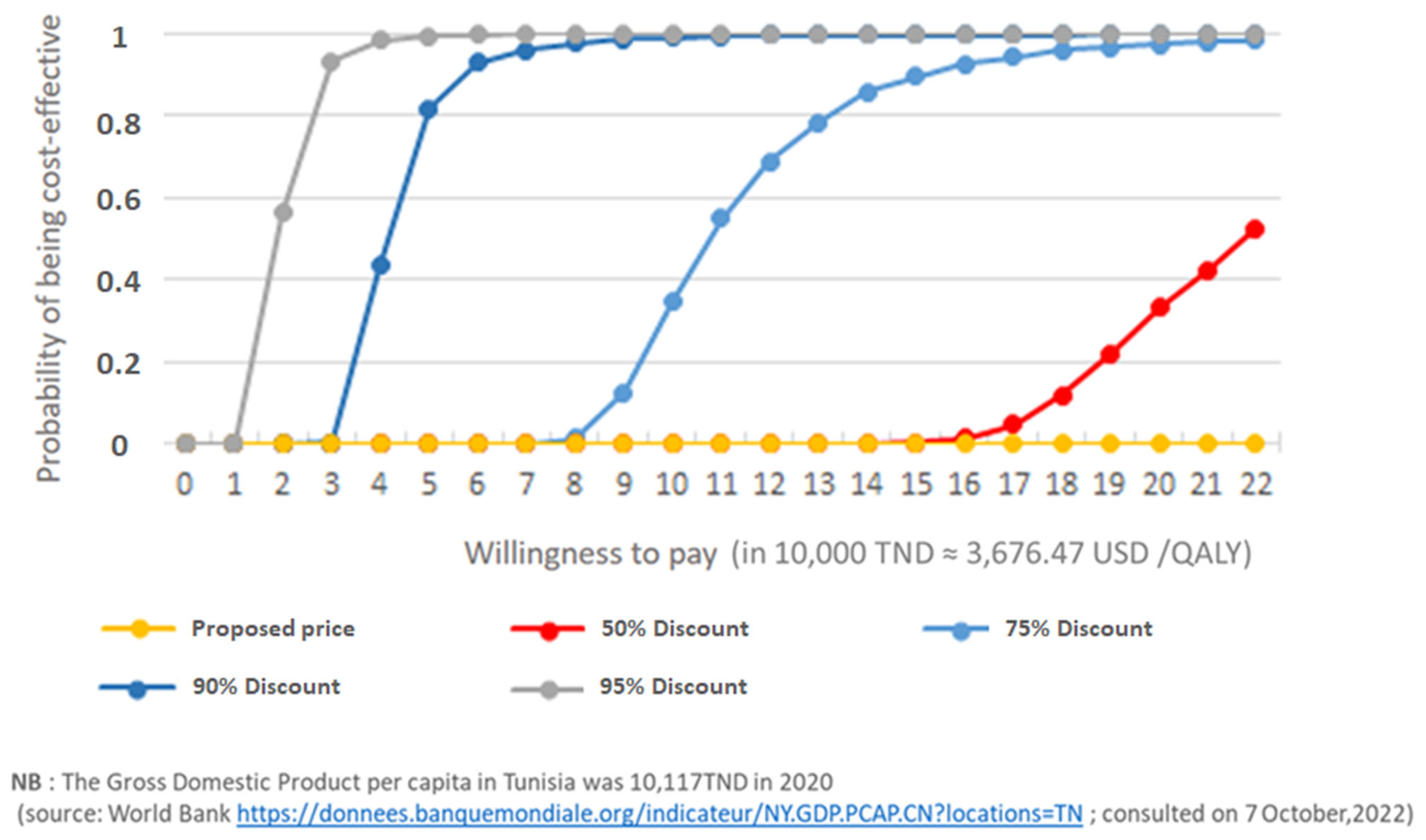
- Sensitivity analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.W.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pike, E.; Hamidi, V.; Saeterdal, I.; Odgaard-Jensen, J.; Klemp, M. Multiple treatment comparison of seven new drugs for patients with advanced malignant melanoma: A systematic review and health economic decision model in a Norwegian setting. BMJ Open 2017, 7, e014880. [Google Scholar] [CrossRef] [PubMed]
- Gorry, C.; McCullagh, L.; Barry, M. Economic Evaluation of Systemic Treatments for Advanced Melanoma: A Systematic Review. Value Health 2020, 23, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- National Authority for Assessment and Evaluation in Healthcare (INEAS). ZELBORAF® (Vemurafenib) as Monotherapy in the Treatment of BRAF V600 Mutation-Positive Unresectable or Metastatic Melanoma; INEA: Tunis, Tunisia, 2024; 63p. [Google Scholar]
- López de Argumedo, M.; Reviriego, E.; Gutiérrez, A.; Bayón, J.C. Actualización del Sistema de Trabajo Compartido para Revisiones Sistemáticas de la Evidencia Científica y Lectura Crítica (Plataforma FLC 3.0); Ministerio de Sanidad, Servicios Sociales e Igualdad, Servicio de Evaluación de Tecnologías Sanitarias del País Vasco, Informes de Evaluación de Tecnologías Sanitarias, OSTEBA: Barakaldo, Spain, 2017. [Google Scholar]
- Shih, V.; Ten Ham, R.M.; Bui, C.T.; Tran, D.N.; Ting, J.; Wilson, L. Targeted Therapies Compared to Dacarbazine for Treatment of BRAFV600E Metastatic Melanoma: A Cost-Effectiveness Analysis. J. Skin Cancer 2015, 2015, e505302. [Google Scholar] [CrossRef] [PubMed]
- Curl, P.; Vujic, I.; van ‘t Veer, L.J.; Ortiz-Urda, S.; Kahn, J.G. Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma. PLoS ONE 2014, 9, e107255. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.L.; Corrêa, F.d.M.; Fernandes, R.R.A.; Zimmerman, I.R. Cost Utility of Target Therapies Compared to Dacarbazine for First-Line Treatment of Advanced Non-Surgical and Metastatic Melanoma in the Brazilian National Health System. Value Health Reg. Issues 2019, 20, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Beale, S.; Dickson, R.; Bagust, A.; Blundell, M.; Dundar, Y.; Boland, A.; Marshall, E.; Plummer, R.; Proudlove, C. Vemurafenib for the treatment of locally advanced or metastatic BRAF V600 mutation-positive malignant melanoma: A NICE single technology appraisal. PharmacoEconomics 2013, 31, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rodriguez, D.; De Diego Blanco, S.; Perez, M.; Rubio-Terres, C. Cost-Effectiveness of Drug Treatments for Advanced