The Potential Role of Therapeutic Drug Monitoring for Safe and Effective Anti-Infective Therapy with Manipulated Dosage Forms
Abstract
1. Introduction
2. Manipulation of Solid Oral Dosage Forms
2.1. Classification and Features of Dosage Forms
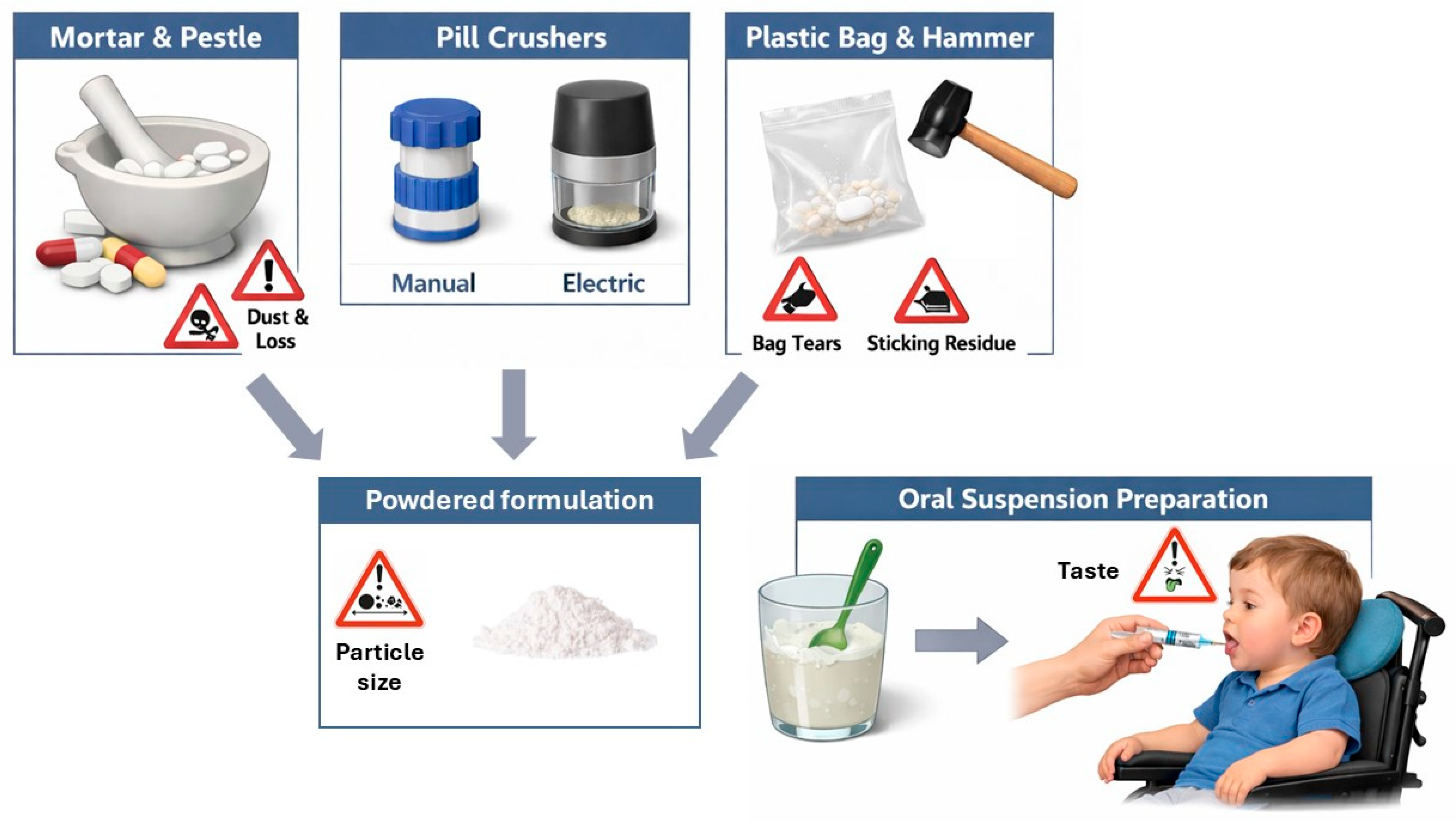
2.2. Practical Considerations on Crushing Oral Dosage Forms
2.3. Administration Problems
3. Case Studies
3.1. Isentress® 400 mg Film Coated Tablets (Raltegravir)
3.2. Prevymis® 240 mg Film Coated Tablets (Letermovir)
3.3. Zyvox® 600 mg Film-Coated Tablets (Linezolid)
| Isentress® 400 mg Film Coated Tablets | Prevymis® 240 mg Film Coated Tablets | Zyvox® 600 mg Film-Coated Tablets |
|---|---|---|
| Tablet core Microcrystalline cellulose (E460) Lactose monohydrate Calcium phosphate dibasic anhydrous Hypromellose 2208 Poloxamer 407 Sodium stearyl fumarate Magnesium stearate | Tablet core Microcrystalline cellulose (E460) Croscarmellose sodium (E468) Povidone (E1201) Colloidal anhydrous silica (E551) Magnesium stearate (E470b) | Tablet core Maize starch (corn derived) Microcrystalline cellulose (E460) Hydroxypropylcellulose (E463) Sodium starch glycollate type A Magnesium stearate (E572) |
| Film coat Polyvinyl alcohol Titanium dioxide Polyethylene glycol 3350 Talc Red iron oxide Black iron oxide | Film-coat Lactose monohydrate Hypromellose (E464) Titanium dioxide (E171) Triacetin (E1518) Iron oxide yellow (E172) Carnauba wax (E903) | Film coat Opadry, white, YS-1-18202-A(e) comprising: Hypromellose (E464) Titanium dioxide (E171) Macrogol 400 Carnauba wax (E903) |
| Drug (Product) | Dosage Form and Release Characteristics | Type of Manipulation | Key Pharmacokinetic Findings | Clinical Implications | Role of TDM |
|---|---|---|---|---|---|
| Raltegravir (Isentress® 400 mg) [72] | Film-coated tablet; immediate-release; soluble film, low solubility in acidic environment | Chewing/ grinding | Chewed tablets compared to intact tablets: Cmax (5404 ± 3032 vs. 3128 ± 2588 ng/mL, p = 0.004) and AUC0–4 (11.634 ± 7.288 versus 7.007 ± 5.803 ng h/mL, p = 0.011) and reduced PK variability | Improved patient compliance and more consistent absorption, despite lack of disintegration of intact tablets | Manipulation did not compromise target attainment and reduced variability |
