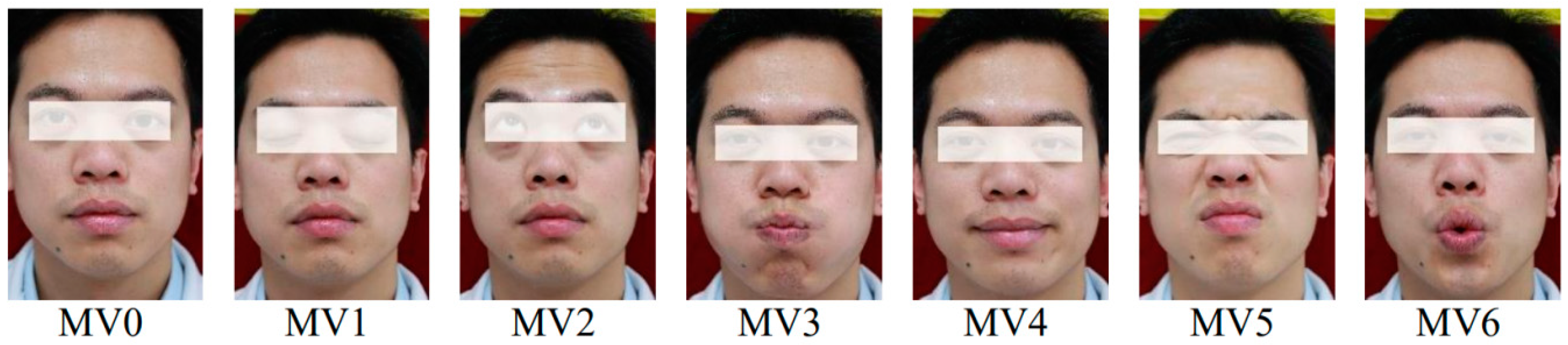
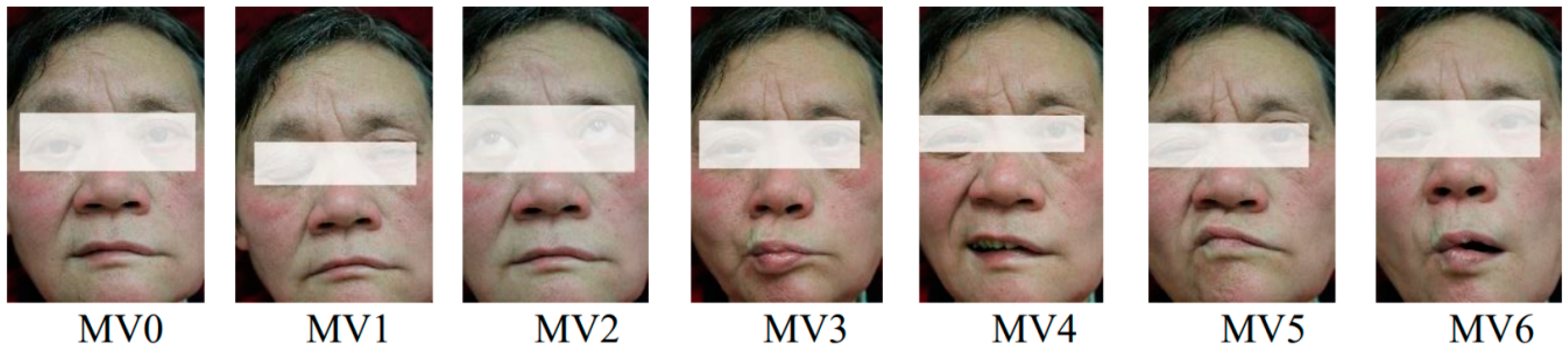
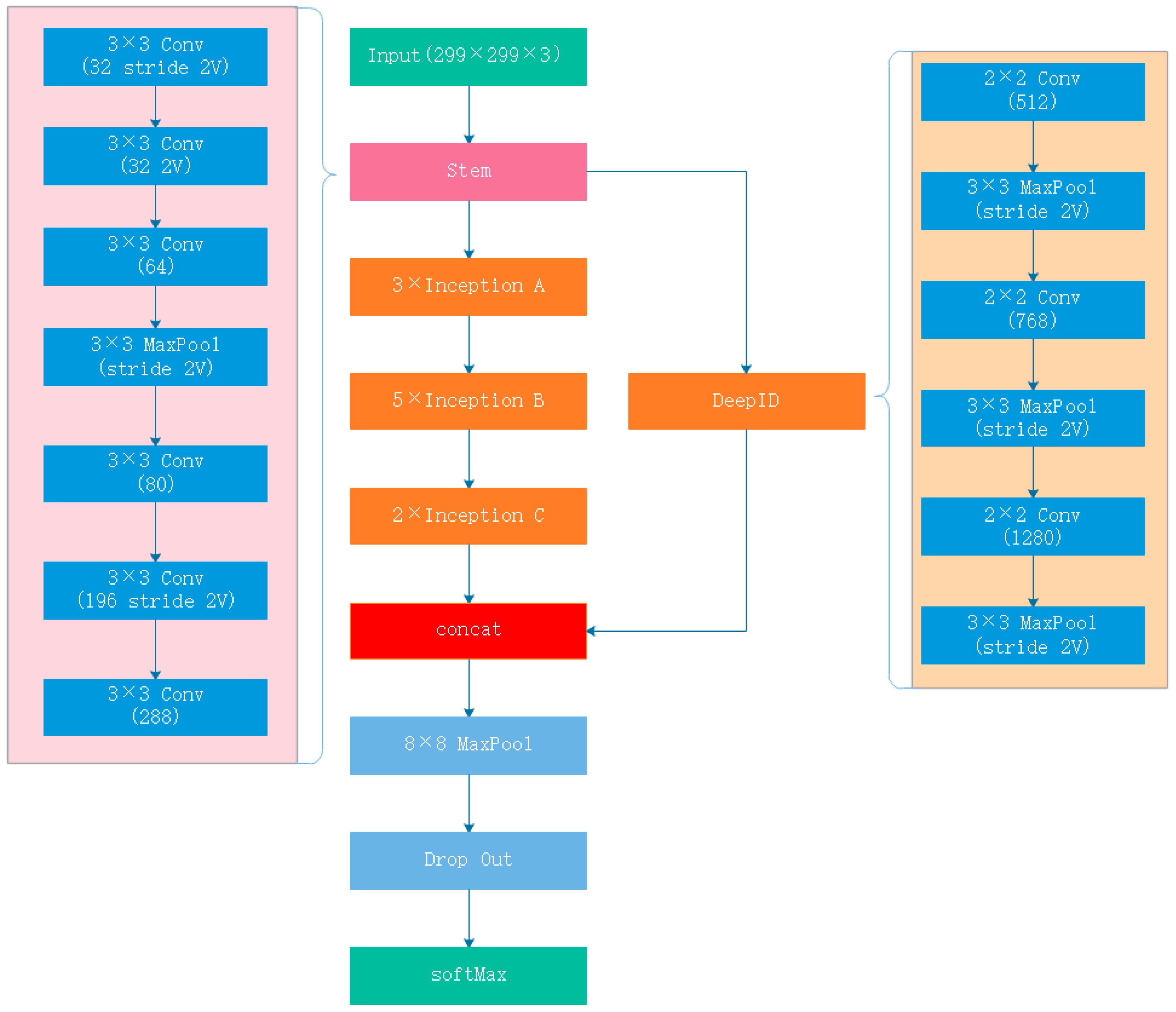
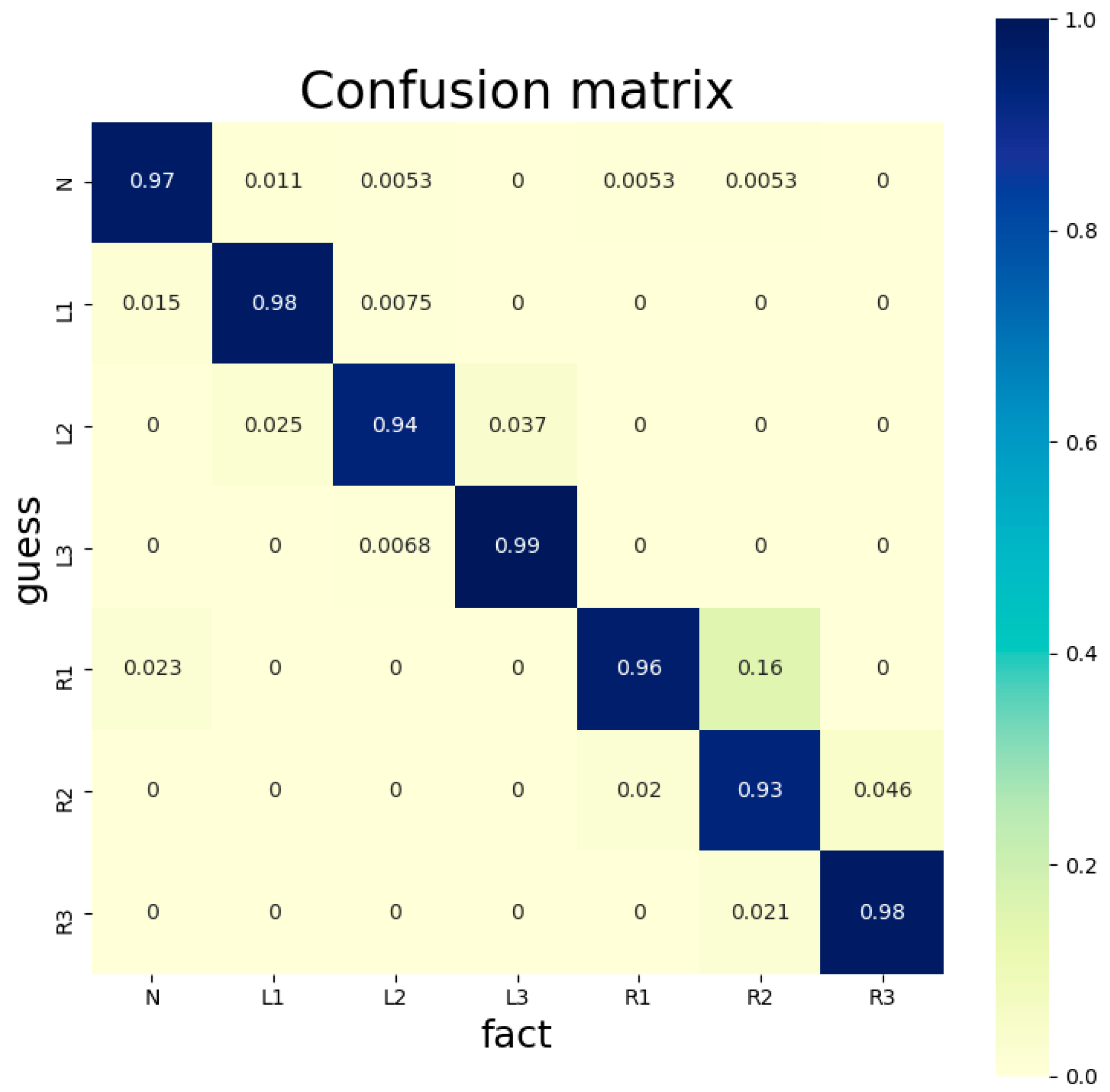
1. Introduction
Facial nerve paralysis (FNP) is one of the most common facial neurological dysfunctions, in which the facial muscles appear to droop or weaken. Such cases are often accompanied by the patient having difficulty chewing, speaking, swallowing, and expressing emotions. Furthermore, the face is a crucial component of beauty, expression, and sexual attraction. As the treatment of FNP requires an assessment to plan for interventions aimed at the recovery of normal facial motion, the accurate assessment of the extent of FNP is a vital concern. However, existing methods for FNP diagnosis are inaccurate and nonquantitative. In this paper, we focus on computer-aided FNP grading and analysis systems to ensure the accuracy of the diagnosis.