Melanoma: A Systematic Literature Review. PharmacoEconomics 2017, 35, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Instance Nationale de l’Evaluation et de l’Accréditation en Santé (INEAS). Choix Méthodologiques pour les Études Pharmaco-Économiques à L’INEAS [Methodological Choices for the Pharmaco-Economic Analyses at the INEAS]; INEAS: Tunis, Tunisia, 2020; 46p. (In French) [Google Scholar]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Franken, M.G.; Leeneman, B.; Gheorghe, M.; Uyl-de Groot, C.A.; Haanen, J.B.; van Baal, P.H. A systematic literature review and network meta-analysis of effective-ness and safety outcomes in advanced melanoma. Eur. J. Cancer Oxf. Engl. 2019, 123, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Claxton, K.; Sculpher, M.J. Decision Modelling for Health Economic Evaluation, 2011st ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Tran, A.; Fogarty, G.; Nowak, A.; Espinoza, D.; Rowbotham, N.; Stockler, M.; Morton, R. A systematic review and meta-analysis of utility estimates in melanoma. Br. J. Dermatol. 2018, 178, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé Ministère des Affaires Sociales. Convention régissant la facturation des services de soins fournis aux assurés sociaux au sein des établissements publics de soins. [Agreement governing the billing of health care services provided to insured persons in public health care institutions]. 2018/80. 2018. unpublished work. (In French) [Google Scholar]
- Ministère de la Santé Ministère des Affaires Sociales. Ministère de la Santé Ministère des Affaires Sociales. Avenant de la convention régissant la facturation des services de soins fournis aux assurés sociaux au sein des établissements publics de soins. [Endorsement to the agreement governing the billing of healthcare services provided to insured persons in public healthcare structures]. 2020/29. 2020. unpublished work. (In French) [Google Scholar]
- Ministère des Affaires Sociales, Caisse Nationale d’Assurance Maladie. Convention sectorielle des laboratoires d’analyses médicales. [Sectoral agreement for medical analysis laboratories]. 24 Décembre 2020. 2020. unpublished work. (In French) [Google Scholar]
- Ministère des Affaires Sociales, Caisse Nationale d’Assurance Maladie. Convention sectorielle des médecins de libre pratique. [Sectoral agreement for private practitioners]. Novembre 2020 34. 2020. unpublished work. (In French) [Google Scholar]
- Caisse Nationale d’Assurance Maladie (CNAM). Statistiques [Statistics]. Available online: http://www.cnam.nat.tn/stat.jsp (accessed on 18 August 2021).
