Abstract
The pulmonary administration of pharmaceutical aerosols to patients is affected by age-dependent variations in the anatomy of the upper airways and the inhalation pattern. Considering this aspect, different upper airway models, representing the geometries of adults and preschool children, and a conventional induction port according to the European Pharmacopeia were used for in vitro testing of dry powder inhalers with single dosed capsules (Cyclohaler®, Handihaler® and Spinhaler®). Deposition measurements were performed using steady flow rates of 30 and 60 L/min for the Handihaler®/Spinhaler® and 30, 60 and 75 L/min for the Cyclohaler®. The inhalation volume was set at 1 L. For the Cyclohaler®, the in vitro testing was supplemented by a pediatric inhalation profile. Slight differences of pulmonary deposition between the idealized adult (11%–15%) and pediatric (9%–11%) upper airway model were observed for the Cyclohaler®. The applied pediatric inhalation profile resulted in a reduction of pulmonary deposition by 5% compared to steady conditions and indicated the influence of the inhalation pattern on the amount of pulmonary deposited particles. The comparison of two pediatric upper airway models showed no differences. The performance of the Handihaler® was similar to the Cyclohaler®. The Spinhaler® showed an insufficient performance and limited reproducibility in our investigations.
1. Introduction
Diseases of the respiratory tract are very common, especially in industrialised countries. Bronchial asthma, manifested in typical symptoms such as coughing and wheezing caused by airway obstruction, is one of the most frequent diseases occurring in the first years of life. However, the prevalence of asthma in children is much higher compared to adults [1,2]. In the first instance, the therapy demands a pulmonary administration to avoid systematic side effects of the active pharmaceutical ingredients (API). In general, two main routes of pulmonary administration can be distinguished: nasal and oral inhalation. Regarding age, physiological features and mental capacity, the nasal route is the option of choice in the treatment of newborns, infants and older children up to the age of 4, by using facemasks and nebulisers as delivery devices [3]. Recently published data described the use of high-flow nasal cannula for the aerosol delivery to children [4]. The oral administration of aerosols is principally limited to preschool children (mainly by using valved holding chambers), older children and adults, while dry powder inhalers (DPI) or metered dose inhalers (MDI) are usually applied as drug delivery systems in these age groups [3]. The preschool pediatric population that this study focused on is defined by a range of age between 2 and 5 years. The delivery of pharmaceutical aerosols via DPIs is the preferred method in pulmonary therapy for this age group [5]. During oral administration, the inhalable API has to overcome a number of anatomical barriers to reach the lung as target organ. In the oral cavity, the inhaled drug particles reach the pharynx, where they are subject to a strong change of direction caused by the throat geometry. Subsequently, the API particles are transported via the larynx and the trachea to the target site of the peripheral airways. In the case of bronchial asthma, the most prescribed medications are inhalable β2-sympathomimetics, such as salbutamol sulfate, which has been used in this study. This API acts as agonist at β2-receptors of smooth muscles located in the whole lung and especially in the peripheral airways, the bronchioles [6]. Thus, the structure and physiology of the upper and peripheral airways are essential aspects in the field of respiratory diseases. In this context, the main factors influencing the delivery of pharmaceutical aerosols to patients of all ages need to be taken into consideration. These factors can be generally divided into three factors: the patient, the drug delivery device and the administered formulation [7]. The patient’s inter- and intra-individual variability of the upper airway geometry is related to age and can be described as the most crucial factor in pulmonary drug delivery [8].
In childhood, the lung is not yet fully developed and several differences between children and adults can be observed [9]. The pharynx, larynx and trachea are smaller in children than in adults, while other parts, like the tongue related to the entire oral cavity, are even more expanded compared to adult dimensions [10]. In addition to the anatomical differences, the peak inspiratory flow rate (PIF) and the inhalation volume of children are lower than in adults, but the inhalation time is shorter compared to an adult inhalation process [11,12]. The capability of preschool children to reach sufficient flow rates increases with age [13]. However, the inhalation pattern differs in children with acute asthma [14] and plays an important role to ensure an adequate treatment of respiratory diseases. In case of DPIs, the patient has to provide sufficient flow rates to induce the deaggregation of the powder mixture (separation of micronized API particles from carrier particles). The required energy for this process has to be supplied by the inhalation force (high PIF and inhalation volume) of the patient [15] and depends on the respective DPI, which is another important factor.
The maximum flow rate generated by the patient is affected by the resistance of the device, which is closely linked to the structure and mechanism of deaggregation. The higher the resistance of the DPI, the higher is the force needed to ensure a sufficient pressure drop within the device and the lower the maximum flow rate [16].
Considering that DPIs are described as appropriate delivery devices for children of preschool age and older [3], there is a need for systematical investigations of the influencing parameters given above. On the one hand, costly in vivo studies including scintigraphic images using radioactive labeled material or pharmacokinetic studies of absorption and elimination can be carried out [17]. On the other hand, the performance of in vitro studies is desirable to assess the aerodynamic particle size distribution of aerosols and to get information about the pulmonary deposition of the API. Standard methods to determine the deposition pattern and the inhaler performance described in the European Pharmacopeia (induction port, preseparator, 4 kPa pressure drop and 4 L volume) poorly reflect in vivo conditions and serve solely as quality control tool. Preliminary in vitro investigations showed a discrepancy of the deposition data in adults and children [18]. In the particular case of children, there is a lack of sufficient in vivo data to verify results produced by in vitro studies. Regarding an enhanced in vitro and in vivo correlation, physical upper airway models representing the anatomy of different age groups in more detail can be used to determine the particle deposition.
A number of upper airway models utilized for several age groups are described in the literature [19,20]. For instance, the idealized adult upper airway model (“Alberta throat”, Figure 1a) [21] is widespread in the field of inhaler performance testing and showed good agreement with in vivo data. For pediatric use, the majority of the described upper airway models represent premature infants [22] and newborns [23]. Physical upper airway models applicable for older children are comparatively rarely described [10,24,25,26]. Golshahi et al. [24] recently introduced a child throat imitating the geometry of children at the age of 6–14 years. This is the main difference between the idealized pediatric upper airway model [10] (Figure 1b) and the pediatric realistic upper airway model [25] (Figure 1c) representing the age group of 4–5 years old children, used in this study. Both investigated upper airway models were developed based on MRI data and represent children from 4–5 years of age. Preliminary experiments using passive multidose DPIs (Easyhaler® and Novolizer®) exhibited, that the idealized pediatric upper airway model leads to reproducible results of inhaler performance and it is recommended as a feasible implement for DPI testing [27].