| Letermovir (Prevymis® 240 mg) [79] | Film-coated tablet; immediate-release; pH-independent soluble film | Crushing and administration via nasogastric tube (pediatrics) | Crushed tablets compared to intact tablets: Cmax 1470 (1003–1903) vs. 1528 (1356–1777) ng/mL, Tmax 1.00 (0.75–1.50) vs. 1.00 (1.00–2.50) h and AUC 5367 (3667–7128) versus 5450 (4282–8908) ng · h/mL | Manipulation feasible for patients unable to swallow tablets; useful in pediatric and transplant settings | TDM potentially useful in complex clinical settings (e.g., drug interactions, hepatic impairment) |
| Linezolid (Zyvox® 600 mg) [84] | Film-coated tablet; conventional release; soluble coating | Tablet splitting (300 mg vs. 600 mg) | Whole tablets compared to split tablets: Cmax 0.019 ± 0.006 vs. 0.022 ± 0.01 mg/mL/mg, p = 0.272; AUC0–5 0.0744 ± 0.036 vs. 0.0739 ± 0.029 mg h/mL·mg, p = 0.962) | Enables dose reduction, flexibility and cost savings, particularly in elderly patients | TDM-guided dose reduction ensured maintenance of Ctrough targets and reduced toxicity risk |
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CI | Confidence Interval |
| Cmax | Peak plasma concentration |
| CMV | Cytomegalovirus |
| FDC | Fixed-dose combination |
| HIV | Human Immunodeficiency Virus |
| HPMC | Hydroxypropyl methyl cellulose |
| HPMC-AS | Hydroxypropyl methyl cellulose Acetate Succinate |
| IV | Intravenous |
| MCC | Microcrystalline cellulose |
| PO | Oral |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinyl pyrrolidone |
| STR | Single tablet regimen |
| TDM | Therapeutic Drug Monitoring |
| Tmax | Time to reach Cmax |
| WHO | World Health Organization |
References
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental Factors Influencing the Development and Spread of Antibiotic Resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.A.; Davey, P.G.; Grundmann, H. Mortality and Hospital Stay Associated with Resistant Staphylococcus Aureus and Escherichia Coli Bacteremia: Estimating the Burden of Antibiotic Resistance in Europe. PLoS Med. 2011, 8, e1001104. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Hillier, S.; Roberts, Z.; Dunstan, F.; Howard, A.; Palmer, S. Antibiotic-Resistant Infections in Primary Care Are Symptomatic for Longer and Increase Workload: Outcomes for Patients with E. Coli UTIs. Br. J. Gen. Pract. 2006, 56, 686–692. [Google Scholar]
- Ashiru-Oredope, D.; Cunningham, N.; Casale, E.; Muller-Pebody, B.; Hope, R.; Brown, C.S.; Hopkins, S. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR); Public Health England Publications: London, UK, 2021.