- Ministre de la Santé Publique. Arrêté du ministre de la santé publique du 1er juin 2006, fixant la nomenclature générale des actes professionnels des médecins, biologistes, médecins dentistes, psychologues cliniciens, sages-femmes et auxiliaires médicaux. [Order of the Minister of Public Health of 1 June 2006, establishing the general nomenclature of professional acts for physicians, biologists, dentists, clinical psychologists, midwives and medical auxiliaries]. J. Off. République Tunis 2006, 9, 1514. (In French) [Google Scholar]
- Ministre des Finances et Ministre de la Santé Publique. Arrêté du 7 juillet 2008 (portant modification de l’arrêté du 19 décembre 1996) fixant les tarifs de prise en charge des malades payants dans les structures sanitaires publiques et à l’arrêté du ministre de la santé publique du 1er juin 2006, fixant la nomenclature générale des actes professionnels des médecins, biologistes, médecins dentistes, psychologues cliniciens, sages-femmes et auxiliaires médicaux. [Order of 7 July 2008 (amending the order of 19 December 1996) setting the rates for the treatment of paying patients in public health facilities and the order of the Minister of Public Health of 1 June 2006, setting the general nomenclature of professional acts for doctors, biologists, dentists, clinical psychologists, midwives and medical auxiliaries]. J. Off. République Tunis 2008, 11, 2077. (In French) [Google Scholar]
- Briggs, A.H.; Weinstein, M.C.; Fenwick, E.A.L.; Karnon, J.; Sculpher, M.J.; Paltiel, A.D. ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med. Decis. Mak. 2012, 32, 722–732. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Regional Office for Europe. Expert Meeting on Market Transparency to Improve Access to High-Priced Innovative Medicines: 18 February 2020, National Institute for Health and Disability Insurance, Brussels, Belgium (No. WHO/EURO: 2020-5582-45347-64895). Available online: https://iris.who.int/bitstream/handle/10665/359002/WHO-EURO-2020-5582-45347-64895-eng.pdf?sequence=1&isAllowed=y (accessed on 10 May 2024).
- Scottish Intercollegiate Guidelines Network (SIGN). Cutaneous Melanoma. (SIGN publication no. 146). August 2023. Available online: http://www.sign.ac.uk (accessed on 6 May 2024).
- National Institute for Health and Care Excellence (NICE). Melanoma: Assessment and Management. Available online: https://www.nice.org.uk/guidance/ng14 (accessed on 10 May 2024).
- Toumi, M. Introduction to Market Access for Pharmaceuticals; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Gandjour, A. Reference Pricing and Price Negotiations for Innovative New Drugs in a Multidimensional Framework. PharmacoEconomics 2013, 31, 221–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. WHO Guideline on Country Pharmaceutical Pricing Policies. 2020. Available online: https://www.who.int/publications/i/item/9789240011878 (accessed on 15 May 2024).
- Dahmani, H.; Fradi, I.; Achour, L.; Toumi, M. Maghreb Research Group Pharmaceutical pricing and reimbursement policies in Algeria, Morocco, and Tunisia: Comparative analysis. J. Mark. Access Health Policy 2023, 11, 2244304. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fair Pricing Forum 2021: Forum Discussion Paper: Pricing Approaches Sensitive to Health Systems’ Ability to Pay and the Need for Accelerating Towards Health Sustainable Development Goal. World Health Organization. Report No.: WHO/MHP/HPS/MIA/2021.02. 2021. Available online: https://apps.who.int/iris/handle/10665/348288 (accessed on 10 May 2024).