This study focuses on in vitro performance testing of reusable DPIs with disposable single dosed capsules (Cyclohaler®, Handihaler® and Spinhaler®) using improved upper airway models representing adults and preschool children compared to a conventional impactor inlet. The inhalation parameters flow rate and volume were varied (steady flow rates) and, in the case of the Cyclohaler®, a time-varying inhalation profile was used to determine the particle deposition in the different oropharyngeal and pulmonary compartments.
2. Experimental Section
2.1. Materials
2.1.1. Dry Powder Inhalers
Three dry powder inhalers were investigated: Cyclohaler® (Cyclocaps Salbutamol®, PB Pharma, Meerbusch, Germany), Handihaler® (Spiriva Handihaler®, Boehringer Ingelheim, Ingelheim, Germany) and Spinhaler® (Intal®, Fisons Arzneimittel, Cologne, Germany). The devices differ in their mechanism of powder deaggregation caused by the construction of the inhaler and the airflow resistance. Whereas Cyclohaler® and Handihaler® contain a fine grid to disperse the powder particles; the Spinhaler® is equipped with a propeller, which is operated by the air stream of the patient. With an airflow resistance of 0.055 (cm H2O)0.5 l min−1 for the Cyclohaler® [28] and 0.051 (cm H2O)0.5 l min−1 for the Spinhaler® [16], they belong to the low resistant dry powder inhaler. In contrast, the Handihaler® (0.158 (cm H2O)0.5 l min−1) is counted among the group of high resistant DPIs [29]. The present studies were performed using capsules from the commercially available product Cyclocaps Salbutamol® to eliminate the influence of the powder formulation on the deposition pattern. Finally, the native capsule piercing mechanism of the Cyclohaler® could not be ensured for the Handihaler® and the Spinhaler®, which was a limitation of our study. The capsules contain salbutamol sulfate as API and α-lactose monohydrate as drug carrier in an interactive mixture.
2.1.2. Upper Airway Models
In addition to the conventional setup according to European Pharmacopeia consisting of a 90° induction port and a preseparator [30], three physical upper airway models were tested. An idealized adult upper airway model [21], an idealized pediatric upper airway model [10] and a realistic pediatric upper airway model [25] were compared (Figure 1). These physical upper airway models consider the anatomical dimensions of the oral cavity, pharynx, larynx and trachea, which were determined on the basis of NMR (Magnetic Resonance Spectroscopy) and CT (Computed Tomography) data of adults and children of the respective age group. For the realistic pediatric model, data of 11 children at the age of 4–5 years and for the idealized pediatric model MRT scans of 5 years old children were used to calculate the dimensions of the different regions located in the upper airways.
2.2. Methods
2.2.1. Time-Varying Inhalation Profile
In order to get preliminary information about inhalation parameters of the patient using the Cyclohaler®, a time-varying inhalation profile was recorded during the inhalation through an empty device by a 4 years old male child (height of 110 cm and body weight of 20 kg). The boy was guided to inhale in an upright position in accordance to handling instructions of the marketed product. For this experiment, the inhaler was placed in an air tight plastic box, which had an associated pneumotachograph (Masterscope Viasys/Jaeger, Germany) on one side. On the opposite side, the inhaler mouth piece was accessible for the child (Figure 2). The peak inspiratory flow rate (PIF) and the inhalation volume over the time were recorded.
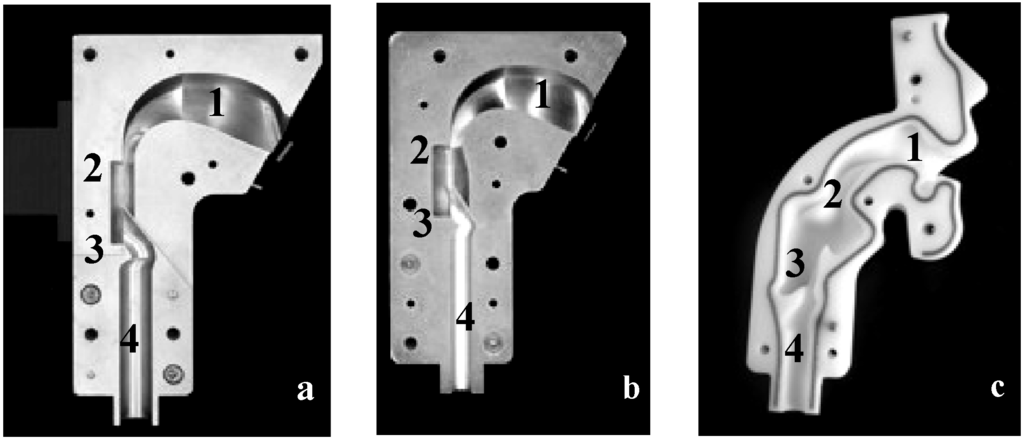
Figure 1.
Physical upper airway models including the different regions 1, oral cavity; 2, pharynx; 3, larynx; and 4, trachea. (a) idealized adult upper airway model; (b) idealized pediatric upper airway model representing 4–5 years old children; and (c) realistic pediatric upper airway model representing 4–5 years old children.
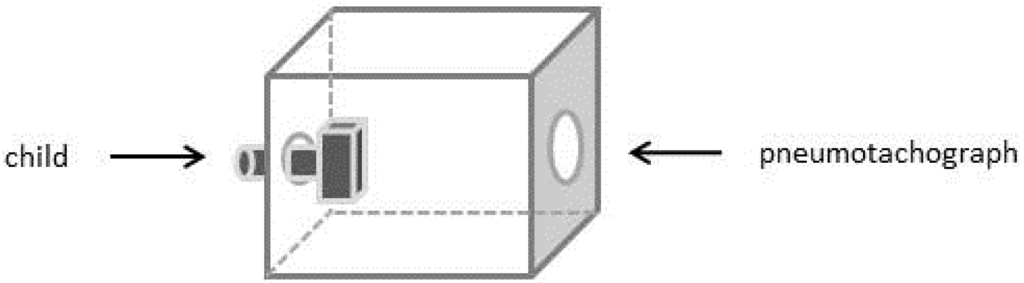
Figure 2.