- McCarthy, K.; Avent, M. Oral or Intravenous Antibiotics? Aust. Prescr. 2020, 43, 45–48. [Google Scholar] [CrossRef]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Cyriac, J.M.; James, E. Switch over from Intravenous to Oral Therapy: A Concise Overview. J. Pharmacol. Pharmacother. 2014, 5, 83–87. [Google Scholar] [CrossRef]
- Kuper, K.M. Competence Assessment Tools for Health-System Pharmacies; Americal Society of Heatlh System Pharmacists: Bethesda, MD, USA, 2018. [Google Scholar]
- Tan, Z.Y.; Liang, W.X.; Zhang, N.; Liang, B.B.; Bai, N.; Cai, Y. Efficacy and Safety of Earlier Switching to an Oral Antibiotic Therapy for the Treatment of Gram-Positive Bloodstream Infections: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2025, 80, 2344–2360. [Google Scholar] [CrossRef]
- Banko, H.; Goldwater, S.H.; Adams, E. Smoothing the Path for Intravenous (IV) to Oral (PO) Conversion: Where Have We Come in 11 Years? Hosp. Pharm. 2009, 44, 959–967. [Google Scholar] [CrossRef]
- McMeekin, N.; Geue, C.; Briggs, A.; Rombach, I.; Li, H.K.; Bejon, P.; McNally, M.; Atkins, B.L.; Ferguson, J.; Scarborough, M. Cost-Effectiveness of Oral versus Intravenous Antibiotics (OVIVA) in Patients with Bone and Joint Infection: Evidence from a Non-Inferiority Trial. Wellcome Open Res. 2020, 4, 108. [Google Scholar] [CrossRef]
- Galanter, W.; Lambert, B.L. Analysis of Computer Alerts Suggesting Oral Medication Use during Computerized Order Entry of i.v. Medications. Am. J. Health-Syst. Pharm. 2010, 67, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Rivelsrud, M.C.; Hartelius, L.; Bergström, L.; Løvstad, M.; Speyer, R. Prevalence of Oropharyngeal Dysphagia in Adults in Different Healthcare Settings: A Systematic Review and Meta-Analyses. Dysphagia 2023, 38, 76–121. [Google Scholar] [CrossRef]
- Brennan, M.J.; Kopecky, E.A.; Marseilles, A.; O’Connor, M.; Fleming, A.B. The Comparative Pharmacokinetics of Physical Manipulation by Crushing of Xtampza® ER Compared with OxyContin®. Pain Manag. 2017, 7, 461–472. [Google Scholar] [CrossRef]
- Tahaineh, L.M.; Gharaibeh, S.F. Tablet Splitting and Weight Uniformity of Half-Tablets of 4 Medications in Pharmacy Practice. J. Pharm. Pract. 2012, 25, 471–476. [Google Scholar] [CrossRef]
- Quinzler, R.; Gasse, C.; Schneider, A.; Kaufmann-Kolle, P.; Szecsenyi, J.; Haefeli, W.E. The Frequency of Inappropriate Tablet Splitting in Primary Care. Eur. J. Clin. Pharmacol. 2006, 62, 1065–1073. [Google Scholar] [CrossRef]
- Gee, M.; Hasson, N.K.; Hahn, T.; Ryono, R. Effects of a Tablet-Splitting Program in Patients Taking HMG-CoA Reductase Inhibitors: Analysis of Clinical Effects, Patient Satisfaction, Compliance, and Cost Avoidance. J. Manag. Care Pharm. 2002, 8, 453–458. [Google Scholar] [CrossRef][Green Version]
- Duncan, M.C.; Castle, S.S.; Streetman, D.S. Effect of Tablet Splitting on Serum Cholesterol Concentrations. Ann. Pharmacother. 2002, 36, 205–209. [Google Scholar] [CrossRef]
- Faikoglu, G.; Saygisever-Faikoglu, K.; Otmar Ozcan, F.; Uskur, T.; Okan Yillar, D.; Berk, B.; Kelicen Ugur, P. The Pharmacological Perspective on Tablet Splitting or Crushing. Pharm. Pharmacol. Int. J. 2022, 10, 22–26. [Google Scholar] [CrossRef]
- Fouad, A.; Quintiliani, R.; Nicolau, D.P.; Asempa, T.E. Relative Bioavailability of Crushed Tebipenem Administered through a Nasogastric Tube with and without Enteral Feeding. J. Antimicrob. Chemother. 2023, 78, 205–208. [Google Scholar] [CrossRef]