Facial nerve paralysis grading systems have long been an important clinical assessment tool; examples include the House–Brackmann system (HB) [
1], the Toronto facial grading system [
2,
3], the Sunnybrook grading system [
4], and the Facial Nerve Grading System 2.0 (FNGS2.0) [
5]. However, these methods are highly dependent on the clinician’s subjective observations and judgment, which makes them problematic with regard to integration, feasibility, accuracy, reliability, and reproducibility of results.
Computer-aided analysis systems have been widely employed for FNP diagnosis. Many such systems have been created to measure facial movement dysfunction and its level of severity, and rely on the use of objective measurements to reduce errors brought about through the use of subjective methods.
Anguraj et al. [
6] utilized Canny edge detection to locate a mouth edge and eyebrow, and Sobel edge detection to find the edges of the lateral canthus and the infraorbital region. Nevertheless, these edge detection techniques are very vulnerable to noise. Neely [
7,
8,
9] and Mcgrenary [
10] used a dynamic video image analysis system which analyzed patients’ clinical images to assess FNP. They used very simple neural networks on FNP, which validated the technology’s potential. Although their results were consistent with the HB scoring system, they had a very small dataset and their system’s image processing was computationally intensive. He et al. [
11] used optical-flow tracking and texture analysis to solve the problem using image processing to capture the asymmetry of facial movements by analyzing the patients’ video data, but this is computationally intensive. Wachtman et al. [
12] measured asymmetry using static images, but their method is sensitive to extrinsic facial asymmetry caused by orientation, illumination, and shadows.