Schematic presentation of the experimental setup for the generated time-varying inhalation profile; on the left side the pediatric patient; in the middle the Cyclohaler® incorporated in an air tight plastic box; on the right side the pneumotachograph.
2.2.2. Deposition Measurements—Steady Flow Rates
According to the standard method of the European Pharmacopeia (Ph. Eur. 2.9.18) for the aerodynamic assessment of fine particles, the experimental setup consisted of a next generation impactor (NGI, Copley Scientific Limited, Nottingham, UK) including a conventional inlet and preseparator. A flow meter (TSI 4000, TSI, Aachen, Germany) and a vacuum pump were used to provide a steady flow rate. The inhalation volume was adjusted by the process time to each flow rate.
For further experiments the inlet and preseparator described in Ph. Eur. were replaced by the physical upper airway models, which were connected to the NGI by a mixing inlet (Figure 3). The inner surfaces of the upper airway models and the cups of the NGI were coated by a 3% (w/w) solution of Brij® (Serva Eletrophoresis, Heidelberg, Germany) and glycerol to avoid a possible resumption of deposited particles in the airstream and to consider the wet conditions in the mucosal environment [27]. Ten capsules were used for each experiment. The deposited salbutamol sulfate was collected and quantified by high performance liquid chromatography (HPLC). The results were assessed based on the oropharyngeal deposition as amount of API collected in the upper airway model (including the inlet and preseparator according to Ph. Eur.), the pulmonary deposition as particles smaller than 5 μm and the emitted dose. The pulmonary deposition was calculated using the cut off diameters of the respective flow rate [30].
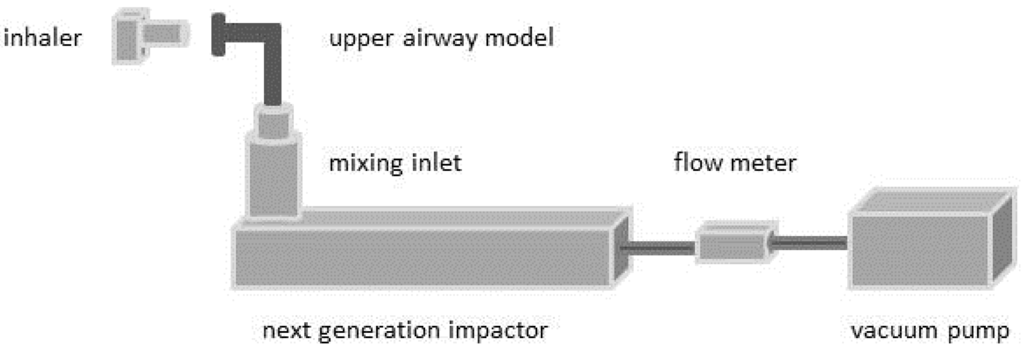
Figure 3.
Schematic presentation of the experimental setup used for deposition measurements.
The performance of the Cyclohaler® was investigated using three different steady flow rates (30, 60 and 75 L/min) and all described upper airway models (except the realistic pediatric model). The inhalation volume was adjusted at 1 L. The Handihaler® and the Spinhaler® were investigated at two steady flow rates (30 and 60 L/min) using the idealized adult and pediatric model. Table 1 shows the investigated flow rates and the achieved pressure drops within the different devices.

Table 1.
Pressure drops of the inhalers at different flow rates using a water column and dosing tube connected to the inhaler; mean ± SD; n = 3.
| Flow rate [L/min] | Pressure drop [kPa] ± s Cyclohaler® | Pressure drop [kPa] ± s Handihaler® | Pressure drop [kPa] ± s Spinhaler® |
|---|---|---|---|
| 30 | 0.22 ± 0.01 | 1.59 ± 0.05 | 0.14 ± 0.01 |
| 60 | 0.94 ± 0.03 | 6.14 ± 0.02 | 1.04 ± 0.01 |
| 75 | 1.17 ± 0.02 | - | - |
2.2.3. Deposition Measurements—Time-Varying Inhalation Profile
The pediatric time-varying inhalation profile was used for in vitro deposition studies. The experimental setup (Figure 3) was extended by an electronic lung device (ASL 5000, Ingmar Medical, Pittsburgh, PA, USA), which was connected via a mixing inlet to the NGI. The electronic lung simulated the inhalation profile only inside the DPI and the upper airway model. Within the NGI a constant flow rate of 60 L/min was assured. These deposition measurements were performed using the idealized and realistic pediatric upper airway model and the Cyclohaler® as drug delivery device.
2.2.4. High Performance Liquid Chromatography
The deposition of salbutamol sulfate was evaluated by HPLC (LaChrom Elite, VWR International, Darmstadt, Germany) using a Nucleodur C18 gravity 250/4 (Macherey & Nagel, Dueren, Germany) column [31]. Acetonitrile/acetic acid pH 3 (52/48, v/v) was chosen as isocratic eluent with a flow rate of 0.45 mL/min. The detection wavelength was set at 276 nm (UV/Vis-detector) and the oven temperature was adjusted at 35 °C.
3. Results and Discussion
3.1. Time-Varying Inhalation Profile—Experimental Plan
Only limited data regarding in vivo inhalation parameters (PIF and inhalation volume) of preschool children are available for the particular inhaler. Bronsky et al. [28] described that 91% of 32 children at the mean age of 9.1 years were able to generate flow rates of at least 60 L/min through the Aerolizer®, which is quite similar to the Cyclohaler® used in this study. Tiddens et al. [32] investigated the inhalation pattern of 96 humans at the age of 6 to 54 years using different types of inhalers (low, medium and high resistant inhalation devices). In the case of low resistant devices, children inhaled a mean volume of only 1.2 L. Both, the described flow rate as well as the inhalation volume do not match the requirements of the Ph. Eur. (2.9.18).
Based on this lack of information a time-varying inhalation profile of a 4 years old male child was recorded during inhalation through the Cyclohaler® (Figure 4, grey line). The additional information obtained by recording the inhalation profile formed the basis for the experimental plan and the chosen process parameters.