- Druckenbrod, R.W.; Healy, D.P. In Vitro Delivery of Crushed Ciprofloxacin through a Feeding Tube. Ann. Pharmacother. 1992, 26, 494–495. [Google Scholar] [CrossRef]
- Huesgen, E.; DeSear, K.E.; Egelund, E.F.; Smith, R.; Max, B.; Janelle, J. A HAART-Breaking Review of Alternative Antiretroviral Administration: Practical Considerations with Crushing and Enteral Tube Scenarios. Pharmacotherapy 2016, 36, 1145–1165. [Google Scholar] [CrossRef]
- Roskam-Kwint, M.; Bollen, P.; Colbers, A.; Duisenberg-Van Essenberg, M.; Harbers, V.; Burger, D. Crushing of Dolutegravir Fixed-Dose Combination Tablets Increases Dolutegravir Exposure. J. Antimicrob. Chemother. 2018, 73, 2430–2434. [Google Scholar] [CrossRef]
- Brown, K.; Thomas, D.; McKenney, K.; Reeder, M.; Simonson, R.B.; Bicer, C.; Nettles, R.E.; Crauwels, H. Impact of Splitting or Crushing on the Relative Bioavailability of the Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Single-Tablet Regimen. Clin. Pharmacol. Drug Dev. 2019, 8, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Hocqueloux, L.; Lefeuvre, S.; Bois, J.; Brucato, S.; Alix, A.; Valentin, C.; Peyro-Saint-Paul, L.; Got, L.; Fournel, F.; Dargere, S.; et al. Bioavailability of Dissolved and Crushed Single Tablets of Bictegravir, Emtricitabine, Tenofovir Alafenamide in Healthy Adults: The SOLUBIC Randomized Crossover Study. J. Antimicrob. Chemother. 2023, 78, 161–168. [Google Scholar] [CrossRef]
- Ragonnet, G.; Laroche, H.; Néant, N.; Benkouiten, S.; Dos Santos, M.C.; Faucher-Zaegel, O.; Solas, C.; Bregigeon-Ronot, S. Enteral Administration of Crushed Rilpivirine in a Patient with HIV: A Case Report. Br. J. Clin. Pharmacol. 2024, 90, 895–899. [Google Scholar] [CrossRef]
- Chrdle, A.; Jerhotová, Z.; Vacík, M.; Linka, M.; Chmelík, V. Crushed Dolutegravir/Abacavir/Lamivudine given via Nasogastric Tube in Gastric Outlet Obstruction Caused by Cancer Resulted in Rapid Viral Load Suppression. Int. J. STD AIDS 2019, 30, 94–98. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Zhao, W.; Sidhu, D.S.; Viani, R.M.; Trinh, R.; Liu, W. A Phase 1 Study to Evaluate the Effect of Crushing, Cutting Into Half, or Grinding of Glecaprevir/Pibrentasvir Tablets on Exposures in Healthy Subjects. J. Pharm. Sci. 2018, 107, 1724–1730. [Google Scholar] [CrossRef]
- van Seyen, M.; Samson, A.D.; Cullen, L.; Eastick, K.; Knol, H.; Colbers, A.; Burger, D.M. Crushed Application of Sofosbuvir and Velpatasvir in a Patient with Swallowing Disorder. Int. J. Antimicrob. Agents 2020, 55, 105934. [Google Scholar] [CrossRef]
- Mogul, A.; Teixeira, E.; McAuliffe, L.; Promrat, K.; Zullo, A.R. Effectiveness of Crushed Sofosbuvir-Velpatasvir in a Patient with Dysphagia. Am. J. Health-Syst. Pharm. 2020, 77, 417–418. [Google Scholar] [CrossRef]
- Pijnenburg, D.W.M.; van Seyen, M.; Abbink, E.J.; Colbers, A.; Drenth, J.P.H.; Burger, D.M. Pharmacokinetic Similarity Demonstrated after Crushing of the Elbasvir/Grazoprevir Fixed-Dose Combination Tablet for HCV Infection. J. Antimicrob. Chemother. 2020, 75, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Huffman, V.; Andrade, D.C.; Sherman, E.; Niu, J.; Eckardt, P.A. Treatment of Chronic Hepatitis C Virus Infection with Crushed Ledipasvir/Sofosbuvir Administered through a Percutaneous Endoscopic Gastrostomy Tube in a Patient with HIV Coinfection. Am. J. Health-Syst. Pharm. 2021, 78, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Dodds Ashley, E.S.; Zaas, A.K.; Fang, A.F.; Damle, B.; Perfect, J.R. Comparative Pharmacokinetics of Voriconazole Administered Orally as Either Crushed or Whole Tablets. Antimicrob. Agents Chemother. 2007, 51, 877–880. [Google Scholar] [CrossRef]