- Vargas-Pelaez, C.M.; Rover, M.R.M.; Soares, L.; Blatt, C.R.; Mantel-Teeuwisse, A.K.; Rossi, F.A.; Restrepo, L.G.; Latorre, M.C.; López, J.J.; Bürgin, M.T.; et al. Judicialization of access to medicines in four Latin American countries: A comparative qualitative analysis. Int. J. Equity Health 2019, 18, 68. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Base Value | Range |
|---|---|---|
| Discount rate | 5% | 3–8% |
| HR PFS | 0.38 | 0.32–0.45 |
| HR OS | 0.81 | 0.68–0.96 |
| Probability of serious AEs related to dacarbazine | 0.016 | 0.013–0.02 |
| Probability of serious AEs related to vemurafenib | 0.029 | 0.025–0.034 |
| Utility PFS state (1 day–3 months) | 0.69 | 0.665–0.715 |
| Utility PFS state (4–12 months) | 0.905 | 0.858–0.952 |
| Utility PFS state (≥12 months) | 0.910 | 0.863–0.957 |
| Utility PD state | 0.45 | 0.403–0.497 |
| Dacarbazine administration costs (TND) | 159.21 | 127.43–191.16 |
| Vemurafenib acquisition costs (TND) | 6178.76 | 4944.47–7407.68 |
| Follow-up costs in PFS state (dacarbazine cohort) | 65.11 | 52.00–78.04 |
| Follow-up costs in PFS state (vemurafenib cohort) | 63.06 | 50.40–75.54 |
| Follow-up costs in PD state | 42.79 | 34.19–51.23 |
| Cost of managing grade 3–4 AEs of dacarbazine | 423.89 | 338.97–507.70 |
| Cost of managing grade 3–4 AEs of vemurafenib | 423.89 | 338.97–507.70 |
| Proportion of patients with BRAF mutation | 0.5 | 0.4–0.6 |
| Interventions | Utilization Rate per Cycle | Unit Cost (PHF) | Monthly Cost (PHF) | Unit Cost (CNAM/Public Scheme | Unit Cost (CNAM /Third-Party Payment and Reimbursement Schemes) | Monthly Cost CNAM (Weighted Average) |
|---|---|---|---|---|---|---|
| Administration costs of dacarbazine | 4.34 | 17.65 | 76.63 | Included in the daily fee * | 44.12 | 173.72 |
| Follow-up costs (dacarbazine cohort) | ||||||
| Consultation with a specialist | 1.45 | 5.15 | 7.46 | Included in the daily fee * | 16.54 | 23.95 |
| Biological tests | 1.45 | 20.00 | 28.94 | Included in the daily fee * | 34.93 | 22.316 |
| Abdominal and pelvic computed tomography (CT) scan | 0.33 | 88.24 | 29.41 | 73.53 | 115.81 | 30.68 |
| Bone scan | 0.008 | 49.63 | 0.411 | 66.18 | 66.18 | 0.55 |
| Magnetic resonance imaging (MRI) | 0.008 | 147.06 | 1.23 | 125.00 | 110.29 | 0.99 |
| Follow-up costs (vemurafenib cohort) | ||||||
| Consultation with a specialist | 1 | 5.15 | 5.15 | 12.87 | 16.54 | 14.47 |
| Biological tests | 1 | 21.18 | 21.18 | 0 | 37.06 | 16.31 |
| Abdominal and pelvic CT scan | 0.33 | 88.24 | 29.41 | 73.53 | 115.81 | 30.68 |
| Bone scan | 0.008 | 49.63 | 0.41 | 66.18 | 66.18 | 0.55 |
| MRI | 0.008 | 147.06 | 1.23 | 125.00 | 110.29 | 0.99 |
| Interventions | Monthly Rate | Unit Costs PHF | Monthly Cost PHF | Unit Cost CNAM Public Scheme | Unit Cost CNAM Third-Party Payment and the Reimbursement Schemes | Monthly Cost CNAM (Weighted Average) |
|---|---|---|---|---|---|---|
| Surgery | 0.008 | 25.74 | 0.21 | 82.72 | 110.29 | 0.69 |
| Outpatient palliative treatment | 0.167 | 17.65 | 12.18 | 17.65 | 44.12 | 4.88 |
| Palliative treatment requiring hospitalization | 0.05 | 613.24 | 30.68 | 613.24 | 613.24 | 30.68 |
| Radiotherapy | 0.008 | 477.94 | 3.98 | 477.94 | 477.94 | 3.98 |
| Definition | Value | Source/Note | |
|---|---|---|---|
| Efficacy estimate for vemurafenib | Overall survival (95%CI) | Progression-free survival (95%CI) | Franken et al., 2019 [15] |
| 0.81 (0.68–0.96) | 0.38 (0.32–0.45) | ||