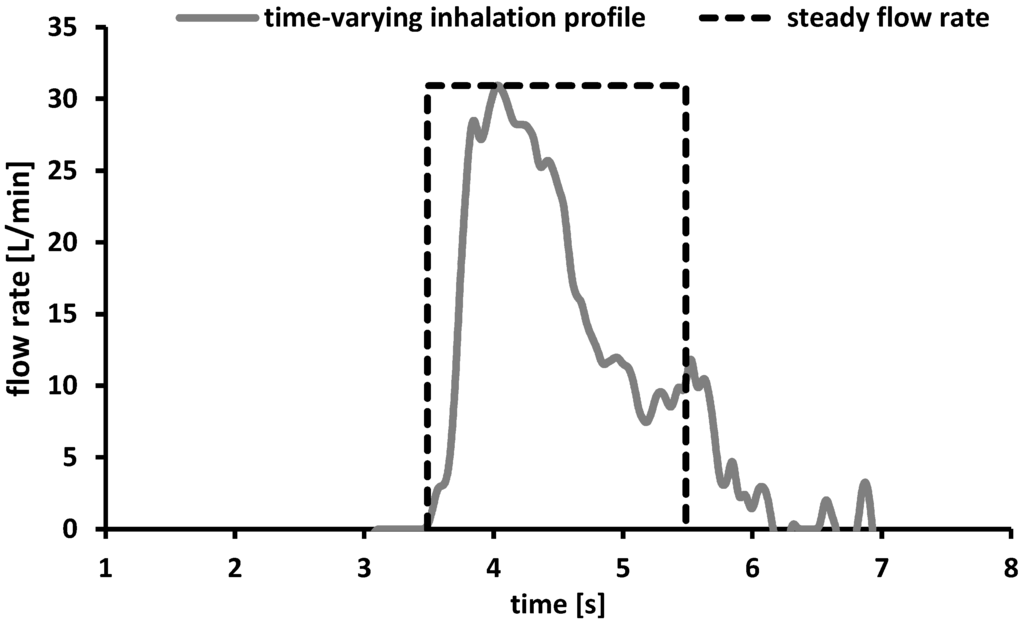
Figure 4.
Time-varying inhalation profile of a 4 years old child (grey line) and schematic presentation of a steady flow rate of 30 L/min and an inhalation volume of 1 L expressed as function of the inhalation time (dashed black line).
The child achieved the maximum PIF of 30 L/min within the first 0.46 s while the complete inhalation period took 3.46 s. The inhalation volume calculated as a function of the area under the curve was about 1 L and is comparable to the data described in literature [32]. The maximum PIF of only 30 L/min was lower than the flow rates reported in literature [28] and would result in an insufficient therapy using the Cyclohaler® as drug delivery device [33]. To cover a broad range of possible flow rates, the process parameters for further deposition measurements under steady conditions were chosen as follows. The minimum flow rate was set to 30 L/min to consider the PIF resulted from the time-varying inhalation profile. As maximum flow rate 75 L/min was selected as approach to the required conditions according to the Ph. Eur. and a flow rate of 60 L/min should consider the data provided by literature [28]. On the basis of the results discussed above, the inhalation volume was adjusted at 1 L.
To get an indication of the differences between steady flow rates and time-varying inhalation profiles, a systematical presentation of a steady flow rate proceed (30 L/min, volume 1 L) is given above (Figure 4). The maximum flow rate was achieved directly after starting the process and formed a plateau until the end of the process (regulated by the adjusted inhalation volume) and subsequently dropped down rapidly.
3.2. Deposition Measurements—Steady Flow Rates
In the treatment of respiratory diseases some key parameters have to be evaluated to ensure a sufficient therapy: the emitted dose as amount of API particles leaving the mouth piece, the oropharyngeal deposition and the pulmonary deposition, representing the amount of API, which can theoretically reach the lung and causes the therapeutic effect. The pulmonary deposition is affected by a multitude of factors, like the age-specific anatomy or the flow rate and requires a systematical investigation considered in this study.
Figure 5 includes the results of the in vitro deposition testing of the Cyclohaler® using steady flow rates and different impactor inlets. The emitted dose ranged from 59% ± 1.2% to 68% ± 2.2% for all investigated upper airway models (including the Ph. Eur. inlet) and flow rates. These values were far below the labeled dose. A change in flow rate from 30 L/min to 60 or 75 L/min resulted in no further increase of emitted dose in all investigated impactor inlets.
Regarding the oropharyngeal deposition, a tendency of lower deposition values in the idealized adult model compared to the idealized pediatric upper airway model and the Ph. Eur. inlet could be observed. But due to the partially high standard deviations, no differences in oropharyngeal deposition between the three investigated impactor inlets could be shown. Each deposition determination led to high values for oropharyngeal deposition and comparatively low amount of particles passing the airway model. These results were transferable to data described in the literature investigating a low to medium resistant multidose DPI [27] and they could be explained by inadequate powder deaggregation caused by the low resistance and the insufficient force of the drug delivery device.
The pulmonary deposition showed no influence induced by the oropharyngeal deposition and the emitted dose. The idealized pediatric upper airway model resulted in a lower pulmonary deposition compared to the Ph. Eur. inlet and the idealized adult model (except Ph. Eur. inlet using 30 L/min). At a steady flow rate of 60 L/min, a higher pulmonary deposition was achieved for the Ph. Eur. inlet (13% ± 0.9%) and the idealized adult upper airway model (15% ± 0.4%) compared to the idealized pediatric model (9% ± 0.4%). By increasing the steady flow rate from 30 to 75 L/min an enhancement of the pulmonary deposition could be obtained. The Ph. Eur. inlet showed the highest increase from 7% ± 0.7% to 14% ± 1.1%. The idealized adult upper airway model exhibited a rise from 11% ± 0.4% to 15% ± 0.5%. The pulmonary deposition using the idealized pediatric model was slightly increased from 9% ± 0.4% to 11% ± 1.1%. This relies on stronger deaggregation processes as consequence of higher energy values caused by higher flow rates.