- Bass, S.N.; Lam, S.W.; Bauer, S.R.; Neuner, E.A. Comparison of Oral Vancomycin Capsule and Solution for Treatment of Initial Episode of Severe Clostridium Difficile Infection. J. Pharm. Pract. 2015, 28, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ayachi, H.; Levillain, J.P. To Crush or Not to Crush, That Is the Question. Int. J. Clin. Pharm. 2016, 38, 526–527. [Google Scholar]
- Sagaria, N.; Mengato, D. “To Crush or Not to Crush, That Is the Question!” An Algorithm Can Help with the Proper Administration of Solid Oral Medicines in Patients Where the Oral Route Is Compromised. Eur. J. Hosp. Pharm. 2021, 30, e3. [Google Scholar]
- Stegemann, S.; Gosch, M.; Breitkreutz, J. Swallowing Dysfunction and Dysphagia Is an Unrecognized Challenge for Oral Drug Therapy. Int. J. Pharm. 2012, 430, 197–206. [Google Scholar] [CrossRef]
- Bryson, S.P. Patient-Centred, Administration Friendly Medicines for Children—An Evaluation of Children’s Preferences and How They Impact Medication Adherence. Int. J. Pharm. 2014, 469, 257–259. [Google Scholar] [CrossRef]
- Hermes, M.; Barnscheid, L.; Garsuch, V.; Schoettler, P.; Dominguez-Hirschi, J.; Breitkreutz, J. Drug Prescription for Children: Results of a Study on the Practical Effect of the EU Regulation No. 1901/2006—“Better Medicines for Children” since Its Coming into Effect in January 2007. Pharm. Ind. 2010, 72, 1334–1344. [Google Scholar]
- Sangnim, T.; Sriamornsak, P.; Singh, I.; Huanbutta, K. Swallowing Gel for Patients with Dysphagia: A Novel Application of Chitosan. Gels 2021, 7, 108. [Google Scholar] [CrossRef]
- Smith, L.; Leggett, C.; Borg, C. Administration of Medicines to Children: A Practical Guide. Aust. Prescr. 2022, 45, 188–192. [Google Scholar] [CrossRef]
- Stoltenberg, I.; Breitkreutz, J. Orally Disintegrating Mini-Tablets (ODMTs)—A Novel Solid Oral Dosage Form for Paediatric Use. Eur. J. Pharm. Biopharm. 2011, 78, 462–469. [Google Scholar] [CrossRef]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of Uncoated Mini-Tablets in Young Children: Results from a Prospective Exploratory Cross-over Study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Temer, A.C.; Teixeira, M.T.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Silva, I.C.; Taveira, S.F.; Marreto, R.N.; Cunha-Filho, M. Subdivision of Tablets Containing Modified Delivery Technology: The Case of Orally Disintegrating Tablets. J. Pharm. Innov. 2018, 13, 261–269. [Google Scholar] [CrossRef]
- Caussin, M.; Mourier, W.; Philippe, S.; Capet, C.; Adam, M.; Reynero, N.; Jouini, C.; Colombier, A.S.; Kadri, K.; Landrin, I.; et al. L’écrasement Des Médicaments En Gériatrie: Une Pratique « artisanale » Avec de Fréquentes Erreurs Qui Nécessitait Des Recommandations. Rev. Med. Interne 2012, 33, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, A.B.A.; Møller-Sonnergaard, J.; Christrup, L.L.; Højsted, J.; Hansen, R. Patients’ Evaluation of Shape, Size and Colour of Solid Dosage Forms. Pharm. World Sci. 2001, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, B.; Abebe, A. Determination of Tensile Strength of Shaped Tablets. Powder Technol. 2021, 383, 11–18. [Google Scholar] [CrossRef]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable Acceptance of Mini-Tablets Compared with Syrup: A Randomized Controlled Trial in Infants and Preschool Children. J. Pediatr. 2013, 163, 1728–1733. [Google Scholar] [CrossRef]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of Uncoated Mini-Tablets in Neonates—A Randomized Controlled Trial. J. Pediatr. 2015, 167, 893–896.e2. [Google Scholar] [CrossRef]