For our method, a new FNP classification standard was established based on FNGS2.0 and asymmetry. FNGS2.0 is a widely used assessment system which has been found to be highly consistent with clinical observations and judgment, achieving 84.8% agreement with neurologist assessments [
13].
Using deep learning to detect facial landmarks in our previous method has shown promising results. Deep convolutional neural networks (DCNNs) [
14] show potential for general and highly variable tasks on image classification [
15,
16,
17,
18,
19]. Deep learning algorithms have recently been shown to exceed human performance in visual tasks like playing Atari games [
20] and recognizing objects [
16]. In this paper, we outline the development of a CNN that matches neurologist performance for human facial nerve paralysis using only image-based classification.
GoogleNet Inception v3 CNN architecture [
18] was pretrained on approximately 1.28 million images (1000 object categories) for the 2014 ImageNet Large Scale Visual Recognition Challenge [
16]. Sun et al. [
21] proposed an effective means for learning high-level overcomplete features with deep neural networks called DeepID CNN, which classified faces according to their identities.
At the same time, DCNNs have had many outstanding achievements as diagnostic aids. Rajpurkar et al. [
22] developed a 34-layer CNN which exceeds the performance of board-certified cardiologists in detecting a wide range of heart arrhythmias from electrocardiograms recorded using a single-lead wearable monitor. Hoochang et al. [
23] used a CNN combined with transfer learning on computer-aided detection. They studied two specific computer-aided detection (CADe) problems, namely thoraco-abdominal lymph node (LN) detection and interstitial lung disease (ILD) classification. They achieved state-of-the-art performance on mediastinal LN detection and reported the first fivefold cross-validation classification results on predicting axial CT slices with ILD categories. Esteva et al. [
15] used a pretrained GoogleNet Inception v3 CNN on skin cancer classification, which matched the performance of dermatologists in three key diagnostic tasks: melanoma classification, melanoma classification using dermoscopy, and carcinoma classification. Sajid et al. [
24] used a CNN model to classify facial images affected by FNP into the five distinct degrees established by House and Brackmann. Sajid used a Generative Adversarial Network (GAN) to prevent overfitting in training. His research demonstrates the potential of deep learning on FNP classification, even though his final classification accuracy results were not very good (89.10–96.90%, depending on the class). Meanwhile, they used a traditional grading standard to directly label the data which may cause erroneous labeling. They also used four complicated image preprocessing steps, which cannot be automated and which require a lot of time and effort during the clinical diagnosis phase for data labeling.
In the process of realizing a reliable computer-aided analysis system, we also proposed a method for FNP quantitative assessment [
25]. We used a DCNN to obtain facial features, then we used asymmetry algorithm to calculate FNP degree. In this work, we validated the effectiveness of DCNN. However, there is currently no work related to the hierarchical classification of FNP using DCNN.
The difficulty of FNP classification lies first and foremost in image classification, followed by face recognition. To design a responsive and accurate CNN for FNP classification, we combined a GoogleNet Inception v3 CNN and a DeepID CNN to design a new CNN called Inception-DeepID-FNP (IDFNP) CNN. As it is difficult to obtain a large enough training dataset, direct training of our model would cause overfitting results, so we need to use transfer learning methods [
26] to eliminate overfitting, as given the amount of expected data available, this was considered to be the optimal choice. We trained the IDFNP CNN by training on ImageNet with no final classification layer and then retrained it using our dataset. This method is optimal given the amount of data available.
Compared with other classification methods, we set up our own dataset classification standards. We used deep learning to directly classify FNP, which allows each FNP image to be processed more quickly, has more accurate classification, and has lower image quality requirements. In order to improve the liability and accuracy of our labeling results, we used a triple-check method to complete the labeling of the image dataset. At the same time, we combined image classification with face recognition.
Using the proposed system, clinicians can quickly obtain the degree of facial paralysis under different movements and make a prediagnosis of facial nerve condition, which can then be used as a reference for final diagnosis. At the same time, we also developed a mobile phone application that enables patients to perform self-evaluations, which can help them avoid unnecessary visits to hospitals.
The remainder of this paper is structured as follows.
The proposed methodology is presented in
Section 2. The experiments and results are given in
Section 3. The results and related discussion are presented in
Section 4. The conclusions about this study are given in
Section 5.