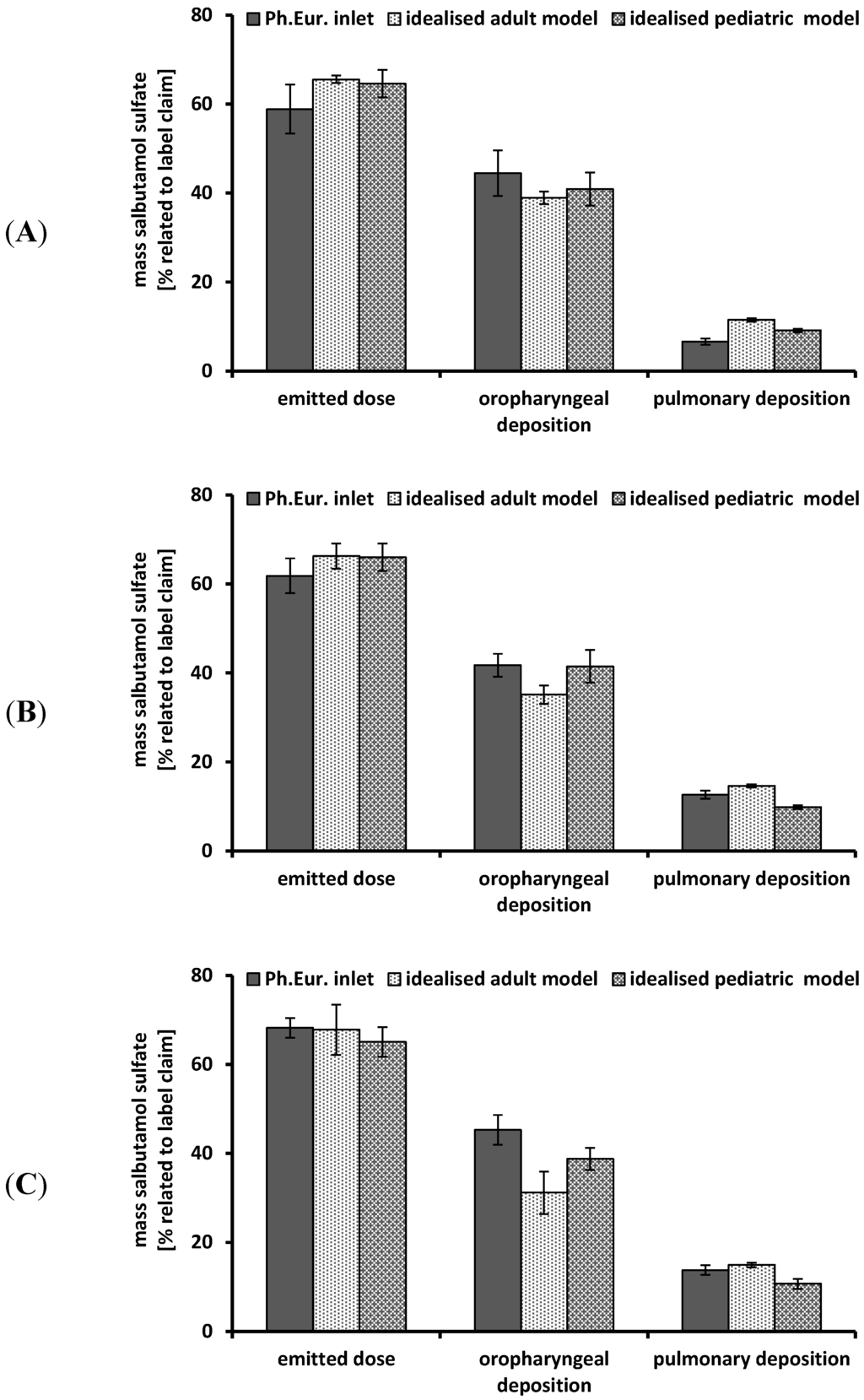
Figure 5.
Performance of the Cyclohaler® at different flow rates using different impactor inlets; Ph. Eur. inlet consisting of induction port and preseparator; idealized adult and pediatric upper airway model; realistic pediatric upper airway model. (A) flow rate 30 L/min; (B) flow rate 60 L/min; (C) flow rate 75 L/min; emitted dose, oropharyngeal deposition and pulmonary deposition were related to label claim; mean ± SD, n = 3.
3.3. Deposition Measurements—Time-Varying Inhalation Profile
The deposition measurements under steady conditions resulted in a low, flow-dependent pulmonary deposition. In the case of the Cyclohaler®, this observation was reviewed by a time-varying inhalation profile of a 4 years old boy, shown in Figure 4. Furthermore, the in vitro testing was extended by using a realistic pediatric upper airway model.
Figure 6 shows the performance results of the Cyclohaler® obtained by simulating the inhalation profile in the upper airway model and the inhalation device. No differences between the realistic and the idealized pediatric upper airway model could be detected. Despite of the more detailed structure of the realistic model, the oropharyngeal deposition was comparable. In both cases the pulmonary deposition was about 5% related to label claim. The idealized pediatric upper airway model seemed to be sufficient for in vitro DPI testing and led to feasible results.
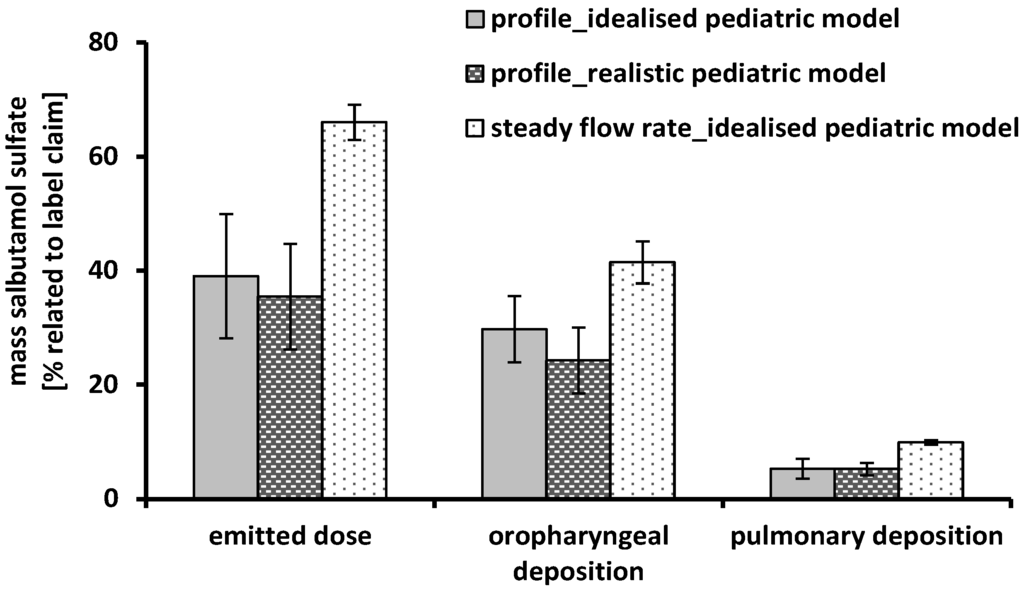
Figure 6.