- Brotherman, D.P.; Bayraktaroglu, T.O.; Garofalo, R.J. Comparison of Ease of Swallowing of Dietary Supplement Products for Age-Related Eye Disease. J. Am. Pharm. Assoc. 2004, 44, 587–593. [Google Scholar] [CrossRef]
- Channer, K.S.; Virjee, J.P. The Effect of Size and Shape of Tablets on Their Esophageal Transit. J. Clin. Pharmacol. 1986, 26, 141–146. [Google Scholar] [CrossRef]
- Kelly, J.; Wright, D. Administering Medication to Adult Patients with Dysphagia. Nurs. Stand. 2009, 23, 62–68. [Google Scholar] [CrossRef]
- Vanham, D.; Spinewine, A.; Hantson, P.; Wittebole, X.; Wouters, D.; Sneyers, B. Drug-Drug Interactions in the Intensive Care Unit: Do They Really Matter? J. Crit. Care 2017, 38, 97–103. [Google Scholar] [CrossRef]
- Bakker, T.; Klopotowska, J.E.; Dongelmans, D.A.; Eslami, S.; Vermeijden, W.J.; Hendriks, S.; ten Cate, J.; Karakus, A.; Purmer, I.M.; van Bree, S.H.W.; et al. The Effect of Computerised Decision Support Alerts Tailored to Intensive Care on the Administration of High-Risk Drug Combinations, and Their Monitoring: A Cluster Randomised Stepped-Wedge Trial. Lancet 2024, 403, 439–449. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Seok, S.H.; Park, E.S. Enhanced Permeability and Oral Absorption of Panax Notoginseng Saponins by Borneol. J. Drug Deliv. Sci. Technol. 2021, 66, 102819. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Ph.Eur. Glossary. In European Pharmacopoeia; EDQM Council of Europe: Strasbourg, France, 2024; Volume 1502, p. 965. [Google Scholar]
- European Medicines Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use Table of Contents. Ema 2014, 44, 1–24. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 1 January 2026).
- Committee for Medicinal Products for Human Use (CHMP). European Medicines Agency Guideline on the Pharmacokinetic and Clinical Evaluation of Modified Release Dosage Forms (EMA/CPMP/EWP/280/96); Committee for Medicinal Products for Human Use (CHMP): Amsterdam, The Netherlands, 2014. [Google Scholar]
- Rathbone, M.J. Modified-Release Drug Delivery Technology; CRC Press: Boca Raton, FL, USA, 2008; Volume 1, ISBN 9781420044355. [Google Scholar]
- Administration Centre for Drug US Department of Health and Human Services Food and Drug; CDER Evaluation and Research. Guidance for Industry Dissolution Testing of Immediate Release Solid Oral Dosage Forms. 1997. Available online: https://www.fda.gov/media/70936/download (accessed on 1 January 2026).
- Fodil, M.; Nghiem, D.; Colas, M.; Bourry, S.; Poisson-Salomon, A.S.; Rezigue, H.; Trivalle, C. Assessment of Clinical Practices for Crushing Medication in Geriatric Units. J. Nutr. Health Aging 2017, 21, 904–908. [Google Scholar] [CrossRef]
- Allerton, G.L. Pill Crushing Device and System 2014. USPTO Patent US8684290B1, 1 April 2014. Available online: https://worldwide.espacenet.com/patent/search/family/050348743/publication/US8684290B1?q=pn%3DUS8684290B1 (accessed on 1 January 2026).
- Banov, F. The Art of Flavoring with Fabiana Banov: Mortar & Pestle. Available online: https://podcasts.apple.com/us/podcast/the-art-of-flavoring-with-fabiana-banov/id1436042984?i=1000537162275 (accessed on 1 January 2026).