| Adverse events | |||
| Probability of serious adverse events associated with dacarbazine per cycle | 0.016 | Pike et al., 2017 [3] | |
| Relative risk of serious adverse events associated with vemurafenib compared to dacarbazine | 1.75 (1.51–2.03) | Franken et al., 2019 [15] | |
| Probability of serious adverse events associated with vemurafenib per cycle | 0.0287 | ||
| Quality-adjusted life years (QALY) weight | |||
| Utility (PFS) | 0.69 (0–3 month) 0.905 (3–12 month) 0.910 (>12 month) | Tran et al., 2019 [17] | |
| Utility (PD) | 0.45 | Tran et al., 2019 [17] | |
| Costs per cycle | |||
| CNAM perspective (USD) | PHF perspective (USD) | ||
| Acquisition cost of dacarbazine | 152.43 | Regulatory authorities | |
| Acquisition cost of vemurafenib | 6170.61 | Regulatory authorities | |
| Administration cost of dacarbazine | 173.72 | 76.63 | |
| Monitoring costs in PFS (vemurafenib cohort) | 63.04 | 57.31 | |
| Monitoring costs in PFS (Dacarbazine cohort) | 65.09 | 67.47 | |
| Monitoring costs in PD | 40.25 | 37.78 | |
| Costs associated with the management of serious adverse events (AEs) of vemurafenib | 12.15 | ||
| Costs associated with the management of serious AEs of dacarbazine | 6.94 | ||
| Cost of BRAF mutation testing | 177.21 | Institut Pasteur–Tunis | |
| Intervention | Total Cost (USD) | Effectiveness (QALYs) | Effectiveness (LYG) | Incremental Cost | Incremental Effectiveness QALY | Incremental Effectiveness LYG | ICER USD/ QALY | ICER USD/ LYG |
|---|---|---|---|---|---|---|---|---|
| PHF perspective | ||||||||
| Dacarbazine | 1865.90 | 0.76 | 1.4 | NA | NA | NA | NA | NA |
| Vemurafenib | 103,298.53 | 1.38 | 1.78 | 101,474.12 | 0.62 | 0.38 | 163,893.64 | 266,553.31 |
| CNAM perspective | ||||||||
| Dacarbazine | 2332.72 | 0.76 | 1.4 | NA | NA | NA | NA | NA |
| Vemurafenib | 103,418.90 | 1.38 | 1.78 | 101,057.35 | 0.62 | 0.38 | 163,238.60 | 265,445.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jameleddine, M.; Harzallah, N.; Grati, H.; Odabachian Jebali, M.C.; Chemli, J.; García Martí, S.; Soto, N.; Pichon-Riviere, A.; Hamouda, C. INEAS’s Cost-Effectiveness Analysis of Vemurafenib: Paving the Way for Value-Based Pricing in Tunisia. J. Mark. Access Health Policy 2024, 12, 294-305. https://doi.org/10.3390/jmahp12040023
Jameleddine M, Harzallah N, Grati H, Odabachian Jebali MC, Chemli J, García Martí S, Soto N, Pichon-Riviere A, Hamouda C. INEAS’s Cost-Effectiveness Analysis of Vemurafenib: Paving the Way for Value-Based Pricing in Tunisia. Journal of Market Access & Health Policy. 2024; 12(4):294-305. https://doi.org/10.3390/jmahp12040023
Chicago/Turabian StyleJameleddine, Mouna, Nabil Harzallah, Hela Grati, Marie Christine Odabachian Jebali, Jaafar Chemli, Sebastián García Martí, Natalie Soto, Andrés Pichon-Riviere, and Chokri Hamouda. 2024. "INEAS’s Cost-Effectiveness Analysis of Vemurafenib: Paving the Way for Value-Based Pricing in Tunisia" Journal of Market Access & Health Policy 12, no. 4: 294-305. https://doi.org/10.3390/jmahp12040023
APA StyleJameleddine, M., Harzallah, N., Grati, H., Odabachian Jebali, M. C., Chemli, J., García Martí, S., Soto, N., Pichon-Riviere, A., & Hamouda, C. (2024). INEAS’s Cost-Effectiveness Analysis of Vemurafenib: Paving the Way for Value-Based Pricing in Tunisia. Journal of Market Access & Health Policy, 12(4), 294-305. https://doi.org/10.3390/jmahp12040023