Performance of the Cyclohaler® using the idealized pediatric upper airway model, the realistic pediatric upper airway model, the time-varying inhalation profile (grey and dashed black column) and a steady flow rate of 60 L/min (scored white column); emitted dose, oropharyngeal deposition and pulmonary deposition were related to label claim; mean ± SD, n = 3.
Another part of the present investigations was the comparison of steady flow rates against the time-varying inhalation profile. Regarding the emitted dose, a high difference between the two inhalation conditions could be observed. The amount of delivered API was 39% ± 10.9% using the inhalation profile and 66% ± 3.1% with the steady flow rate of 60 L/min. These results could possibly be explained by the different flow rates achieved in the DPI and the upper airway models accompanied by an additionally inhomogeneity proceed of the flow rate during the inhalation of the child. The maximum PIF was reached only for a short time of about 0.1 s and led to an insufficient and varying deflation of the capsules incorporated in the device. The oropharyngeal deposition was slightly higher under steady conditions (41% ± 3.7%) compared to the inhalation profile (30% ± 5.8%) and is mainly generated by a flow-dependent high intensity of particle impaction. However, this effect could be counterbalanced by a higher amount of particles smaller than 5 μm (pulmonary deposition) in the case of the steady flow rate. Despite of equal conditions in the NGI (steady flow rate of 60 L/min), there was an increase in pulmonary deposition from 5% ± 1.8% up to 10% ± 0.4% by using a steady flow rate of 60 L/min instead of the inhalation profile (maximum PIF of 30 L/min). The main reasons for this finding has been the enhanced deaggregation of the powder particles primarily occurring in DPI as result of the higher flow rate [34,35] and the much higher emitted dose for steady flow rates.
3.4. Transfer to Further DPIs
The results achieved from performance testing of the Cyclohaler® were transferred to two further DPIs, Handihaler® and Spinhaler®. The measurements were limited to steady flow rates (30 and 60 L/min) and the idealized adult and pediatric upper airway models.
Figure 7 shows the emitted dose, oropharyngeal and pulmonary deposition obtained by performance testing of the Handihaler® and the Spinhaler®. In the case of the Handihaler® no differences between the adult and the pediatric upper airway model could be determined. There was only a slight decrease from 19% ± 0.6% to 16% ± 0.3% in pulmonary deposition using the pediatric model and 60 L/min as steady flow rate. These results were comparable to data obtained by the Cyclohaler®. In this context, the device resistance and the pressure drop within the devices (Table 1) has to be taken into account. The Handihaler® is assigned to high resistant DPIs, demonstrated by the fact that a flow rate of only about 40 L/min was needed to cause a pressure drop of 4 kPa within the device [29]. In the case of the Cyclohaler® more than 100 L/min were necessary. This led to various energy inputs, which was closely related to the oropharyngeal and pulmonary deposition. The Handihaler® performance was not affected by flow rate; only the standard deviations were higher using a flow rate of 30 L/min. The Spinhaler® showed low values for the emitted dose. More than half of the powder remained in the capsules and led to high variations of the deposition pattern. This may be due to the exchange of the formulation, which has not been adapted for the device. Compared to the other investigated devices, the Spinhaler® showed the worst performance expressed as low emitted dose, high amount of API deposited in the upper airway models (72%–85% related to emitted dose) and low pulmonary deposition. Differences between the adult and pediatric upper airway model were less distinctive using low flow rates (30 L/min). At a flow rate of 60 L/min the pulmonary deposition in the pediatric model (9% ± 1.5%) was lower than in the adult model (18% ± 1.9%).
Recently reported innovations in the field of aerosol delivery were dealing with changes in the inhaler geometry (mouth piece or capsule chamber) by including a three-dimensional rod array [36,37]. This technology showed an improvement in inhaler performance of several devices, like the here investigated Handihaler® [38].
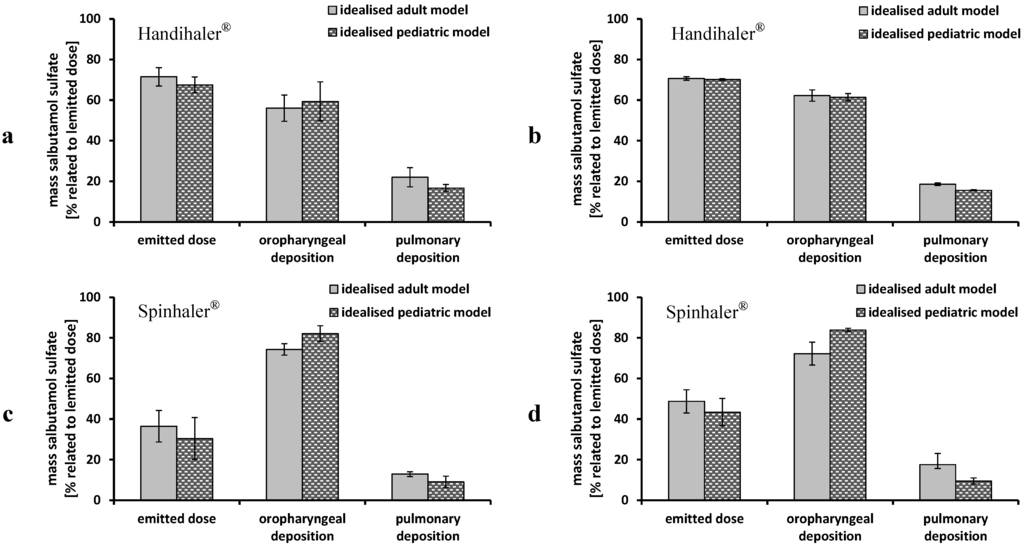
Figure 7.
Performance of Handihaler® and Spinhaler® using the idealized adult (grey columns) and pediatric (dashed black columns) upper airway models and steady flow rates; (a) Handihaler® 30 L/min; (b) Handihaler® 60 L/min; (c) Spinhaler® 30 L/min; and (d) Spinhaler® 60 L/min; emitted dose, oropharyngeal deposition and pulmonary deposition were related to emitted dose; mean ± SD, n = 3.