- Palugan, L.; Cerea, M.; Vecchio, C.; Maroni, A.; Foppoli, A.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Gazzaniga, A. Newly Designed Punch for Scored Tablets: Evaluation by an Expert System Based on Quality by Design. J. Drug Deliv. Sci. Technol. 2021, 65, 102729. [Google Scholar] [CrossRef]
- Coldani, M.E.; Palugan, L.; Foppoli, A.; Cerea, M.; Pinto, J.F. Evaluation of Different Techniques for Wet Granulation and Pelletization Processes Using Milk as Innovative Pharmaceutical Excipient for Pediatric Use. Int. J. Pharm. 2024, 666, 124836. [Google Scholar] [CrossRef]
- Cerea, M.; Zheng, W.; Young, C.R.; McGinity, J.W. A Novel Powder Coating Process for Attaining Taste Masking and Moisture Protective Films Applied to Tablets. Int. J. Pharm. 2004, 279, 127–139. [Google Scholar] [CrossRef]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste Masking Technologies in Oral Pharmaceuticals: Recent Developments and Approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef]
- Nahata, M.C.; Allen, L.V. Extemporaneous Drug Formulations. Clin. Ther. 2008, 30, 2112–2119. [Google Scholar] [CrossRef]
- Mennella, J.A.; Beauchamp, G.K. Optimizing Oral Medications for Children. Clin. Ther. 2008, 30, 2120–2132. [Google Scholar] [CrossRef]
- Cattaneo, D.; Baldelli, S.; Cerea, M.; Landonio, S.; Meraviglia, P.; Simioni, E.; Cozzi, V.; Fucile, S.; Gazzaniga, A.; Clementi, E.; et al. Comparison of the in Vivo Pharmacokinetics and in Vitro Dissolution of Raltegravir in HIV Patients Receiving the Drug by Swallowing or by Chewing. Antimicrob. Agents Chemother. 2012, 56, 6132–6136. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA) Prevymis—EMA/80635/2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/prevymis (accessed on 29 August 2025).
- Kuhn, A.; Puttkammer, J.; Madigan, T.; Dinnes, L.; Khan, S.; Ferdjallah, A.; Kohorst, M. Letermovir as Cytomegalovirus Prophylaxis in a Pediatric Cohort: A Retrospective Analysis. Transplant. Cell. Ther. 2023, 29, 62.e1–62.e4. [Google Scholar] [CrossRef]
- Richert-Przygonska, M.; Jaremek, K.; Debski, R.; Konieczek, J.; Lecka, M.; Dziedzic, M.; Bogiel, T.; Styczynski, J.; Czyzewski, K. Letermovir Prophylaxis for Cytomegalovirus Infection in Children After Hematopoietic Cell Transplantation. Anticancer. Res. 2022, 42, 3607–3612. [Google Scholar] [CrossRef]
- Galaverna, F.; Baccelli, F.; Zama, D.; Tridello, G.; Masetti, R.; Soncini, E.; Mura, R.; Barzaghi, F.; Colombini, A.; Prunotto, G.; et al. Letermovir for Cytomegalovirus Infection in Pediatric Patients Undergoing Allogenic Hematopoietic Stem Cell Transplantation: A Real-Life Study by the Infectious Diseases Working Group of Italian Association of Pediatric Hematology-Oncology (AIEOP). Bone Marrow Transplant. 2024, 59, 505–512. [Google Scholar] [CrossRef]
- Körholz, K.F.; Füller, M.A.; Hennies, M.; Holterhus, M.; Hagedorn, S.; Ahlmann, M.; Thorer, H.; Burkhardt, B.; Groll, A.H. Letermovir for Prophylaxis and Pre-Emptive Therapy of Cytomegalovirus Infection in Paediatric Allogeneic Haematopoietic Cell Transplant Patients. Pediatr. Drugs 2023, 25, 225–232. [Google Scholar] [CrossRef]
- MSD Prevymis Tablets 240 mg Related Materials Book. Available online: https://www.pmda.go.jp/drugs/2018/P20180402006/index.html (accessed on 20 November 2024).
- Deleenheer, B.; Spriet, I.; Maertens, J. Pharmacokinetic Drug Evaluation of Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic Stem Cell Transplantation. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1197–1207. [Google Scholar] [CrossRef]
- Suetsugu, K.; Shigematsu, T.; Nakamura, T.; Hirota, T.; Ieiri, I. Clinical Pharmacokinetics and Pharmacodynamics of Letermovir in Allogenic Hematopoietic Cell Transplantation. Clin. Pharmacokinet. 2024, 63, 945–964. [Google Scholar] [CrossRef]
- Pfizer ZYVOX (Linezolid) Injection (Linezolid) Tablets (Linezolid) for Oral Suspension. Pfizer Drug Manual. 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021132s027lbl.pdf (accessed on 1 January 2026).
- Cattaneo, D.; Fusi, M.; Cozzi, V.; Baldelli, S.; Bonini, I.; Gervasoni, C.; Clementi, E. Supra-Therapeutic Linezolid Trough Concentrations in Elderly Patients: A Call for Action? Clin. Pharmacokinet. 2021, 60, 603–609. [Google Scholar] [CrossRef]
- Cattaneo, D.; Gervasoni, C.; Cozzi, V.; Castoldi, S.; Baldelli, S.; Clementi, E. Therapeutic Drug Management of Linezolid: A Missed Opportunity for Clinicians? Int. J. Antimicrob. Agents 2016, 48, 728–731. [Google Scholar] [CrossRef]
- Tietjen, A.K.; Kroemer, N.; Cattaneo, D.; Baldelli, S.; Wicha, S.G. Population Pharmacokinetics and Target Attainment Analysis of Linezolid in Multidrug-Resistant Tuberculosis Patients. Br. J. Clin. Pharmacol. 2022, 88, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Datapharm Ltd. Electronic Medicine Compendium. Available online: https://www.medicines.org.uk/emc/product/1688 (accessed on 1 January 2026).