4. Discussion
As we see from
Table 7, neurological agreement exceeds our method in MV2, MV5, and MV6. However, neurologists take too long examining FNP images, as each such examination takes at least 10 s. Our method takes a few milliseconds per FNP image and is thus more efficient, while its accuracy is comparable to that of neurologists. Our previous method takes much longer per FNP image by calculating facial asymmetry with traditional computational methods, while only its accuracy in MV0 on RgAs is higher. Furthermore, our previous method requires more standard images like face angle, image clarity, and lighting conditions.
As we see from
Table 8, the accuracy of FNP classification when using Sajid’s method was 92.6%. The accuracy of FNP in Neely’s method [
28] is 95%, which is lower than our method. HC [
28] used RBF with 0/1 disagreement to measure accuracy of FNP movements. Even with 1 disagreement, which allows for more experimental errors, the result is significantly worse than ours. Wang [
29,
30] used SVM with RBF to measure accuracy. The result showed our method is better than their method in MV2–6. In MV1, their accuracy is not much higher than ours. Although they didn’t calculate the accuracy of MV0, we can still see from the rest of the results that our method yields superior results.
As we see from
Table 9, these models have strong generalization ability for different datasets, but because their design was optimized for their main, that is, image classification, the final training results of these models are not as good as our model. We also see that Inception-v3, upon which our own design was based, achieved only 93.3% accuracy. Therefore, there is still considerable potential for the optimization of this excellent image classification model for specific applications, especially with residual network derivatives like Inception-ResNet-v2.
Meanwhile, on the basis of our findings, clinicians can quickly obtain the degree of facial paralysis according to different facial movements. Clinicians can make a prediagnosis of facial nerve paralysis based on patients’ facial movements, which will be used as a reference for their final diagnosis. For example, the result of one patient in MV1 (Eye closed), MV2 (Eyebrows raised), and MV4 (Grinning) was L3, and the result of the patient in other movements was N or L1, which corresponds to a prediagnosis that severe paralysis is present in the in left orbicularis oculi muscle.
5. Conclusions
In this paper, we presented a neural network model called IDFNP for FNP image classification, which uses a deep neural network and can achieve accuracy which is comparable to that of neurologists. Key to the performance of the model is an FNP annotated dataset and a deep convolutional network which can classify facial nerve paralysis and facial nerve paresis effectively and accurately. IDFNP combines Inception-v3, which achieves a great result in image classification, and DeepID, which is highly efficient in facial recognition.
The contributions of our method can be summarized as follows: Firstly, a symmetry-based annotation scheme for FNP images with seven different classes is presented. Secondly, using deep neural network on FNP images and cropping the face from the FNP images can eliminate facial deformation for FNP patients and minimize the influence of environmental factors. Thirdly, transfer learning avoids overfitting effectively for a limited range of FNP images. Combining an image classification CNN, such as Inception-v3, and a face recognition CNN like DeepID improves accuracy for the FNP dataset and achieves the same diagnostic accuracy as a neurologist. Fourthly, our method is validated against the performance of other well-known methods, which serves as proof that IDFNP is suitable for FNP classification and can effectively assist neurologists in clinical diagnosis.
In terms of clinical diagnosis, future work will be needed to apply IDFNP performance to other facial diseases or diseases which can be identified visually. On the one hand, more detailed diagnosis of facial paralysis would further aid neurologists in their work. In the future, we plan to undertake a more in-depth study of the position and the degree of disease. On the other hand, we can extend our findings to other conditions. For example, one of the symptoms of a stroke is facial asymmetry, which is very similar to the symptoms of FNP. If IDFNP can diagnose strokes and distinguish various degrees of facial stroke images and facial nerve paralysis images, then preventive treatment for strokes based on facial images can be realized. Given that modern smartphones and PCs are power tools of deep learning, with the help of the IDFNP results, citizens will have an enhanced ability to obtain an automated assessment for these diseases that may prompt them to visit a specialized physician.
The evaluation results produced by our methods are mostly consistent with the subjective assessment of doctors. Our methods can help clinicians to decide on a specific therapy for each patient, and for the most affected region of the face as reference.
Given that more and more FNP patients are being treated, high-accuracy diagnosis from FNP images can save expert clinicians and neurologists considerable time and decrease the frequency of misdiagnosis. Furthermore, we hope that this technology will enable greater widespread use of FNP images through photography as a diagnostic tool in places where access to a neurologist is limited.