4. Conclusions
The majority of respiratory diseases demand a pulmonary administration of APIs to patients of all age groups. Hence, the therapy should consider age-specific characteristics of the main affecting elements: patient, device and formulation. This research focused on the influencing factors caused by the patient and the drug delivery device. Therefore, a time-varying inhalation profile was recorded during inhalation using the Cyclohaler®. It showed a maximum PIF of 30 L/min, which was lower than flow rates described in literature [28,33]. For the Cyclohaler®, comparative in vitro studies were performed using the pediatric inhalation profile and steady flow rates to investigate the influence of different inhalation conditions. This resulted in a lower pulmonary deposition of 5% ± 1.8% using the inhalation profile compared to 10% ± 0.4% achieved with steady flow rates. Time-varying inhalation profiles are promising tools to obtain information about the inhalation behavior of the patient and could be used for adjusting in vitro investigations to special age groups, especially children. However, this study is limited by the fact that only one trained 4 years old child was included. Apart from inhalation parameters influencing the delivery of inhalable particles, the age-specific anatomy of the oropharynx together with the amount of API particles concentrated in this region play an essential role. These particles are not available for lung therapy. In this context, improved upper airway models were included in this study. Using the Cyclohaler®, the idealized adult upper airway model led to similar results as the conventional induction port method of the European Pharmacopeia, but represents the upper airway geometry of adults in more detail and is today a tool frequently used for assessing device performance. With respect to children, an idealized and a realistic upper airway model was investigated, and both showed similar pulmonary deposition of about 5% related to label claim. The realistic pediatric upper airway model provided no additional information. There were slight differences manifested between the idealized adult and pediatric model, while the pulmonary depositions achieved using the pediatric model were always lower. The idealized pediatric upper airway model ensured a good reproducibility, confirming the results obtained by Below et al. [27].
To assess the performance of the Cyclohaler®, the flow dependent change in pulmonary deposition has to be taken into account, as this is an important aspect in inhalation therapy. Diversified flow rates, often appearing in children depending on their mental capacity and emotional state, yield an intra-individual heterogeneous therapy including varying amounts of API reaching the peripheral airways. Finally, the Cyclohaler® offered a restricted robustness with regard to varying flow rates. The high resistant device Handihaler® was less affected by flow rate, but led to deposition data comparable to the Cyclohaler®. The Spinhaler® showed a comparatively worse performance and limited reproducibility.
With focus on recent newly established requirements of the EMA and FDA regarding pediatric medicines, DPI performance testing in children will gain importance. Therefore, this idealized pediatric upper airway model can be recommended for in vitro testing of DPIs with single dosed capsules and is a promising tool in inhaler- and formulation development.
Acknowledgments
The authors would like to thank S. Stich for his helpful ideas as well as D. Bickmann and H. Wachtel for providing the electronic lung test system.
Author Contributions
Sandra Lindert as main author did the experimental work. Antje Below and Joerg Breitkreutz provided the idea and supervised this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bacharier, L.B.; Boner, A.; Carlsen, K.-H.; Eigenmann, P.A.; Frischer, T.; Götz, M.; Helms, P.J.; Hunt, J.; Liu, A.; Papadopoulos, N.; et al. Diagnosis and treatment of asthma in childhood: A PRACTALL consensus report. Allergy 2008, 63, 5–34. [Google Scholar] [CrossRef]
- Bisgaard, H.; Szefler, S. Prevalence of asthma-like symptoms in young children. Pediatr. Pulmonol. 2007, 42, 723–728. [Google Scholar] [CrossRef]
- Walsh, J.; Bickmann, D.; Breitkreutz, J.; Chariot-Goulet, M.; EuPFI. Delivery devices for the administration of paediatric formulations: Overview of current practice, challenges and recent developments. Int. J. Pharm. 2011, 415, 221–231. [Google Scholar] [CrossRef]
- Bressan, S.; Balzani, M.; Krauss, B.; Pettenazzo, A.; Zanconato, S.; Baraldi, E. High-flow nasal cannula oxygen for bronchiolitis in a pediatric ward: A pilot study. Eur. J. Pediatr. 2013, 172, 1649–1656. [Google Scholar] [CrossRef]
- Reflection paper: Formulations of choice for the paediatric formulation. Presented at Committee for Medicinal Products for Human Use (CHMP). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf (accessed on 16 January 2014).
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- Timsina, M.P.; Martin, G.P.; Marriott, C.; Ganderton, D.; Yianneskis, M. Drug delivery to the respiratory tract using dry powder inhalers. Int. J. Pharm. 1994, 101, 1–13. [Google Scholar] [CrossRef]
- Grgic, B.; Finlay, W.H.; Burnell, P.K.; Heenan, A.F. In vitro intersubject and intrasubject deposition measurements in realistic mouth-throat geometries. J. Aerosol Sci. 2004, 35, 1025–1040. [Google Scholar] [CrossRef]
- Amirav, I.; Newhouse, M.T. Deposition of small particles in the developing lung. Paediatr. Respir. Rev. 2012, 13, 73–78. [Google Scholar] [CrossRef]
- Bickmann, D.; Wachtel, H.; Kröger, R.; Langguth, P. Examining Inhaler Performance Using A Child’s Throat Model. Available online: http://www.rddonline.com/publications/articles/article.php?ArticleID=1189&return=1 (accessed on 15 December 2013).