| Dosage Form | Drug Release Mode | Details | Examples of Typical Excipients Used | Manipulation (Crushing/ Chewing) | |
|---|---|---|---|---|---|
| Uncoated tablet | Immediate-release | Uncoated powder/granules | Tablet | Diluent (cellulose microcrystalline, mannitol, lactose, starch), disintegrant (cross-caramellose, cross-povidone), lubricant (magnesium stearate), anti-sticking (talc), glidant (colloidal silica), surfactant (sodium lauryl sulphate) | Ok |
| Modified-release: prolonged (sustained, retard, extended, pulsatile) | Uncoated powder/uncoated granules/coated or uncoated tablets | Tablet | Hydrophilic polymer (hypromellose, hydroxypropyl cellulose), hydrophobic materials (ethylcellulose, stearic acid, lipids) | No | |
| Coated tablet | Immediate-release | Soluble coating | Coating | Hypromellose (HPMC), PVA, PVP, PVP-PVA… | Ok |
| Modified-release: gastro-resistance (delayed, enteric) | Gastric-resistant coating | Coating | pH-dependent solubility materials (methacrylate or methacrylic acid polymers, Eudragit L, HPMC-AS) | No | |
| Modified-release: prolonged (sustained, retard, extended) | Osmotic pump | Coating | Cellulose acetate | No | |
| Modified-release: prolonged (sustained, retard, extended) | Coated tablet | Coating | Ethylcellulose, Eudragit RL or RS | No | |
| Capsule | Immediate-release | Powder | Diluent (cellulose microcrystalline, mannitol, lactose, starch), disintegrant (cross-caramellose, cross-povidone), lubricant (magnesium stearate), anti-sticking (talc), glidant (colloidal silica), surfactant (sodium lauryl sulphate) | Ok | |
| Uncoated granules | Excipients for powder formulation and binders (PVP, hypromellose) | Ok | |||
| Coated granules | Coating | Soluble materials (hypromellose, HPMC, PVA, PVP, PVP-PVA) | Ok | ||
| Modified-release | Coated granules | Coating | Methacrylate or methacrylic acid polymers (e.g., HPMC-AS) | No | |
| Pellets (uncoated/coated) | Core | Excipients for pelletisation and binders (MCC, PVP, hypromellose) | No | ||
| Coating. | Methacrylate or methacrylic acid polymers (e.g., HPMC-AS) Hydrophilic polymer (hypromellose, hydroxypropyl cellulose), hydrophobic materials (ethylcellulose, stearic acid, lipids) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Baldelli, S.; Borgonovo, F.; Foppoli, A.; Gori, A.; Cattaneo, D.; Cerea, M. The Potential Role of Therapeutic Drug Monitoring for Safe and Effective Anti-Infective Therapy with Manipulated Dosage Forms. Pharmaceutics 2026, 18, 176. https://doi.org/10.3390/pharmaceutics18020176
Baldelli S, Borgonovo F, Foppoli A, Gori A, Cattaneo D, Cerea M. The Potential Role of Therapeutic Drug Monitoring for Safe and Effective Anti-Infective Therapy with Manipulated Dosage Forms. Pharmaceutics. 2026; 18(2):176. https://doi.org/10.3390/pharmaceutics18020176
Chicago/Turabian StyleBaldelli, Sara, Fabio Borgonovo, Anastasia Foppoli, Andrea Gori, Dario Cattaneo, and Matteo Cerea. 2026. "The Potential Role of Therapeutic Drug Monitoring for Safe and Effective Anti-Infective Therapy with Manipulated Dosage Forms" Pharmaceutics 18, no. 2: 176. https://doi.org/10.3390/pharmaceutics18020176
APA StyleBaldelli, S., Borgonovo, F., Foppoli, A., Gori, A., Cattaneo, D., & Cerea, M. (2026). The Potential Role of Therapeutic Drug Monitoring for Safe and Effective Anti-Infective Therapy with Manipulated Dosage Forms. Pharmaceutics, 18(2), 176. https://doi.org/10.3390/pharmaceutics18020176