- Amirav, I.; Newhouse, M.T.; Mansour, Y. Measurement of peak inspiratory flow with in-check dial device to simulate low-resistance (Diskus) and high-resistance (Turbohaler) dry powder inhalers in children with asthma. Pediatr. Pulmonol. 2005, 39, 447–451. [Google Scholar] [CrossRef]
- Kamps, A.W.; Brand, P.L.; Jan Roorda, R. Variation of peak inspiratory flow through dry powder inhalers in children with stable and unstable asthma. Pediatr. Pulmonol. 2004, 37, 65–70. [Google Scholar] [CrossRef]
- Adachi, Y.S.; Adachi, Y.; Itazawa, T.; Yamamoto, J.; Murakami, G.; Miyawaki, T. Ability of preschool children to use dry powder inhalers as evaluated by in-check meter. Pediatr. Int. 2006, 48, 62–65. [Google Scholar] [CrossRef]
- Bentur, L.; Mansour, Y.; Hamzani, Y.; Beck, R.; Elias, N.; Amirav, I. Measurement of inspiratory flow in children with acute asthma. Pediatr. Pulmonol. 2004, 38, 304–307. [Google Scholar] [CrossRef]
- Vidgren, M.; Kärkkäinen, A.; Karjalainen, P.; Paronen, P.; Nuutinen, J. Effect of powder inhaler design on drug deposition in the respiratory tract. Int. J. Pharm. 1988, 42, 211–216. [Google Scholar] [CrossRef]
- Clark, A.R.; Hollingworth, A.M. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—Implications for in vitro testing. J. Aerosol Med. 1993, 6, 99–110. [Google Scholar] [CrossRef]
- Borgström, L.; Nilsson, M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: Terbutaline. Pharm. Res. 1990, 7, 1068–1070. [Google Scholar] [CrossRef]
- Isaacs, K.K.; Martonen, T.B. Particle deposition in children’s lungs: Theory and experiment. J. Aerosol Med. 2005, 18, 337–353. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yamada, Y.; Yeh, H.C.; Swift, D.L. Deposition of ultrafine aerosols in a human oral cast. Aerosol Sci. Technol. 1990, 12, 1075–1081. [Google Scholar] [CrossRef]
- Olsson, B. Aerosol particle generation from dry powder inhalers: Can they equal pressurized metered dose inhaler? J. Aerosol Med. 1995, 8, 13–20. [Google Scholar] [CrossRef]
- Stapleton, K.W.; Guentsch, E.; Hoskinson, M.K.; Finlay, W.H. On the suitability of κ–ε turbulence modeling for aerosol deposition in the mouth and throat: A comparison experiment. J. Aerosol Sci. 2000, 31, 739–749. [Google Scholar]
- Minocchieri, S.; Burren, J.M.; Bachmann, M.A.; Stern, G.; Wildhaber, J.; Buob, S.; Schindel, R.; Kraemer, R.; Frey, U.P.; Nelle, M. Development of the premature infant nose throat-model (PrINT-Model)—An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 2008, 64, 141–146. [Google Scholar] [CrossRef]
- Janssens, H.M.; de Jongste, J.C.; Fokkens, W.F.; Robben, S.G.F.; Wouters, K.; Tiddens, H.A. The Sophia anatomical infant nose-throat (Saint) model: A valuable tool to study aerosol deposition in infants. J. Aerosol Med. 2001, 14, 433–441. [Google Scholar] [CrossRef]
- Golshahi, L.; Finlay, W.H. An idealized child throat that mimics average pediatric oropharyngeal deposition. Aerosol Sci. Technol. 2012, 46, I–IV. [Google Scholar] [CrossRef]
- Wachtel, H.; Bickmann, D.; Breitkreutz, J.; Langguth, P. Can pediatric throat models and air flow profiles improve our dose finding strategy? Available online: http://www.rddonline.com/publications/articles/article.php?ArticleID=1435 (accessed on 15 December 2013).
- Corcoran, T.E.; Shortall, B.P.; Kim, I.K.; Meza, M.P.; Chigier, N. Aerosol drug delivery using heliox and nebulizer reservoirs: Results from an MRI-based pediatric model. J. Aerosol Med. 2003, 16, 263–271. [Google Scholar] [CrossRef]
- Below, A.; Bickmann, D.; Breitkreutz, J. Assessing the performance of two dry powder inhalers in preschool children using an idealized pediatric upper airway model. Int. J. Pharm. 2013, 444, 169–174. [Google Scholar] [CrossRef]
- Bronsky, E.A.; Grossmann, J.; Henis, M.J.; Gallo, P.P.; Yegen, Ü.; Della Cioppa, G.; Kottakis, J.; Mehra, S. Inspiratory flow rates and volumes with the Aerolizer dry powder inhaler in asthmatic children and adults. Curr. Med. Res. Opin. 2004, 20, 131–137. [Google Scholar] [CrossRef]
- Al-Showair, R.A.; Tarsin, W.Y.; Assi, K.H.; Pearson, S.B.; Chrystyn, H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir. Med. 2007, 101, 2395–2401. [Google Scholar] [CrossRef]
- European Pharmacopeia Commission. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles (2.9.18). In European Pharmacopeia 7.8; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2013; pp. 274–285. [Google Scholar]
- Lohrmann, M.; Kappl, M.; Butt, H.-J.; Urbanetz, N.A.; Lippold, B.C. Adhesion forces in interactive mixtures for dry powder inhalers—Evaluation of a new measuring method. Eur. J. Pharm. Biopharm. 2007, 67, 579–586. [Google Scholar] [CrossRef]
- Tiddens, H.A.; Geller, D.E.; Challoner, P.; Speirs, R.J.; Kesser, K.C.; Overbeek, S.E.; Humble, D.; Shrewsbury, S.B.; Standaert, T.A. Effect of dry powder inhaler resistance on the inspiratory flow rates and volumes of cystic fibrosis patients of six years and older. J. Aerosol Med. 2006, 19, 456–465. [Google Scholar] [CrossRef]
- Nielsen, K.G.; Skov, M.; Klug, B.; Ifversen, M.; Bisgaard, H. Flow-dependent effect of formoterol dry-powder inhaled from the Aerolizer®. Eur. Respir. J. 1997, 10, 2105–2109. [Google Scholar] [CrossRef]
- Chew, N.Y.; Chan, H.-K. In vitro aerosol performance and dose uniformity between the Foradile® Aerolizer® and the Oxis® Turbohaler®. J. Aerosol Med. 2001, 14, 495–501. [Google Scholar] [CrossRef]
- De Boer, A.H.; Winter, H.M.; Lerk, C.F. Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers. Part 1. Inhalation characteristics, work of breathing and volunteer’s preference in dependence of the inhaler resistance. Int. J. Pharm. 1996, 130, 231–244. [Google Scholar] [CrossRef]
- Behara, S.R.; Farkas, D.; Hindle, M.; Longest, P.W. Development of a high efficiency dry powder inhaler: Effects of capsule chamber design and inhaler surface modifications. Pharm. Res. 2013. [Google Scholar] [CrossRef]
- Behara, S.R.; Longest, P.W.; Farkas, D.; Hindle, M. Development and comparison of new high-efficiency dry powder inhalers for carrier-free formulations. J. Pharm. Sci. 2013. [Google Scholar] [CrossRef]
- Son, Y.-J.; Longest, P.W.; Tian, G.; Hindel, M. Evaluation and modification of commercial dry powder inhalers for the aerolization of a submicrometer excipient enhanced growth (EEG) formulation. Eur. J. Pharm. Sci. 2013, 49, 390–399. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).